Abstract
Ca2+ influx is a central component of the receptor-evoked Ca2+ signal. A ubiquitous form of Ca2+ influx comes from Ca2+ channels that are activated in response to depletion of the endoplasmic reticulum Ca2+ stores and are thus named the store-operated Ca2+-influx channels (SOCs). One form of SOCs is the Transient Receptor Potential Canonical (TRPC) channels. A major question in the field of Ca2+ signaling is the molecular mechanism that regulates the opening and closing of these channels. All TRPC channels have a Homer binding ligand and two conserved negative charges that interact with two terminal lysines of the Stromal Interacting Molecule 1 (STIM1). The Homer and STIM1 sites are separated by only four amino acid residues. Based on available results, we propose a molecular mechanism by which Homer couples TRPC channels to IP3 receptors (IP3Rs) to keep these channels in the closed state. Dissociation of the TRPCs-Homer-IP3Rs complex allows STIM1 access to the TRPC channels negative charges to gate open these channels.
Introduction
The receptor-evoked Ca2+ signal begins with the activation of phospholipase C (PLCβ or PLCγ) to generate inositol trisphosphate (IP3), which releases Ca2+ from intracellular stores, primarily the endoplasmic reticular stores (Berridge and Irvine, 1989). The IP3-mediated Ca2+ release launches the Ca2+ signal that involves cyclical activation of Ca2+ channels and Ca2+ pumps to generate multitude of Ca2+ signals in the form of Ca2+ puffs, blinks and sparks that can coalesce into cyclical Ca2+ increases to generate Ca2+ oscillations and/or to propagate Ca2+ in the form of waves (Berridge, 2006). These Ca2+ signals control a plethora of physiological functions essential for cell survival, specialized cell functions, and eventually cell death (Berridge et al., 2003). A crucial component of all receptor-evoked Ca2+ signals is Ca2+ influx across the plasma membrane. Ca2+ influx is activated in response to Ca2+ release from internal stores, most commonly the endoplasmic reticulum (ER) (Parekh and Putney, 2005). Ca2+ influx is essential to maintain the physiological Ca2+ oscillations and to provide the Ca2+ for replenishment of the Ca2+ store at the end of the stimulated period (Kiselyov et al., 2006). Ca2+ influx also directly mediates many cellular functions on time scales from msec, such as exocytosis (Pang and Sudhof, 2010), to days, such as gene regulation (Di Capite et al., 2009) and cell death (Supnet and Bezprozvanny, 2010).
The receptor-evoked store-operated Ca2+ influx is mediated by two types of Ca2+ influx channels: the Orai family (Hogan et al., 2010) and TRPC family channels (Lee et al., 2010a; Worley et al., 2007b), and are gated by the endoplasmic reticulum (ER) Ca2+ sensor STIM1 (Liou et al., 2005; Roos et al., 2005). The TRPC channels are also gated by the scaffolding protein Homer1 (Kim et al., 2006; Worley et al., 2007a; Yuan et al., 2003). This short review highlights the potential role of Homer1 and STIM1 in the closing and opening of the TRPC channels.
The Homers
The Homers are a family of three scaffolding proteins: Homer1, Homer2 and Homer3, with numerous isoforms that were discovered in the brain as immediate early genes (Soloviev et al., 2000; Xiao et al., 2000). The Homer1a isoform is rapidly upregulated in response to synaptic activity induced by seizure, or during induction of long-term potentiation. Homer1a is also selectively induced in cells of the hippocampus when rodents engage in exploratory behavior (Brakeman et al., 1997; Kato et al., 1998). All Homers have an Ena VASP Homology 1 (EVH1) domain. Homer1a has a short C-terminal extension, while long-form Homers have a C-terminal coiled-coil domain and two leucine zippers (Tadokoro et al., 1999; Xiao et al., 1998) and Fig. 1A). The N-terminal EVH1 domain of the different Homers are about 60–70% conserved, while the coiled-coil domains show only about 20% sequence identity. The coiled-coils serve to assemble the Homers into elongated tetramers (Hayashi et al., 2006). The tetrameric Homer is likely required to form a lattice with other scaffolds that bind Ca2+ signaling proteins in cellular microdomains (Kim and Sheng, 2004; Sala et al., 2005; Tadokoro et al., 1999; Tu et al., 1999; Xiao et al., 2000). The Homers EVH1 domain interacts with and regulates the activity of several Ca2+ signaling proteins listed in Fig. 1B that reside in Ca2+ signaling complexes. The monomeric Homer1a then disrupts signaling complexes and functions by acting as a negative regulator of the long Homers (Roche et al., 1999; Xiao et al., 1998).
Fig. 1. The Homers and their localization.
