Abstract
Microbial parasites of animals include bacteria, viruses, and various unicellular eukaryotes. Because of the difficulty in studying these microorganisms in both humans and disease vectors, laboratory models are commonly used for experimental analysis of host-parasite interactions. Drosophila is one such model that has made significant contributions to our knowledge of bacterial, fungal, and viral infections. Despite this, less is known about other potential parasites associated with natural Drosophila populations. Here, we surveyed sixteen Drosophila populations comprising thirteen species from four continents and Hawaii and found that they are associated with an extensive diversity of trypanosomatids (Euglenozoa, Kinetoplastea). Phylogenetic analysis finds that Drosophila-associated trypanosomatids are closely related to taxa that are responsible for various types of leishmaniases and more distantly related to the taxa responsible for human African trypanosomiasis and Chagas disease. We suggest that Drosophila may provide a powerful system for studying the interactions between trypanosomatids and their hosts.
Introduction
A century of basic research in Drosophila genetics, physiology, ecology, and evolution has solidified its status as a model organism for biological research. Work in Drosophila informs applied research across a variety of disciplines, including drug discovery [1], the genetic basis of human diseases [2], [3], and the genomics of insect resistance to pesticides [4]. One area where Drosophila has made a particularly strong impact is the study of the animal response to microbial pathogens [5]. For example, the discovery that the intracellular bacterium Wolbachia reduces viral growth in Drosophila melanogaster [6] raises the possibility that Wolbachia-infected Aedes aegypti mosquitoes will be an effective control against dengue virus transmission [7], [8]. Drosophila has also proven to be a valuable model for human diseases. Cystic fibrosis (CF) in humans is commonly associated with infection by the opportunistic pathogen Pseudomonas aeruginosa. Drosophila has been developed as a model for CF and it was found that other bacterial taxa isolated from CF patients can modify P. aeruginosa’s role in CF afflicted Drosophila [9]. This polymicrobial view of CF infection is now being applied to human patients [10], [11]. Thus, the utility of using Drosophila as a model for host-microbe interactions is well established. However, one area where it has rarely been applied is the study of animal-trypanosomatid interactions.
Trypanosomatids (Euglenozoa, Kinetoplastea) are unicellular eukaryotic parasites of invertebrates, vertebrates, and plants [12]. Parasitic trypanosomatids can be primarily restricted to one host (monoxenous) or cycle between two hosts (dixenous). Several dixenous trypanosomatids are clinically important human pathogens that are vectored by insects. Among these are Trypanosoma brucei, Trypanosoma cruzi and various species of Leishmania, which are the causative agents of human African trypanosomiasis, Chagas disease, and the leishmaniases, respectively. These three neglected tropical diseases account for over 60,000 human deaths per year [13].
Monoxenous trypanosomatids have been detected in Diptera, Hemiptera, Hymenoptera, Lepidoptera, and Siphonaptera [14], [15] although the true diversity of insect-associated trypanosomatids is likely far from realized [14], [16]. Indeed, extensive surveys within Heteroptera have found that nearly a quarter of all individuals are infected, many with previously undescribed trypanosomatid strains [17], [18], [19], [20]. These surveys challenge the “one host – one parasite” view of trypanosomatid infection [21] and suggest that the factors defining host-parasite specificity are not well understood in monoxenous trypanosomatids. While the negative effects, if any, of most trypanosomatids are unknown, a common parasite of bumble bees, Crithidia bombi, imposes dramatic fitness consequences on hibernating queens, which subsequently leads to reduced colony-founding success [22].
Despite an abundance of studies on Drosophila interactions with bacteria, fungi, and viruses [5], Drosophila-trypanosomatid interactions have been neglected. Trypanosomatids were first found in Drosophila confusa [23] and were subsequently found to be prevalent in natural fly populations in both Europe and the United States [15], [24], [25]. Infections spread quickly through laboratory populations and then are maintained over the course of at least 250 days [26]. Trypanosomatids are observed in the intestines and the Malpighian tubules of flies and in laboratory media and bananas that have been used by infected individuals [15], [26]. To our knowledge, only one study has attempted to define the molecular basis of immune response to trypanosomatid infection in Drosophila [27]. The authors find that host survival and antimicrobial peptide production is dependent upon both trypanosomatid species and whether the parasite is ingested orally or injected directly into hemolymph.
Because Drosophila is an established model organism for studying host-microbe interactions and because trypanosomatids are important parasites of both humans and insects, we sought to characterize the diversity of trypanosomatids associated with Drosophila. Over 3000 species of Drosophila and related genera inhabit every continent except Antarctica, and these taxa utilize a great variety of substrates as feeding and breeding sites [28], [29]. In this study, we wanted to survey a wide breadth of host phylogenetic, geographic, and ecological diversity in order to capture the extent of trypanosomatid diversity associated with natural populations of Drosophila. Fourteen different species of flies from four continents were collected, with special emphasis placed on flies obtained from a variety of feeding sites, including fruits, flowers, cacti, and mushrooms. We show that Drosophila-associated trypanosomatids are closely related to other monoxenous insect trypanosomatids and follow the same patterns of host-infectivity and geographic distribution. We end with a discussion of how research into Drosophila-trypanosomatid interactions can inform trypanosomatid work in general and suggest that Drosophila represents a powerful and underused model for studying the transmission and virulence of these often neglected parasites.
Methods
Fly Collection, Dissection, and DNA Extraction
Drosophila samples were collected with the help of many colleagues around the world (see Acknowledgments, Table 1, Dataset S1). No specific permits were required for the described field studies and owners of private residences provided informed consent before collections took place. All samples were obtained from naturally occurring substrates, and no artificial baits were used to attract flies. For collections done in Northern California, adults were immediately transferred to sterile no-nutrient media (2% agar in water) and transported to the University of California, Davis for dissection. For more remote field collections, flies were stored in 100% ethanol for transport. For freshly collected flies, the entire gut was dissected. However, for flies stored in ethanol, dissection was not feasible because weakening of the fly tissues caused the gut to fragment. For these samples, the entire fly bodies were externally sterilized before DNA extraction. Specifically, the entire fly bodies were washed twice in 1 ml 2.5% sodium hypochlorite and twice in 1 ml sterile water with each wash consisting of 30 seconds of vortexing at max speed with 0.5 ml of 0.1 mm glass beads. Seven to 20 fly bodies or guts were combined for each sample. The detailed DNA extraction protocol can be found in [30]. Further details regarding sample collection dates, locations, and contents can be found in Dataset S1.