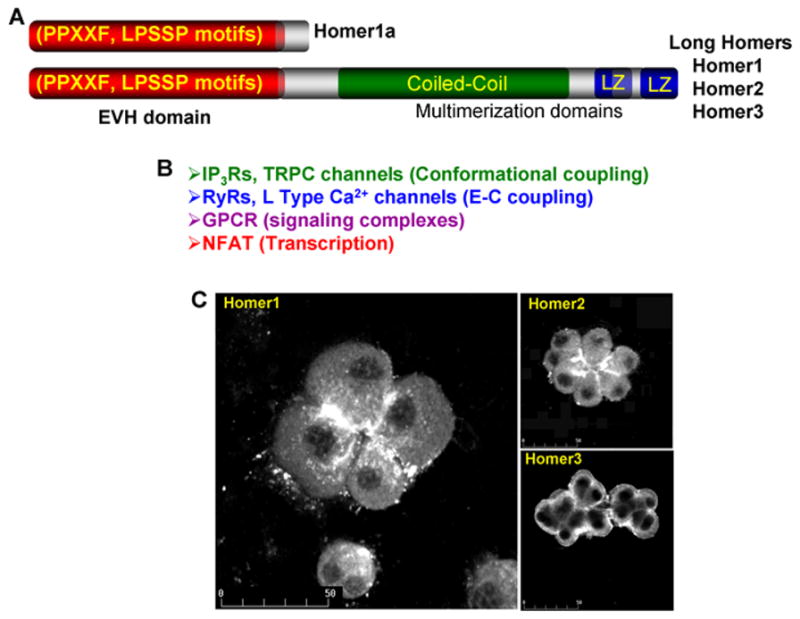
Panel (A) shows the domains of Homer1a and of the long Homers (Homer1, Homer2 and Homer3). Panel (B) lists some of the Ca2+ signaling proteins that have Homer ligands and have been shown to bind to the EVH domain and co-localize with the Homers. Panel (C) shows the localization of Homer1 and Homer2 at the apical pole and Homer3 in the basal pole of mouse pancreatic acini. The images in panel C were taken from (Shin et al., 2003).
Localization of the Homers and interaction with Ca2+ signaling proteins
In neurons, the Homers are found in the Post-Synaptic-Density (PSD) and dendrites, where they interact with the metabotropic glutamate receptors mGluR1 and mGluR5 (Brakeman et al., 1997; Kato et al., 1998; Roche et al., 1999; Tu et al., 1999). Mutation and structural analysis revealed that the EVH1 domain binds to the ligands PPXXF (Barzik et al., 2001; Irie et al., 2002), øPPXF and the LPSSP (Yuan et al., 2003). These ligands are found in the mGluRs and many Ca2+ signaling proteins, including Shank (Tu et al., 1999), PLCβ (Hwang et al., 2005; Nakamura et al., 2004), IP3Rs (Kim et al., 2006; Yuan et al., 2003), TRPC channels (Kim et al., 2006; Yuan et al., 2003), ryanodine receptors (Feng et al., 2002; Hwang et al., 2003; Ward et al., 2004; Westhoff et al., 2003), several L-type Ca2+ channel isoforms (Huang et al., 2007; Olson et al., 2005; Yamamoto et al., 2005) and nuclear factor of activated T cells (NFAT) (Huang et al., 2008).
To regulate Ca2+ signaling, the Homers have to be present in signaling microdomains. The Ca2+ signaling centers in the PSD and dendrites are enriched with Homer proteins (Ango et al., 2000; Dietrich et al., 2005; Fagni et al., 2002; Shiraishi et al., 2003). In polarized cells, Ca2+ signaling complexes are clustered at the apical pole, a region expressing Homer1 and Homer2 (Shin et al., 2003). This is reproduced in Fig. 1C taken from (Shin et al., 2003), which shows that Homer1 and Homer2 are restricted to the apical pole of pancreatic acinar cells, whereas Homer3 is enriched at the basal pole. Ca2+ signaling proteins are also enriched at the apical pole of polarized cells (Kiselyov et al., 2006) and co-localize with Homer1 and Homer2 (Shin et al., 2003). The C-terminal portion of the Homers coiled-coil domain appears to mediate the subcellular localization of the Homers and is required for clustering of Ca2+ signaling proteins (Hayashi et al., 2006).
The three Homers have distinct roles in Ca2+ signaling. The role of Homer3 in Ca2+ signaling is not well understood. The most intriguing recent finding is that Homer2 and Homer3 regulate NFAT (Huang et al., 2008). These Homers compete with the phosphatase calcineurin for binding to NFAT and, in effect, sequester NFAT in the cytosol to prevent its dephosphorylation and, thus, translocation to the nucleus (Huang et al., 2008). In this manner, the Homers can regulate all of the many roles of NFAT in cell differential and function. Interestingly, phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II (CaMKII) regulates its targeting to the plasma membrane and its interaction with cytosolic proteins (Mizutani et al., 2008). CaMKII also phosphorylates NFAT at the site dephosphorylated by calcineurin (Crabtree and Olson, 2002). This raises the possibility that a CaMKII-Homer-NFAT-calcineurin complex operates to control the function of NFAT.