Table 1. Drosophila populations associated with trypanosomatids.
| LibraryName | Number oftrypanosomatidSequences | Species | Diet | Location |
| ANM | 36 | D. ananassae | Morinda fruit | Captain Cook, Hawaii |
| ELA | 65 | D. elegans | Alpinia flowers | Hsinchu, Taiwan |
| ELD | 3 | D. elegans | Brugmansia flowers | Hsinchu, Taiwan |
| FNS | 18 | D. falleni | Russula mushrooms | Stony Brook, NY |
| ICF | 55 | D. immigrans | Citrus fruit | Wolfskill Experimental Orchard, Winters, Ca |
| IMH | 127 | D. sp. aff. immigrans | Hibiscus flowers | Captain Cook, Hawaii |
| MEC | 46 | D. malerkotliana | Terminalia fruit | Seychelles islands, Africa |
| MIC | 8 | Microdrosophila sp. | Shelf fungus | Malaysia |
| MOV | 9 | D. mojavensis and D. arizonae | Agria cactus | Sonora, Mexico |
| NNS | 3 | D. neotestacea | Russula mushrooms | Stony Brook, NY |
| POM | 236 | Unidentified Drosophila sp. | Ipomoea flowers | Waimanu, Hawaii |
| PON | 5 | Unidentified Drosophila sp. | Pandanus fruit | Waimanu, Hawaii |
| SCA | 3 | Scaptodrosophila hibiscii | Hibiscus flowers | Queensland, Australia |
| SPP | 1 | D. melanogaster and D. simulans | Opuntia fruit | Arboretum, Davis, Ca |
| TKM | 290 | D. takahashii | Morinda fruit | Captain Cook, Hawaii |
Further details provided in Dataset S1.
Library Creation and Sequencing
Details of primer design, PCR conditions, sequencing, and quality checking parameters are provided in [31]. Briefly, the D1/D2 loop of the rDNA 28S large subunit (LSU) was amplified using the primers NL1 and NL4 [32] (Dataset S2). The amplified LSU from each sample was sequenced on a Roche GS Junior Titanium machine in the laboratory of Dr. Jonathan Eisen with the assistance of the University of California, Davis Microarray Core Facility. 12819 total reads were generated. The raw sequencing reads were checked using the QIIME platform [33] resulting in 4877 high-quality reads for analysis. This dataset was previously used to describe the yeast communities associated with these same Drosophila populations [31].
Initial Identification of Trypanosomatid Reads and Sequence Alignment
Initial taxonomy assignment was performed by querying each of the 4877 sequences to the entire NCBI database (as of 10/21/2011). 961 sequences had a closest match to either Leishmania donovani (911 sequences) or Crithidia fasciculata (50 sequences). These sequences will be the focus of this study. The remaining sequences have a nearest blast hit to fungi, Drosophila, or plants [31].
To understand why so many trypanosomatid sequences were amplified with primers primarily used for fungal identification, the NCBI primer blast tool was used to determine the specificity of the NL1 and NL4 primers. Both the primers were exactly complementary to their proposed binding sites in many fungi including common Drosophila associates such as species of Hanseniaspora and Saccharomyces [31] (data not shown). When these primers were queried against the genomes of the trypanosomatids, Leishmania major, Trypanosoma brucei and Crithidia fasciculata, it was found that the NL4 primer is exactly complementary to its proposed binding sites, whereas three mismatches occur with the NL1 primer (data not shown).
The 961 putative trypanosomatid sequences have an average length of 488 base pairs (min = 469, max = 561). 24 sequences were assumed to be chimeric and removed because an NCBI Blast search found that the 100 final base pairs were not closely related to any trypanosomatid. The remaining 937 sequences were aligned using maffT and the genafpair option to produce an alignment of 673 columns [34], [35]. Since many positions toward the end of the alignment (roughly corresponding to the D2 region) contained mostly gaps, the final 163 columns were removed. Despite removing these 163 columns from the alignment, an average of only 67 nucleotides was removed from each read (min = 41, max = 88).
25 sequences were identified as chimeric using the UCHIME chimera checker in mothur [36]. The remaining 912 sequences were re-aligned using maffT producing an alignment of 502 positions. Seven additional sequences were identified as chimeric in this alignment. After their removal, maffT was used to produce the final alignment of 905 sequences and 500 columns. The final dataset consists of 15 libraries with an average of 60 sequences each (min = 1, max = 284). Seven of the libraries contain ten or fewer sequences. The 905 sequences used in the final analysis are available through NCBI under the accession numbers KC182802 to KC183706. All sequences and alignments are available through figshare (http://dx.doi.org/10.6084/m9.figshare.106978).
OTU Generation, Diversity Measurements and Phylogenetic Analysis
Prior to community and phylogenetic analyses, similar sequences were grouped into operational taxonomic units (OTUs). This is done because each sequence represents a different individual within the microbial community and similar sequences come from closely related individuals. OTUs are therefore surrogates for the different microbial taxa within the community. The software package mothur was used to generate OTUs from the chimera-checked alignment [36]. OTUs were formed at the 3% divergence level (97% similarity) using the average neighbor clustering algorithm and the countends = F option during the calculation of the distance matrix. This is the same similarity threshold used for the bacterial [30] and yeast [31] communities associated with these Drosophila populations. OTU clustering produced 17 OTUs with an average size of 52.2 sequences (SD = 91.3). The largest OTU contains 314 sequences, nine OTUs contain only one sequence, and 11 OTUs contain 10 or fewer sequences (Table 2, Dataset S3). OTUs were also clustered at the “unique” cutoff (identical sequences are grouped together; 0% divergence). This produced 414 OTUs with an average size of 2.2 sequences (SD = 7.1). The largest OTU contains 92 sequences, 365 OTUs contain only one sequence, and 405 OTUs contain 10 or fewer sequences (Table 3, Dataset S4).
Table 2. Distribution of trypanosomatids within and between Drosophila populations at the 3% divergence level (97% similarity).
| Sympatric | Sympatric | Sympatric | Sympatric | |||||||||||||
| Number of Sequences in OTU | ANM | IMH | POM | PON | TKM | ELA | ELD | FNS | NNS | ICF | SPP | MEC | MIC | MOV | SCA | |
| 314 | 32 | 1 | 262 | 17 | 2 | Both | ||||||||||
| 182 | 180 | 1 | 1 | Both | ||||||||||||
| 159 | 3 | 16 | 22 | 64 | 2 | 6 | 1 | 42 | 1 | 2 | Both | |||||
| 144 | 127 | 1 | 2 | 3 | 10 | 1 | Both | |||||||||
| 47 | 26 | 12 | 8 | 1 | Allopatric | |||||||||||
| 38 | 2 | 28 | 8 | Allopatric | ||||||||||||
Sympatic populations were collected within the same geographic area during the same time period. All other population combinations are considered allopatric. Final column indicates if the OTU was found in either sympatric populations, allopatric populations, or both allopatric and sympatric populations. Only OTUs that are present in multiple populations are shown. Data for remaining OTUs can be found in Dataset S3.
Table 3. Distribution of trypanosomatids within and between Drosophila populations at the 0% divergence level (100% similarity).
| Sympatric | Sympatric | Sympatric | Sympatric | |||||||||||||
| Number ofSequences in OTU | ANM | IMH | POM | PON | TKM | ELA | ELD | FNS | NNS | ICF | SPP | MIC | MEC | MOV | SCA | |
| 92 | 13 | 78 | 1 | Both | ||||||||||||
| 58 | 56 | 1 | 1 | Both | ||||||||||||
| 55 | 8 | 47 | Sympatric | |||||||||||||
| 41 | 1 | 24 | 1 | 15 | Both | |||||||||||
| 31 | 3 | 28 | Sympatric | |||||||||||||
| 9 | 6 | 3 | Allopatric | |||||||||||||
| 8 | 4 | 1 | 3 | Allopatric | ||||||||||||
| 6 | 1 | 5 | Allopatric | |||||||||||||
| 3 | 1 | 2 | Allopatric | |||||||||||||
| 2 | 1 | 1 | Allopatric | |||||||||||||
| 2 | 1 | 1 | Sympatric | |||||||||||||
| 2 | 1 | 1 | Sympatric | |||||||||||||
Sympatic populations were collected within the same geographic area during the same time period. All other population combinations are considered allopatric. Final column indicates if the OTU was found in either sympatric populations, allopatric populations, or both allopatric and sympatric populations. Only OTUs that are present in multiple populations are shown. Data for remaining OTUs can be found in Dataset S4.
A representative sequence for each 3% divergence OTU was chosen using the get.oturep function in mothur which selects the sequence that has the minimum total distance to all the other sequences within that OTU. Kinetoplastid LSU sequences were taken from NCBI for phylogenetic comparison (Dataset S5). These include several species of monoxenous insect trypanosomatids (Leptomonas, Herpetomonas, and Crithidia) and numerous dixenous insect-vectored trypanosomatids (Leishmania, Endotrypanum, and various Trypanosoma species). The free living outgroup to the order trypanosomatida (Bodo saltans of the order Bodonida) was used as the outgroup in this analysis [12]. These Kinetoplastid sequences were aligned to the degapped, aligned representative sequences using maffT, the genafpair algorithm, and the add function. Because several of these sequences are very long (over 10,000 bases) and span the entire ribosomal repeat region (which includes the 5S, 18S, and 28S rDNA genes and the ITS1 and ITS2 interspacer regions), this alignment was trimmed to include only the 28S rDNA (LSU) region. jModelTest was used on this trimmed alignment to determine the optimal model of nucleotide substitution (GTR+G) [37], [38]. Bayesian analysis was performed using MrBayes v3.1.2 [39]. Two independent chains were run for 10,000,000 generations resulting in an average standard deviation of split frequencies of 0.0040. Tracer v1.5.0 was used to confirm stationarity of the log likelihoods [40], and the first 25% of the 10,000 total trees were used as burnin. Results were visualized using Dendroscope [41].
As many of the libraries have very few sequences, diversity analyses will be limited to interpretations that can be determined by shared presence. Alpha and beta diversity measurements and UniFrac analysis [42] were not performed.
Characterization of the SSU in a Single Population of Drosophila
For 14 of the 15 populations in which trypanosomatids were discovered, no whole flies or DNA remained. However, for one population (Drosophila ananassae collected in Hawaii; ANM in Table 1), numerous flies remained from the initial collection and could be used to study between individual and within individual variation in trypanosomatid infection. Additionally, a different diagnostic gene, the 18S rDNA small subunit (SSU), can be used to refine the phylogenetic placement of the Drosophila-associated trypanosomatids. The SSU is a commonly used diagnostic marker for trypanosomatid identification [43].
DNA was extracted from 21 individual flies. Single flies were homogenized in 250 ul of HB buffer (50 mM Tris, 400 ml NaCL, 20 mM EDTA, 0.5% SDS, pH 7.5) followed by a three hour 55C Proteinase K incubation and ethanol precipitation. Successful DNA extraction was confirmed by PCR amplification using the Drosophila specific primers COII-F and COII-R (Dataset S2). Any samples that did not amplify were not used in further analyses. The amplified COII gene was sequenced to confirm host identity and is available through NCBI under the accession number KC183710.