In addition to binding of NFAT, Homer2 also regulates signaling by G-protein-coupled receptors (GPCRs) through regulation of the GTPase-activating protein (GAP) activity of Regulators of G proteins Signaling (RGS) proteins and of PLCβ (Shin et al., 2003). Deletion of Homer2 in mice reveals that Homer2 is involved in the pathway that underlies response to cocaine and alcohol (Kalivas et al., 2004; Szumlinski et al., 2005; Szumlinski et al., 2003). In G protein signaling in the unstimulated state, Gαq is bound with GDP and is associated with Gβγ. Receptor stimulation catalyzes the exchange of GDP for GTP on Gαq (Freissmuth et al., 1989; Gilman, 1987). Termination of the signal requires hydrolysis of GTP by Gαq that is markedly accelerated by RGS proteins (Ishii and Kurachi, 2003; Ross and Wilkie, 2000) and PLCβ (Ross, 2008). Homer2 accelerates the GAP activity of both RGS proteins and PLCβ to attenuate the intensity of the Ca2+ signal (Shin et al., 2003).
Homer1 and gating of TRPC channels
Of all the Homers, the function of Homer1 is best documented and understood. Homer1 binds to mGluRs (Beneken et al., 2000) and to the N-terminus of the IP3Rs (Yuan et al., 2003), linking them to the Shank proteins to form a Ca2+ signaling complex that regulates Ca2+ release (Fagni et al., 2002; Sala et al., 2005). Homer proteins also affect interaction and communication of several other Ca2+ signaling receptors and proteins in the PSD, as reviewed in (Worley et al., 2007a). Of these interactions, the interaction of Homer1 with TRPC channels and regulation of channel activity by Homer1 have been explored extensively.
A role of Homer1 in the regulation of Ca2+ influx was revealed by the increased spontaneous Ca2+ influx in cells from which Homer1 was deleted (Yuan et al., 2003). TRPC channels express the Homer binding ligand øPPXF in their C-terminus. Accordingly, Homer proteins bind to all TRPC channels and assemble them into complexes with IP3Rs (Yuan et al., 2003). Disruption of the Homer binding ligand of the TRPC channels prevents their binding to Homer. Most notably, disruption of the TRPC1-Homer-IP3Rs complex by mutation in the Homer ligand or by expression of Homer1a results in spontaneously active TRPC channels (Yuan et al., 2003). Moreover, cell stimulation results in IP3-dependent dissociation of the TRPCs-Homer1-IP3Rs (Kim et al., 2006). These findings clearly indicate that Homer1 is essential to maintain the TRPC channels in the closed state. At the basal state, TRPC channels are present in a complex with IP3Rs that is formed by the long Homer1b/c to keep the channel inactive. Upon cell stimulation, the complexes are dissociated to allow activation of TRPC channels.
STIM1
STIM1 was discovered by a search for the molecular identity of the Ca2+ influx pathway and its regulators. In particular, STIM1 is the molecule that mediates the communication between ER Ca2+ load and activation of the Ca2+ influx channels at the plasma membrane (Liou et al., 2005; Roos et al., 2005). The suggestion that Ca2+ influx channels are activated in response to Ca2+ release from the ER was based on the assumption that reloading of the ER occurs at resting [Ca2+]i (Putney, 1986). Reloading at resting [Ca2+]i was shown experimentally for the first time by direct measurement of [Ca2+]i (Muallem et al., 1986) with parallel measurement of reloading the Ca2+ stores after their complete discharge (Muallem et al., 1986; Pandol et al., 1987) and analysis of unidirectional Ca2+ fluxes across the ER and plasma membrane (Muallem et al., 1988; Pandol et al., 1987). Subsequent demonstration that both passive depletion of the stores by inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps with thapsigargin and receptor stimulation activate the same Ca2+ influx pathway established the relationship between ER Ca2+ load and gating of Ca2+ influx channels (Takemura et al., 1989). The molecule that transmits the information of the ER Ca2+ load to the plasma membrane Ca2+ influx channels remained a mystery until the finding of STIM1 (Liou et al., 2005; Roos et al., 2005). The role of STIM1 in Ca2+ influx was established by demonstrating that deletion of STIM1 resulted in inhibition of Ca2+ influx and clustering of STIM1 at plasma membrane microdomains in response to Ca2+ release from the ER (Liou et al., 2005; Roos et al., 2005). This was later shown to occur in all cells examined.