To test flies for the presence of trypanosomatids, the primers SSU1 and SSU2 were used to amplify the trypanosomatid rDNA small subunit (SSU) (Dataset S2) [44] using the following PCR protocol: Initial denaturation at 95C for 3 min followed by 30 amplification cycles (95C for 30 s, 55C for 1 min, 72C for 2 min 30 s) and a final extension at 72C for 10 min. Six individual flies tested positive for trypanosomatid infection. From these individuals, amplified DNA was ligated into the pCRII vector using the Invitrogen TOPO TA cloning kit and transformed into chemically competent DH5-alpha cells. Fifty total colonies were picked and sequenced using Life Technologies™ Big Dye ® Terminator v3.1. Between one and 17 colonies were sequenced per individual (Table 4).
Table 4. Distribution of trypanosomatids within and between individual flies in Hawaiian Drosophila ananassae.
| Individual Fly ID | ||||||
| 2 | 4 | 6 | 8 | 13 | 21 | |
| OTU 1 | 1 | |||||
| OTU 2 | 2 | |||||
| OTU 5 | 5 | |||||
| OTU 7 | 6 | 1 | ||||
| OTU 35 | 5 | 14 | 5 | 11 | ||
| Total Clones from Individual | 5 | 17 | 11 | 5 | 1 | 11 |
OTUs named based upon the number of sequences within that OTU.
Initially, each clone was sequenced using only the SSU1 primer. These sequences were aligned and clustered using the programs and settings outlined above. A representative sequence from each of the five 3% divergence OTUs was chosen and sequenced using internal sequencing primers to ensure near-complete coverage of the SSU gene (Dataset S2). These sequences were aligned to taxa from an existing SSU dataset [43], the bumble bee parasite Crithidia bombi, the human parasites Trypanosoma cruzi and Trypanosoma brucei, and to their closest NCBI blast hit (as of 10/10/2012) using maffT and the genafpair algorithm. Fifteen sequences from the subfamily Leishmaniinae were removed from [43] prior to alignment. As with the LSU phylogeny, the free living Bodo saltans (of the order Bodonida) was used as the outgroup [12]. jModelTest was used on this alignment to determine the optimal model of nucleotide substitution (GTR+I+G) [37], [38]. Bayesian analysis was performed using MrBayes v3.1.2 [39]. Two independent chains were run for 10,000,000 generations resulting in an average standard deviation of split frequencies of 0.0025. Tracer v1.5.0 was used to confirm stationarity of the log likelihoods [40], and the first 25% of the 10,000 total trees were used as burnin. Results were visualized using Dendroscope [41]. The nearly full-length, assembled representative sequences from each SSU OTU are available through NCBI under the accession numbers KC183711 to KC183715. All sequences and alignments are available through figshare (http://dx.doi.org/10.6084/m9.figshare.106978).
Results
Phylogenetic Position of Drosophila-associated trypanosomatids
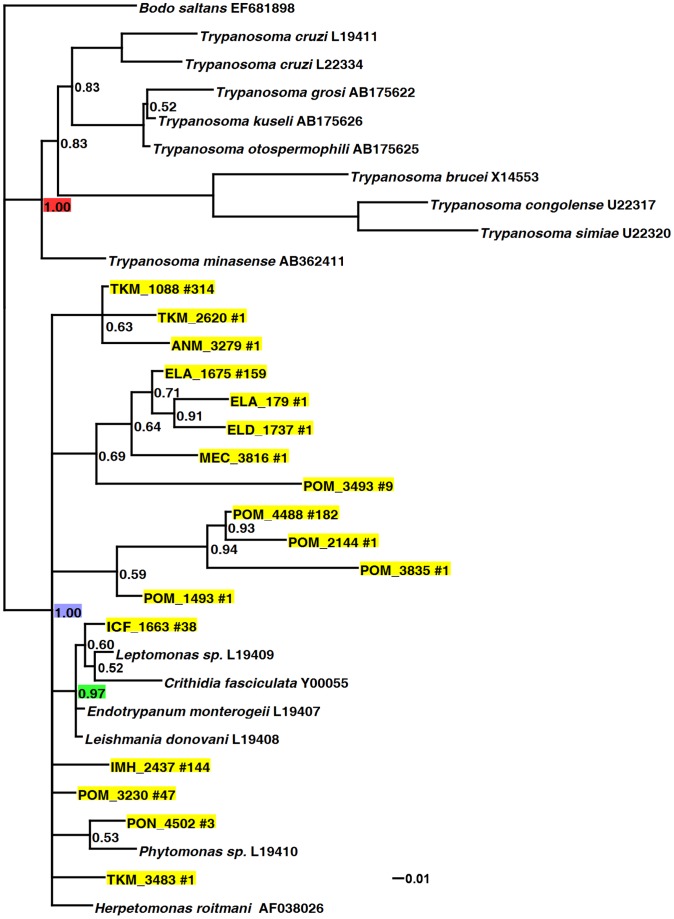
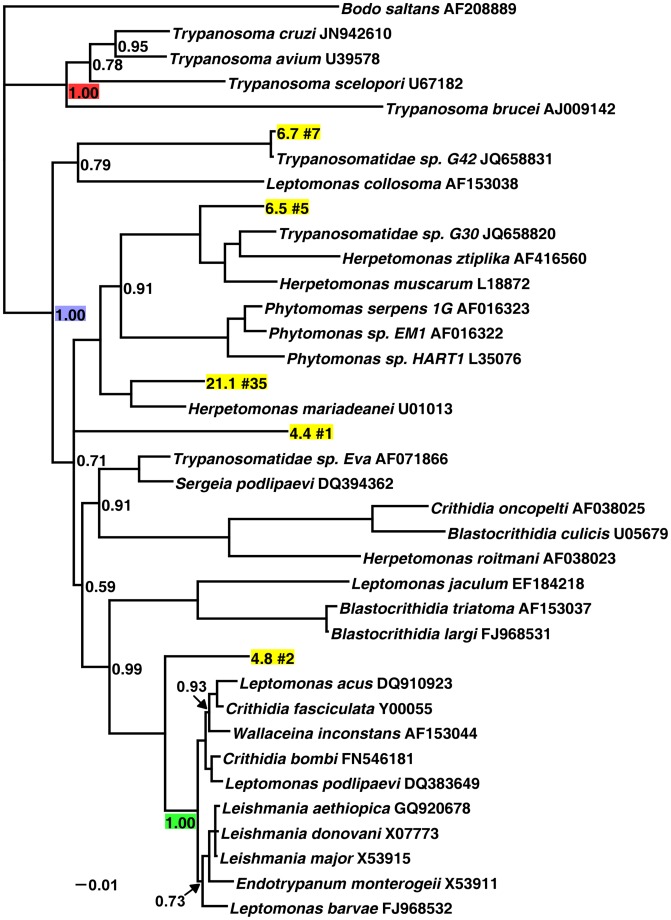
The trypanosomatids found with Drosophila are closely related to other insect-associated trypanosomatids and are phylogenetically distinct from the dixenous human pathogens in the genus Trypanosoma. In both the SSU and the LSU phylogenetic trees, we find strong support for the clade containing the genera Leishmania, Phytomonas, Crithidia, Herpetomonas, Endotrypanum, and the Drosophila-associated sequences (Figures 1 and 2; blue node). In congruence with the current understanding of trypanosomatid phylogenetics [12], [14], both trees find strong support for the Trypanosoma genus (red node).
Figure 1. Bayesian analysis of the ribosomal large subunit (LSU) of Drosophila associated trypanosomatids.
LSU data was obtained from 15 geographically dispersed Drosophila populations. Nodes with less than 50% posterior probability are collapsed. Nodes without a support value shown have 100% posterior probability. The red node identifies the genus Trypanosoma, the blue node identifies the non-Trypanosoma trypanosomatids, and the green node identifies the subfamily Leishmaniinae [45]. Representative sequences of each Drosophila-associated OTU are highlighted in yellow. Each representative sequence has a unique identifier followed by the number of sequences within that OTU. Unhighlighted taxa are comparison sequences obtained from NCBI and are followed by their accession number. Raw data, alignments, and the NEWICK tree file are available on figshare (http://dx.doi.org/10.6084/m9.figshare.106978).
Figure 2. Bayesian analysis of the ribosomal small subunit (SSU) of Drosophila ananassae-associated trypanosomatids.
SSU data was obtained from six individual flies collected in Captain Cook, Hawaii. Nodes with less than 50% posterior probability are collapsed. Nodes without a support value shown have 100% posterior probability. The red node identifies the genus Trypanosoma, the blue node identifies the non-Trypanosoma trypanosomatids, and the green node identifies the subfamily Leishmaniinae [45]. Representative sequences of each Drosophila-associated OTU are highlighted in yellow. Each representative sequence has a unique identifier followed by the number of sequences within that OTU. Unhighlighted taxa are comparison sequences obtained from [43] and NCBI and are followed by their accession number. The raw sequences, alignments, and the NEWICK tree file are available on figshare (http://dx.doi.org/10.6084/m9.figshare.106978).
The SSU phylogeny finds that the trypanosomatids associated with the Hawaiian Drosophila ananassae population do not form a single monophyletic clade but belong to multiple, well supported groups (Figure 2). While the LSU phylogeny suggests this as well, many nodes in that phylogeny are weakly supported (Figure 1). The SSU data finds that no Drosophila ananassae-associated trypanosomatids are within the subfamily Leishmaniinae [45] (Figure 2; green node), which includes the dixenous human pathogen Leishmania and the mosquito parasite Crithidia fasciculata [44], however the LSU phylogeny finds that some of the discovered taxa (the 38 sequences represented by ICF_1663 in Figure 1) are indeed closely related to the subfamily Leishmaniinae. Several trypanosomatids found associated with Drosophila ananassae (the seven sequences represented by 6.7 in Figure 2) are very closely related to taxa found with Reduviidae bugs in Ghana [20].
Trypanosomatids are Widespread and not Restricted to a Single Location
Trypanosomatids were detected in 15 Drosophila populations (Table 1, Dataset S3). These 15 populations come from four different continents (Africa, Australia, North America, and Asia) and seven different geographically isolated locations (Hawaii, both coasts of North America, Australia, Africa, Malaysia, and Taiwan). Drosophila collected from four different feeding substrates (fruit, flowers, cacti, and mushrooms) were found with trypanosomatids. At least 13 different species of Drosophila were associated with detectable levels of trypanosomatids.
Although there were five Drosophila populations with fungal sequencing reads [31] that did not have trypanosomatid reads (Dataset S1), we note that our primers were not 100% complementary to known trypanosomatid genomes (data not shown). Indeed, the number of fungal reads is inversely proportional to the number of trypanosomatid reads in a given population (data not shown) suggesting that competition for primer binding sites may be responsible for some of the apparent variation in trypanosomatid abundance between host populations.
Closely Related Trypanosomatids are Found in Multiple Drosophila Populations
Some populations were collected in the same general location on the same day or, at most, within the same month. Although different host species were sampled, their physical proximity allows for the opportunity of direct cross infection between populations. These population combinations (ANM:IMH:POM:PON:TKM, FNS:NNS, ELA:ELD, and ICF:SPP) shall henceforth be called “sympatric”. All other combinations are separated by at least 1,500 kilometers and shall be called “allopatric”.
When calculated at the 3% divergence level, eight OTUs are found more than once, and the six largest OTUs (out of 17 total) are found in multiple populations. All six of these are found in allopatric populations (Table 2, Dataset S3). The most widespread OTU (comprised of 159 sequences) is found in six allopatric regions ranging from Hawaii, Australia, Taiwan, Africa, and both coasts of North America.
OTUs were also calculated at the 0% divergence level. That is, sequences had to be identical (in the aligned, trimmed dataset) to be grouped together. Although divergence at diagnostic genes may not accurately reflect divergence at genomic loci important for host adaptation, this is the most stringent cut-off available given our data. At the 0% divergence level, 49 sequences were found more than once (Table 3, Dataset S4). Of these, 12 were found in multiple populations and eight in allopatric populations. D. takahashii and D. ananassae (both collected from Morinda fruit at Captain Cook, Hawaii) share many OTUs.