STIM1 is a type 1, single transmembrane-span protein with its N-terminus in the ER lumen and its C-terminus in the cytoplasm. The N-terminus has at least two functional domains, an EF hand low affinity Ca2+ binding domain and a sterile alpha motif (SAM), and at least one regulatory site at cysteine 56. The EF hand Ca2+ binding affinity is relatively low in the range of 0.5–1 mM (Stathopulos et al., 2009) and, when bound with Ca2+, traps STIM1 in the ER. Dissociation of Ca2+ from the EF hand results in the clustering of STIM1 at ER/plasma membrane microdomains (Liou et al., 2005; Roos et al., 2005). The SAM domain functions to facilitate clustering of STIM1 (Stathopulos et al., 2006; Stathopulos et al., 2008). STIM1 cysteine 56 is S-glutathionylated and, thus, may function as an oxidant sensor (Hawkins et al., 2010). Gating of Ca2+ influx channels is achieved by the STIM1 C-terminus, which opens all channels gated by STIM1 (Huang et al., 2006; Lee et al., 2010a; Yuan et al., 2009). The C-terminus starts with an ERM domain that encompasses several functional domains., the most notable of which is a minimal sequence of STIM1(344-442) termed STIM1 Orai-activating region (SOAR) (Yuan et al., 2009), CRAC (Ca2+-release activating Ca2+ current) Activating Domain (CAD) (Park et al., 2009) or coiled-coil domain containing region b9 (CCB9) (Kawasaki et al., 2009). This domain is sufficient to fully activate the Orai channels. SOAR has a coiled-coil domain that interacts with the C-terminal coiled-coil domain of the Orais (Korzeniowski et al., 2010; Muik et al., 2009; Schindl et al., 2009; Yuan et al., 2009) to fully activate them. The CRAC-modulatory domain/Ca2+-dependent inactivation (CMD/CDI) sequence is located downstream of SOAR in the Ezrin/Radixin/Moesin (ERM) domain.. The Orai channels undergo fast Ca2+-dependent inactivation (Lis et al., 2007) that is mediated by the CMD/CDI patch (Derler et al., 2009; Lee et al., 2009; Mullins et al., 2009). The CMD/CDI patch interacts with a calmodulin binding site at the N-terminus of Orai1 (Mullins et al., 2009) and with conserved glutamates at the C-terminus of the three Orais to determine the isoform-specific fast Ca2+-dependent inactivation (Lee et al., 2009). Downstream of the ERM domain, there are several phosphorylation sites that can be phosphorylated by several kinases and appear to control Ca2+ influx during mitosis (Smyth et al., 2009), although these sites have no apparent role during meiosis (Yu et al., 2009). STIM1 is also phosphorylated by Extracellular Regulated Kinase1/2 (ERK1/2), which may control interaction of STIM1 with Orai1 (Pozo-Guisado et al., 2010).
TRPC channels and STIM1
Another domain of STIM1 at end of the C-terminus is the polybasic lysine-rich domain (K-domain). The K-domain has several known roles. The K-domain anchors STIM1 to the plasma membrane and facilitates its clustering (Korzeniowski et al., 2010; Liou et al., 2007). A key function of the K-domain is gating of TRPC channels (Huang et al., 2006; Lee et al., 2010b; Yuan et al., 2007). For a long time, TRPC channels have been considered the primary receptor-stimulated and store-operated Ca2+-influx channels. (Montell, 2005; Parekh and Putney, 2005), the latter based on the findings that their downregulation and inhibition reduce SOC activity (Kiselyov et al., 2007; Villereal, 2006). The discovery of STIM1 and the Orai channels shifted attention to the Orai channels as the primary SOCs (Cahalan et al., 2007; Lee et al., 2010a). Although it is clear that Orai1 is required for all forms of Ca2+ influx (Lee et al., 2010a), growing evidence implicate TRPC channels in SOC and receptor-operated Ca2+-influx channel (ROC) activity and in their regulation by STIM1. For example, inhibition of TRPC3 with Pyrazole 3 inhibited ROCs (Kiyonaka et al., 2009; Shirakawa et al., 2010), and inhibition of TRPC1 (Beech et al., 2003), TRPC3 (Chen et al., 2010), TRPC5 (Xu et al., 2006) and TRPC6 (Saleh et al., 2008) with anti-TRPC channel antibodies inhibited ROCs and SOCs. Knockdown by siRNA of several TRPC channels (Villereal, 2006) and gene deletion in mice of TRPC1 (Hong et al., 2010; Liu et al., 2007), TRPC3 (Kim et al., 2009) and TRPC4 (Freichel et al., 2001; Tiruppathi et al., 2002) reduced ROCs and SOCs.