Multiple Strains within each Drosophila Population and within Individual Flies
Each population had more than one 3% divergence LSU OTU (Table 2, Dataset S3), and these likely represent multiple species. The population harboring the greatest diversity of trypanosomatids (POM) is associated with nine different LSU OTUs. Even though the genome of a single species may contain multiple copies of the LSU, it is unlikely that they have diverged greater than 3%. Indeed, all six copies of this region within Leishmania major, for which the complete genome is available, are identical (data not shown). Surveying the diversity of SSU from individual flies finds that multiple strains (at 3% divergence) can be present within a single individual (Table 4).
Discussion
Our results provide the most extensive survey to date of trypanosomatids associated with Drosophila species. We find that flies from all geographic regions and feeding types are associated with trypanosomatids. Although deeper and more extensive sampling with more specific primers is needed to conclusively say if any populations or species of Drosophila do not harbor trypanosomatids, our data suggests that this is an unlikely possibility. This is in concordance with previous studies [24], [25], and, to our knowledge, all populations of Drosophila that have been explicitly tested for the presence of trypanosomatids have been infected.
The data presented here also represents the most detailed taxonomic characterization of Drosophila-associated trypanosomatids. Microscopy based identifications have been unable to classify taxa to below the order trypanosomatid [24]. The only study which utilized molecular methods of identification focused primarily on the hyper-variable spliced leader sequence and was therefore unable to compare Drosophila–associated trypanosomatids to named taxa [25]. That study also generated three sequences of the trypanosomatid GAPDH gene and found, as we did, that Drosophila-associated trypanosomatids are closely related to other monoxenous insect trypanosomatids.
Unfortunately, we are unable to classify many of the Drosophila-associated trypanosomatids discovered in the global survey (Figure 1) due to the paucity of LSU data from trypanosomatids. Indeed, most of the non-Trypanosoma trypanosomatids included in our analysis (Herpetomonas roitmani, Endotrypanum monterogeii, Leishmania donovani, Phytomonas sp., and Leptomonas sp.) come from a single study [46], and, to our knowledge, no other monoxenous taxa have LSU sequence data publically available for the region overlapping the one sequenced in this study.
In addition to confirming the association of trypanosomatids with Drosophila, these results also provide insight into more general issues regarding the distribution, geographic endemism, and host-specificity of insect-associated trypanosomatids. The “one host – one parasite” paradigm [21] has been challenged recently by extensive surveying of the trypanosomatids associated with insects in Central America [19], China [17], and Africa [20]. Indeed, here we find that species, populations, and individuals can be associated with multiple strains of trypanosomatids. Additionally, geographically distant fly populations are associated with very closely related trypanosomatids suggesting that geographic distances do not provide a substantial barrier to dispersal relative to the evolutionary rate of the genetic marker used (LSU). A similar pattern has been observed for trypanosomatids associated with widely distributed populations of herbivorous insects in the family Pyrrhocoridae [47]. Finally, we find that Drosophila-associated trypanosomatids do not form a single monophyletic clade within the trypanosomatid phylogeny. This, along with the fact that sympatric species of Drosophila share many OTUs, suggests that little host specificity exists over both ecological and evolutionary timescales.
Drosophila has been used previously to study the interaction between insects and unicellular eukaryotic parasites. For example, the development of Plasmodium gallinaceum, a close relative of the human malarial parasite, has been modeled in Drosophila melanogaster by directly injecting Plasmodium ookinetes into the insect’s hemocoel [48]. Use of D. melanogaster genetic knock-outs has led to the discovery of genes in Anopheles gambiae that reduce Plasmodium growth [49]. This was possible despite the fact that Plasmodium does not stably infect the intestines of D. melanogaster [48], and no parasites of the phylum Apicomplexa, of which Plasmodium is a member, have ever been found in Drosophila. While the development of T. brucei, T. cruzi, and Leishmania is relatively well understood within their respective insect vectors [50], [51], [52], none of these insects can be as easily genetically manipulated as D. melanogaster. Because of this, we suggest that modeling trypanosomatid development in D. melanogaster may provide insights similar to those already gained in the Plasmodium-Anopheles system.
It is becoming increasingly clear that vertically-inherited intracellular symbionts can have a strong effect on vector infection status and disease transmissibility [53]. For example, T. brucei is more prevalent in tsetse flies that are co-infected with Sodalis glossinidius suggesting that this endosymbiont increases its host’s capacity to acquire and potentially transmit T. brucei [54]. In contrast, the presence of Wolbachia in D. melanogaster protects against viral infection [6], and this information is currently being used to interrupt the spread of dengue virus by Aedes aegypti mosquitoes [7], [8]. A reduction in pathogen load seems to be a general effect of Wolbachia as indicated by reduced Plasmodium levels in Wolbachia-infected mosquitoes [55]. Given that the intracellular symbionts associated with Drosophila are well characterized [56] and the experimental tools needed for Wolbachia manipulation are available in Drosophila [57], it seems relevant to ask how intracellular symbionts may interact with trypanosomatid parasites co-occurring in the same hosts. The results of such studies could have a substantial positive effect on efforts to control trypanosomatid caused human diseases.
Supporting Information
Drosophila populations associated with trypanosomatids (Detailed Version). A more detailed version of Table 1 describing where, when, and by whom each sample was collected.
(XLSX)
Primer Sequences.
(XLSX)
Detailed OTU information at the 3% divergence cutoff. This excel file contains the OTU assigned to each sequence used in this study along with information regarding the host species, location, environment, and other information regarding the library each sequence belongs to.
(XLSX)
Detailed OTU information at the 0% divergence cutoff. This excel file contains the OTU assigned to each sequence used in this study along with information regarding the host species, location, environment, and other information regarding the library each sequence belongs to.
(XLSX)
Accession numbers.
(XLSX)
Acknowledgments
This work would not have been possible without the help of many colleagues around the world. Wild Drosophila samples were collected for us by Artyom Kopp, Olga Barmina, Marie-Louise Cariou, Bill Etges, Jenna Morgan Lang, Delphine Legrand, Shane McEvey, Chen-Siang Ng, and John True. We thank David Plachetzki for advice on phylogenetic and bioinformatic methods, and Olga Barmina and Matt Rolston for technical help. Dmitri Maslov has been an invaluable resource during data analysis. Artyom Kopp, Dmitri Maslov, Michael Turelli, David Plachetzki, Jonathan A. Eisen, and four anonymous reviewers provided helpful advice for improving the manuscript.
Funding Statement
This work was supported by a UC-Davis Center for Population Biology Graduate Student Research Award to JAC, a UC-Davis President’s Undergraduate Fellowship to PMJ, NSF grant IOS-0815141 and REU supplement to Artyom Kopp, and an Alfred P. Sloan Foundation grant to Jonathan A. Eisen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Newman T, Sinadinos C, Johnston A, Sealey M, Mudher A (2011) Using Drosophila models of neurodegenerative diseases for drug discovery. Expert Opinion on Drug Discovery 6: 129–140. [DOI] [PubMed] [Google Scholar]
- 2. Bier E (2005) Drosophila, the golden bug, emerges as a tool for human genetics. Nature Reviews Genetics 6: 9–23. [DOI] [PubMed] [Google Scholar]
- 3. Mackay TEC, Anholt RRH (2006) Of flies and man: Drosophila as a model for human complex traits. Annual Review of Genomics and Human Genetics 7: 339–367. [DOI] [PubMed] [Google Scholar]
- 4. ffrench-Constant RH, Daborn PJ, Le Goff G (2004) The genetics and genomics of insecticide resistance. Trends in Genetics 20: 163–170. [DOI] [PubMed] [Google Scholar]
- 5. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annual Review of Immunology 25: 697–743. [DOI] [PubMed] [Google Scholar]
- 6. Teixeira L, Ferreira A, Ashburner M (2008) The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila melanogaster. Plos Biology 6: 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–U101. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–U107. [DOI] [PubMed] [Google Scholar]
- 9.Sibley CD, Duan KM, Fischer C, Parkins MD, Storey DG, et al.. (2008) Discerning the Complexity of Community Interactions Using a Drosophila Model of Polymicrobial Infections. Plos Pathogens 4. [DOI] [PMC free article] [PubMed]
- 10. Rogers GB, Hoffman LR, Doring G (2011) Novel concepts in evaluating antimicrobial therapy for bacterial lung infections in patients with cystic fibrosis. Journal of Cystic Fibrosis 10: 387–400. [DOI] [PubMed] [Google Scholar]
- 11.Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME (2012) Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clinical Microbiology Reviews 25: 193–+. [DOI] [PMC free article] [PubMed]
- 12. Simpson AGB, Stevens JR, Lukes J (2006) The evolution and diversity of kinetoplastid flagellates. Trends in Parasitology 22: 168–174. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Dept. of Control of Neglected Tropical Diseases., Crompton DWT, Daumerie D, Peters P, Savioli L, et al. (2010) Working to overcome the global impact of neglected tropical diseases : first WHO report on neglected tropical diseases. Geneva, Switzerland: World Health Organization. ix, 172 p. p.
- 14. Maslov DA, Votypka J, Yurchenko V, Lukes J (2013) Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends in Parasitology 29: 43–52. [DOI] [PubMed] [Google Scholar]
- 15. Mcghee RB, Cosgrove WB (1980) Biology and Physiology of the Lower Trypanosomatidae. Microbiological Reviews 44: 140–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Podlipaev S (2001) The more insect trypanosomatids under study-the more diverse Trypanosomatidae appears. International Journal for Parasitology 31: 648–652. [DOI] [PubMed] [Google Scholar]
- 17. Votypka J, Maslov DA, Yurchenko V, Jirku M, Kment P, et al. (2010) Probing into the diversity of trypanosomatid flagellates parasitizing insect hosts in South-West China reveals both endemism and global dispersal. Molecular Phylogenetics and Evolution 54: 243–253. [DOI] [PubMed] [Google Scholar]
- 18. Westenberger SJ, Sturm NR, Yanega D, Podlipaev SA, Zeledon R, et al. (2004) Trypanosomatid biodiversity in Costa Rica: genotyping of parasites from Heteroptera using the spliced leader RNA gene. Parasitology 129: 537–547. [DOI] [PubMed] [Google Scholar]
- 19. Maslov DA, Westenberger SJ, Xu X, Campbell DA, Sturm NR (2007) Discovery and barcoding by analysis of spliced leader RNA gene sequences of new isolates of trypanosomatidae from Heteroptera in Costa Rica and Ecuador. Journal of Eukaryotic Microbiology 54: 57–65. [DOI] [PubMed] [Google Scholar]
- 20. Votypka J, Klepetkova H, Jirku M, Kment P, Lukes J (2012) Phylogenetic relationships of trypanosomatids parasitising true bugs (Insecta: Heteroptera) in sub-Saharan Africa. International Journal for Parasitology 42: 489–500. [DOI] [PubMed] [Google Scholar]
- 21.Podlipaev SA (1990) Catalogue of world fauna of Trypanosomatidae (Protozoa). Leningrad: Proc. Zool. Inst. U.S.S.R. Acad. Sci. 177 p.