Regulation of TRPC channels by STIM1 was suggested when it was discovered that TRPC1, TRPC4 and TRPC5, but not TRPC3, TRPC6 and TRPC7, interact with STIM1 and that STIM1 is required for the activity of TRPC1 (Huang et al., 2006). Subsequently, a role for STIM1 in the regulation of native and expressed TRPC channels has been demonstrated in several cell types, including the function of TRPC channels in salivary gland (Liu et al., 2007; Ong et al., 2007), vascular (Takahashi et al., 2007) and pulmonary arterial (Ng et al., 2010) smooth muscle, HL-7702 (Zhang et al., 2010), intestinal (Rao et al., 2010), mesangial (Sours-Brothers et al., 2009) and mast cells (Ma et al., 2008).
That STIM1 must be regulating channels other than Orai1 is further concluded from studying the localization of the native Orai1 and TRPC channels and the recruitment of STIM1, in particular in polarized secretory cells where Ca2+ signaling complexes are highly compartmentalized (Kiselyov et al., 2006). In response to store depletion, over-expressed Orai1 and STIM1 always co-cluster at the same puncta and show perfect co-localization (Huang et al., 2006; Korzeniowski et al., 2010; Liou et al., 2007; Mercer et al., 2006; Park et al., 2009; Yuan et al., 2009; Zhang et al., 2005). This is clearly not the case with the native proteins where overexpression artifacts are avoided. Orai1 is expressed almost exclusively at the apical pole of pancreatic and salivary glands acinar cells. By sharp contrast, Ca2+ store depletion resulted in recruitment of only a fraction of STIM1 to the apical pole, while the majority of STIM1 is recruited to the lateral pole that appears to be free of Orai1 (Hong et al., 2010). This indicates that STIM1 interacts with Orai1 and additional Ca2+ influx channels, which turn out to be TRPC channels since TRPC1 is also expressed at the lateral pole, deletion of TRPC1 (Hong et al., 2010; Liu et al., 2007) and TRPC3 (Kim et al., 2009) reduces SOC, and the native STIM1 co-immunoprecipitate with the native TRPC1 and Orai1. Localization and co-immunoprecipitation of the native proteins is illustrated in Fig. 2, which was taken from (Hong et al., 2010). Another recent study reported expression of small amount of Orai1 in the lateral membrane domain of acinar cells that does not express IP3Rs (Lur et al., 2011). However, the role of this Orai1 is not known since the expression level is very low and whether STIM1 is recruited to this domain was not determined. The key finding of luminal membrane domains, identified by both Cadherin and Laminin, that do express high level of STIM1 but no Orai1 and the co-IP of STIM1 with TRPC channels in response to store depletion (Hong et al., 2010), indicates that STIM1 most likely regulates the native TRPC channels.
Fig. 2. Localization of NATIVE STIM1 and Orai1 in polarized cells.
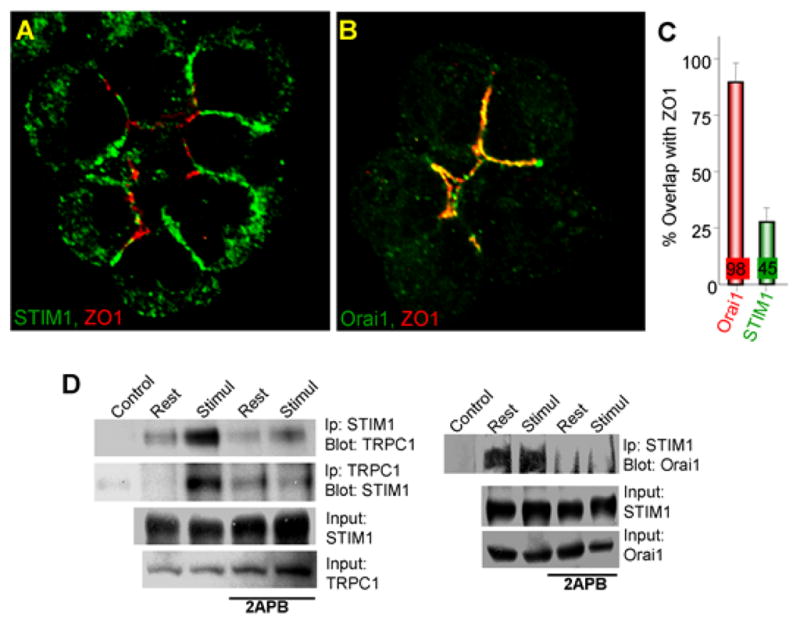
In panels (A–C), ZO1 was used to mark the tight junction at the apical pole of mouse pancreatic acinar cells. Panel (A) shows that small fraction of native STIM1 is at the apical pole and most of native STIM1 is at the lateral plasma membrane and panel (B) shows that native Orai1 is confined exclusively to the apical pole. The fraction of native STIM1 and native Orai1 at the apical pole is shown in panel (C). All experiments in panels (A–C) are with store-depleted cells. Panel (D) shows that native STIM1 and TRPC1 and STIM1 and Orai1 are co-immunoprecipitated, the co-IP of STIM1 and TRPC1 (but not of STIM1 and Orai1) is enhanced by cell stimulation that depletes the Ca2+ stores and the complexes are broken with 2APB, which dissociates STIM1-formed complexes. The figure is reproduced from (Hong et al., 2010).
Opening of TRPC channels by STIM1
To address the question of the mechanism by which STIM1 opens the TRPC channels, we analyzed the role of the K-domain in TRPC channels function since deletion of the K-domain resulted in a dominant negative STIM1 inhibitor of TRPC1 (Huang et al., 2006), although the K-domain is not required for activation of Orai1 by STIM1 (Zeng et al., 2008). The K-domain likely folds as an α-helix with several positive charges at the helix surface, including the two terminal lysines (Huang et al., 2006). Deletion or mutation of the two terminal lysines also resulted in a dominant negative STIM1 that inhibits, rather than activates, TRPC1. One possibility was that the two terminal lysines of STIM1 may interact with negative charges in TRPC channels. A search for a negative patch in TRPC channels identified at least two conserved DD/E residues in the C-terminus of the TRPC channels (Lee et al., 2010b; Zeng et al., 2008). Mutational and functional analysis and complementation showed that the two terminal STIM1 lysines K684 and K685 communicate electrostatically with the conserved DD/E of TRPC channels to gate channel opening (Lee et al., 2010b; Zeng et al., 2008). These findings, therefore, indicate that STIM1 can directly gate the TRPC channels.
The sequence of the TRPC channels’ C-terminal domains, where these conserved DD/E residues are located, is shown in red letters in Fig. 3A. The blue letters show the localization of the Homer ligand. Remarkably, only 4 residues separate the two sites. As outlined above, binding of Homer1 to TRPC channels and coupling them with IP3Rs keep the channels in a closed state. Interaction of the TRPC channels’ DD/E with STIM1(K684,K685) switches the channels to the open state. These findings lead to the model in Fig. 3B. The model proposes that, in the resting state, the ER is filled with Ca2+, which binds to the EF hand of STIM1 to keep STIM1(K684,K685) away from the DD/E residues of the TRPC channels. The N-terminal domain of the IP3R is in the conformation that exposes its Homer1-binding ligand to allow binding to Homer1 that also binds to the Homer1-binding ligand in the C-terminus of the TRPC channels. The TRPC-Homer-IP3R channel complex shields the DD/E from STIM1(K684,K685). Sequestration of STIM1 in the ER and formation of the TRPC-Homer-IP3R channel complex together ensure that the TRPC channels are kept in the closed state. Once the cells are stimulated to generate IP3, binding of IP3 to the IP3Rs dissociates the IP3R-Homer1-TRPC channel complexes. At the same time, IP3 activates the IP3Rs to release ER Ca2+, and dissociation of Ca2+ from STIM1 EF hand results in the clustering of STIM1 with the TRPC channels, so that now STIM1(K684,K685) can access the DD/E residues of the TRPC channels and stabilize the TRPC channels open state. As indicated throughout this review, the model in Fig. 3B is supported by multiple lines of evidence. However, the direct relationship between interaction of STIM1 and Homer1 with TRPC channels has not been explored yet. Such a study should test directly the validity of the model.
Fig. 3. A model for gating of TRPC channels by Homer1 and STIM1.
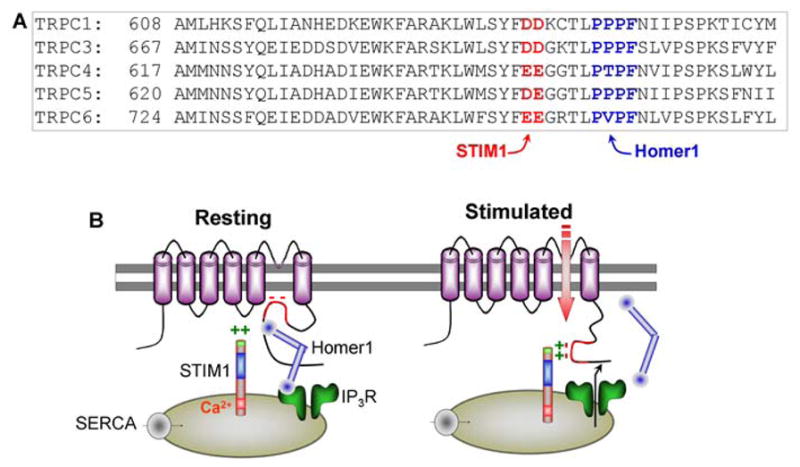
Panel (A) depicts the localization of the Homer1 binding ligand (blue) and the conserved DD/E site that interacts with STIM1(K684,K685) (red) in the C-terminus of the TRPC channels. Panel (B) is a model for the proposed relationship between Homer1 and STIM1 in keeping TRPC channels in closed or open state, respectively. Further details are given in the text.