- 22. Brown MJF, Schmid-Hempel R, Schmid-Hempel P (2003) Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology 72: 994–1002. [Google Scholar]
- 23. Chatton E, Alilaire E (1908) Coexistence d’un Leptomonas (Herpetomonas) et d’un Trypanosoma chez un muscide non vulnenant, Drosophila confusa Staeger. Comptes rendus des Seances de la Societe de Biologie 64: 3. [Google Scholar]
- 24. Ebbert MA, Burkholder JJ, Marlowe JL (2001) Trypanosomatid prevalence and host habitat choice in woodland Drosophila. Journal of Invertebrate Pathology 77: 27–32. [DOI] [PubMed] [Google Scholar]
- 25. Wilfert L, Longdon B, Ferreira AGA, Bayer F, Jiggins FM (2011) Trypanosomatids are common and diverse parasites of Drosophila. Parasitology 138: 858–865. [DOI] [PubMed] [Google Scholar]
- 26. Rowton ED, Mcghee RB (1978) Population-Dynamics of Herpetomonas-Ampelophilae, with a Note on Systematics of Herpetomonas from Drosophila Spp. Journal of Protozoology 25: 232–235. [Google Scholar]
- 27. Boulanger N, Ehret-Sabatier L, Brun R, Zachary D, Bulet P, et al. (2001) Immune response of Drosophila melanogaster to infection with the flagellate parasite Crithidia spp. Insect Biochemistry and Molecular Biology 31: 129–137. [DOI] [PubMed] [Google Scholar]
- 28.Markow TA, O’Grady P (2006) Drosophila: A guide to species identification and use. Oxford, UK Elsevier.
- 29. Markow TA, O’Grady P (2008) Reproductive ecology of Drosophila. Functional Ecology 22: 747–759. [Google Scholar]
- 30.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A (2011) Bacterial Communities of Diverse Drosophila Species: Ecological Context of a Host-Microbe Model System. Plos Genetics 7. [DOI] [PMC free article] [PubMed]
- 31. Chandler JA, Eisen JA, Kopp A (2012) Yeast Communities of Diverse Drosophila Species: Comparison of Two Symbiont Groups in the Same Hosts. AppIied and Environmental Microbiolology 78: 7327–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurtzman CP, Robnett CJ (1997) Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. Journal of Clinical Microbiology 35: 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katoh K, Toh H (2010) Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26: 1899–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Applied and Environmental Microbiology 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Posada D (2008) jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 38. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 39. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 40.Rambaut A, Drummond AJ (2007) Tracer v1.4, Available from http://beast.bio.ed.ac.uk/Tracer.
- 41.Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, et al.. (2007) Dendroscope: An interactive viewer for large phylogenetic trees. Bmc Bioinformatics 8: -. [DOI] [PMC free article] [PubMed]
- 42.Lozupone C, Hamady M, Knight R (2006) UniFrac - An online tool for comparing microbial community diversity in a phylogenetic context. Bmc Bioinformatics 7: -. [DOI] [PMC free article] [PubMed]
- 43. Maslov DA, Yurchenko VY, Jirku M, Lukes J (2010) Two New Species of Trypanosomatid Parasites Isolated from Heteroptera in Costa Rica. Journal of Eukaryotic Microbiology 57: 177–188. [DOI] [PubMed] [Google Scholar]
- 44. Yurchenko VY, Lukes J, Jirku M, Zeledon R, Maslov DA (2006) Leptomonas costaricensis sp n. (Kinetoplastea : Trypanosomatidae), a member of the novel phylogenetic group of insect trypanosomatids closely related to the genus Leishmania. Parasitology 133: 537–546. [DOI] [PubMed] [Google Scholar]
- 45.Jirku M, Yurchenko VY, Lukes J, Maslov DA (2012) New Species of Insect Trypanosomatids from Costa Rica and the Proposal for a New Subfamily within the Trypanosomatidae. Journal of Eukaryotic Microbiology. [DOI] [PubMed]
- 46. Fernandes AP, Nelson K, Beverley SM (1993) Evolution of Nuclear Ribosomal-Rnas in Kinetoplastid Protozoa - Perspectives on the Age and Origins of Parasitism. Proceedings of the National Academy of Sciences of the United States of America 90: 11608–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Votypka J, Klepetkova H, Yurchenko VY, Horak A, Lukes J, et al. (2012) Cosmopolitan distribution of a trypanosomatid Leptomonas pyrrhocoris. Protist 163: 616–631. [DOI] [PubMed] [Google Scholar]
- 48. Schneider D, Shahabuddin M (2000) Malaria parasite development in a Drosophila model. Science 288: 2376–2379. [DOI] [PubMed] [Google Scholar]
- 49. Brandt SM, Jaramillo-Gutierrez G, Kumar S, Barillas-Mury C, Schneider DS (2008) Use of a Drosophila Model to Identify Genes Regulating Plasmodium Growth in the Mosquito. Genetics 180: 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sacks D, Kamhawi S (2001) Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol 55: 453–483. [DOI] [PubMed] [Google Scholar]
- 51. Rassi A, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 52. Vickerman K, Tetley L, Hendry KA, Turner CM (1988) Biology of African trypanosomes in the tsetse fly. Biol Cell 64: 109–119. [DOI] [PubMed] [Google Scholar]
- 53. Weiss B, Aksoy S (2011) Microbiome influences on insect host vector competence. Trends in Parasitology 27: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farikou O, Njiokou F, Mbida JAM, Njitchouang GR, Djeunga HN, et al. (2010) Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes-An epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infection Genetics and Evolution 10: 115–121. [DOI] [PubMed] [Google Scholar]
- 55. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, et al. (2009) A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 56. Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, et al. (2006) Heritable endosymbionts of Drosophila. Genetics 174: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boyle L, O’Neill SL, Robertson HM, Karr TL (1993) Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260: 1796–1799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drosophila populations associated with trypanosomatids (Detailed Version). A more detailed version of Table 1 describing where, when, and by whom each sample was collected.
(XLSX)
Primer Sequences.
(XLSX)
Detailed OTU information at the 3% divergence cutoff. This excel file contains the OTU assigned to each sequence used in this study along with information regarding the host species, location, environment, and other information regarding the library each sequence belongs to.
(XLSX)
Detailed OTU information at the 0% divergence cutoff. This excel file contains the OTU assigned to each sequence used in this study along with information regarding the host species, location, environment, and other information regarding the library each sequence belongs to.
(XLSX)
Accession numbers.
(XLSX)