Conclusions
In this review, the molecular mechanism of gating of TRPC channels by Homer and STIM1 is highlighted. Homer binds to and couples TRPCs to IP3Rs to keep the channels in a closed state, while STIM1 gates open TRPCs in response to depletion of ER Ca2+ stores. STIM1 can also regulate Orai1 channels present in the same cell type. This is exemplified best in polarized cells, where Orai1 is confined to the apical pole while TRPCs and STIM1 present in the apical and lateral membranes. The same can also be seen in other cell types. Presence of multiple store-operated Ca2+ influx channels in the same cells can serves to mediate selective cellular functions. An excellent recent example is the efficient activation of NFAT by Orai1-mediated, but not by TRPC-mediated, Ca2+ influx, while TRPC1-mediated Ca2+ influx activates K+ channels and NFκB (Cheng et al., 2011).
References
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzik M, Carl UD, Schubert WD, Frank R, Wehland J, Heinz DW. The N-terminal domain of Homer/Vesl is a new class II EVH1 domain. Journal of molecular biology. 2001;309:155–169. doi: 10.1006/jmbi.2001.4640. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26:143–154. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: organization and function. Cell calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell calcium. 2007 doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang D, Ma S, He H, Luo Z, Feng X, Cao T, Ma L, Yan Z, Liu D, Tepel M, Zhu Z. Increased rhythmicity in hypertensive arterial smooth muscle is linked to transient receptor potential canonical channels. J Cell Mol Med. 2010;14:2483–2494. doi: 10.1111/j.1582-4934.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca Entry Via Orai1 Regulates Plasma Membrane Recruitment of TRPC1 and Controls Cytosolic Ca Signals Required for Specific Cell Functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin C. A Ca2(+ )release-activated Ca2(+) (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2(+)-dependent inactivation of ORAI1 channels. J Biol Chem. 2009;284:24933–24938. doi: 10.1074/jbc.C109.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE. 2002;2002:RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, Pessah IN. Homer regulates gain of ryanodine receptor type 1 channel complex. J Biol Chem. 2002;277:44722–44730. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Casey PJ, Gilman AG. G proteins control diverse pathways of transmembrane signaling. Faseb J. 1989;3:2125–2131. [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annual review of biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J Neurosci. 2006;26:8492–8501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Li Q, Kim M, Shin D, Feske S, Birnbaumer L, Cheng K, Ambudkar IS, Muallem S. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. Traffic (Copenhagen, Denmark) 2010 doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Kim JY, Dehoff M, Mizuno Y, Kamm KE, Muallem S, Worley PF, Zeng W. Ca2+ signaling in microdomain: Homer1 Mediates the Interaction between RyR2 and Cav1.2 to regulate E-C coupling. Journal of Biological Chemistry. 2007 doi: 10.1074/jbc.M611529200. in press. [DOI] [PubMed] [Google Scholar]
- Huang GN, Huso DL, Bouyain S, Tu J, McCorkell KA, May MJ, Zhu Y, Lutz M, Collins S, Dehoff M, Kang S, Whartenby K, Powell J, Leahy D, Worley PF. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science (New York, NY. 2008;319:476–481. doi: 10.1126/science.1151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Hwang JI, Kim HS, Lee JR, Kim E, Ryu SH, Suh PG. The interaction of phospholipase C-beta3 with Shank2 regulates mGluR-mediated calcium signal. J Biol Chem. 2005;280:12467–12473. doi: 10.1074/jbc.M410740200. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell calcium. 2003;34:177–184. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Irie K, Nakatsu T, Mitsuoka K, Miyazawa A, Sobue K, Hiroaki Y, Doi T, Fujiyoshi Y, Kato H. Crystal structure of the Homer 1 family conserved region reveals the interaction between the EVH1 domain and own proline-rich motif. Journal of molecular biology. 2002;318:1117–1126. doi: 10.1016/S0022-2836(02)00170-5. [DOI] [PubMed] [Google Scholar]
- Ishii M, Kurachi Y. Physiological actions of regulators of G-protein signaling (RGS) proteins. Life Sciences. 2003;74:163–171. doi: 10.1016/j.lfs.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Szumlinski KK, Worley P. Homer2 gene deletion in mice produces a phenotype similar to chronic cocaine treated rats. Neurotox Res. 2004;6:385–387. doi: 10.1007/BF03033313. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochemical and biophysical research communications. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nature reviews. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Shin DM, Kim JY, Yuan JP, Muallem S. TRPC channels: interacting proteins. Handb Exp Pharmacol. 2007:559–574. doi: 10.1007/978-3-540-34891-7_33. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, I, Manjarres M, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS letters. 2010a;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent Function of Transient Receptor Potential Canonical (TRPC) Channels Tunes Their Store-operated Mode. J Biol Chem. 2010b;285:38666–38673. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14687–14692. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 Are Store-Operated Ca(2+) Channels with Distinct Functional Properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lur G, Sherwood MW, Ebisui E, Haynes LP, Feske S, Sutton R, Burgoyne R, Mikoshiba K, Petersen OH, Tepikin AV. IP3 receptors and Orai channels in pancreatic acinar cells: co-localisation and its consequences. The Biochemical journal. 2011 doi: 10.1042/BJ20110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. J Neurosci. 2008;28:5369–5382. doi: 10.1523/JNEUROSCI.4738-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Muallem S, Fimmel CJ, Pandol SJ, Sachs G. Regulation of free cytosolic Ca2+ in the peptic and parietal cells of the rabbit gastric gland. J Biol Chem. 1986;261:2660–2667. [PubMed] [Google Scholar]
- Muallem S, Schoeffield MS, Fimmel CJ, Pandol SJ. Agonist-sensitive calcium pool in the pancreatic acinar cell. II. Characterization of reloading. The American journal of physiology. 1988;255:G229–235. doi: 10.1152/ajpgi.1988.255.2.G229. [DOI] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Ng LC, Airey JA, Hume JR. The contribution of TRPC1 and STIM1 to capacitative Ca(2+) entry in pulmonary artery. Adv Exp Med Biol. 2010;661:123–135. doi: 10.1007/978-1-60761-500-2_8. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007 doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ, Schoeffield MS, Fimmel CJ, Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987;262:16963–16968. [PubMed] [Google Scholar]
- Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiological reviews. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 2010;123:3084–3093. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rao JN, Rathor N, Zou T, Liu L, Xiao L, Yu TX, Cui YH, Wang JY. STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-mediated Ca2+ signaling after wounding. American journal of physiology. 2010;299:C579–588. doi: 10.1152/ajpcell.00066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EM. Coordinating speed and amplitude in G-protein signaling. Curr Biol. 2008;18:R777–R783. doi: 10.1016/j.cub.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annual review of biochemistry. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–4592. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. The Journal of physiology. 2008;586:2463–2476. doi: 10.1113/jphysiol.2008.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C. Recent progress on STIM1 domains controlling Orai activation. Cell calcium. 2009;46:227–232. doi: 10.1016/j.ceca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T. Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci. 2003;22:188–201. doi: 10.1016/s1044-7431(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, Kusano A, Hashimoto E, Nakagawa T, Kaneko S. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Molecular characterisation of two structurally distinct groups of human homers, generated by extensive alternative splicing. Journal of molecular biology. 2000;295:1185–1200. doi: 10.1006/jmbi.1999.3436. [DOI] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med (Maywood) 2009;234:673–682. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell calcium. 2010;47:183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Toda S, Middaugh LD, Worley PF, Kalivas PW. Evidence for a relationship between Group 1 mGluR hypofunction and increased cocaine and ethanol sensitivity in Homer2 null mutant mice. Ann N Y Acad Sci. 2003;1003:468–471. doi: 10.1196/annals.1300.055. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13801–13806. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, Nobori K, Iijima T, Ito H. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochemical and biophysical research communications. 2007;361:934–940. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Villereal ML. Mechanism and functional significance of TRPC channel multimerization. Semin Cell Dev Biol. 2006;17:618–629. doi: 10.1016/j.semcdb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CW, Feng W, Tu J, Pessah IN, Worley PK, Schneider MF. Homer protein increases activation of Ca2+ sparks in permeabilized skeletal muscle. J Biol Chem. 2004;279:5781–5787. doi: 10.1074/jbc.M311422200. [DOI] [PubMed] [Google Scholar]
- Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell calcium. 2003;34:261–269. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell calcium. 2007a;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell calcium. 2007b;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Current opinion in neurobiology. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2653–2659. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sakagami Y, Sugiura S, Inokuchi K, Shimohama S, Kato N. Homer 1a enhances spike-induced calcium influx via L-type calcium channels in neocortex pyramidal cells. Eur J Neurosci. 2005;22:1338–1348. doi: 10.1111/j.1460-9568.2005.04278.x. [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Pan LJ, Zhang ZM. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of SOCs in HL-7702 cells. Amino Acids. 2010;39:195–204. doi: 10.1007/s00726-009-0398-5. [DOI] [PubMed] [Google Scholar]


