Abstract
Dietary omega-3 fatty acids have been demonstrated to have positive physiological effects on lipid metabolism, cardiovascular system and insulin resistance. Type-2 diabetes (T2DM) is known for perturbations in fatty acid metabolism leading to dyslipidemia. Our objective was to investigate beneficial effects of dietary flaxseed oil and fish oil in streptozotocin–nicotinamide induced diabetic rats. Thirty-six adult, male, Wistar rats were divided into six groups: three diabetic and three non-diabetic. Diabetes was induced by an injection of nicotinamide (110 mg/kg) and STZ (65 mg/kg). The animals received either control, flaxseed oil or fish oil (10 % w/w) enriched diets for 35 days. Both diets lowered serum triglycerides and very low-density lipoprotein cholesterol levels and elevated serum high-density lipoprotein cholesterol levels in diabetic rats, while serum total cholesterol and LDL-C levels remained unaffected. Both the diets increased omega-3 levels in plasma and RBCs of diabetic rats. Flaxseed oil diet significantly up-regulated the key transcription factor peroxisome proliferator-activated receptor-α (PPAR-α ) and down-regulated sterol regulatory element-binding protein-1 (SREBP-1) in diabetic rats, which would have increased β-oxidation of fatty acids and concomitantly reduced lipogenesis respectively, thereby reducing TG levels. Fish oil diet, on the contrary lowered serum TG levels without altering PPAR-α while it showed a non-significant reduction in SREBP-1 expression in diabetic rats. Another key finding of the study is the activation of D5 and D6 desaturases in diabetic rats by flaxseed oil diet or fish oil diets, which may have resulted in an improved omega-3 status and comparable effects shown by both diets. The reduced expression of Liver–fatty acid binding protein in diabetic rats was restored by fish oil alone, while both diets showed equal effects on adipocyte fatty acid–binding protein expression. We also observed down-regulation of atherogenic cytokines tumor necrosis factor-α and interleukin-6 by both the diets. In conclusion, dietary flaxseed oil and fish oil have therapeutic potential in preventing lipid abnormalities in T2DM.
Keywords: Flaxseed oil, Fish oil, Streptozotocin, Desaturases, PPAR-α, SREBP-1
Introduction
It is well established that dietary omega-3 fatty acids serve as biological regulators with various physiological roles. They are a fundamental part of the cell membrane, act as signaling molecules, modify gene expression (Price et al. 2000; Deckelbaum et al. 2006; De Caterina and Massaro 2005) and can thereby sustain a status of well-being (Simopoulos 1991; Siddiqui et al. 2004; Connor 2000). Fittingly, omega-3 fatty acids have been established to have a profound correlation with several human diseases. Moreover, omega-3 fatty acids are essential dietary nutrients for normal growth and development (Innis 2004). Omega-3 fatty acids have a variety of physiological actions, like hypotriglyceridemic (McKenney and Sica 2007), anti-inflammatory (Singer et al. 2008) and anti-arrhythmic effects (Reiffel and McDonald 2006). They have been demonstrated to be beneficial in prevention and management of many conditions such as autoimmune and inflammatory (Simopoulos 2002; Mori and Beilin 2004), cardiovascular disease (Kris-Etherton et al. 2003), neurodegenerative diseases (Calon and Cole 2007) and cancer (Hardman 2002). A higher intake of the essential omega-3 fatty acid, α-linolenic acid (ALA) has been suggested to be associated with lower prevalence odds ratio of coronary artery disease (CAD) in US population (Djoussé et al. 2001).
Flaxseed oil, principally ALA, and fish oil, predominantly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the major sources of omega-3 polyunsaturated fatty acids (PUFAs) available today on a commercial basis and are often used as dietary supplements. With the current level of awareness, both of the oils are routinely prescribed or self-administered. Besides, there is an increasing tendency among the people of insistent inclusion of fish and flaxseeds in the daily diet. In a predominantly vegetarian Indian population, flaxseed consumption is a traditional food item. Moreover, ALA remains the only kind of omega-3 fatty acid that can be obtained via diet among vegans.
Type-2 diabetes mellitus (T2DM) is associated with a varied array of biochemical and physiological anomalies, especially in the liver, including abnormal carbohydrate, lipid and protein metabolism (Meyer et al. 1998; Krauss 2004; Gougeon et al. 1994). Szkudelski (2012) emphasized utility of STZ-NIC model for diabetes and reviewed the course of disease development. The abnormalities in lipid metabolism result in higher levels of circulating free fatty acids, hypertriglyceridemia and lower levels of high-density lipoprotein cholesterol (HDL-C) in T2DM patients (Reaven et al. 1988; Ginsberg 1996). Sustained elevations in free fatty acids result in increased insulin resistance, thus helping the progression of T2DM (Boden 2003). Hypertriglyceridemia leads to increase in the levels of triglycerides (TG)-rich lipoproteins, thereby increasing the risk of atherosclerosis in T2DM patients (Tkac et al. 1997). These alterations in lipid metabolism are effected by the abnormalities in the expression of hepatic transcription factors and genes.
Several studies have demonstrated that omega-3 fatty acids can improve lipid metabolism by lowering serum TG. However, there is a lack of evidence regarding these effects obtained in T2DM with dietary supplementation of flaxseed oil or fish oil. Moreover, the molecular mechanisms underlying the effects of these dietary oils have not been well elucidated. We hypothesize that omega-3 fatty acids improve lipid metabolism through regulation of PUFA metabolism, thereby altering transcription factors like peroxisome proliferator-activated receptor-α (PPAR-α), sterol regulatory element-binding protein-1 (SREBP-1) and their downstream genes like delta-5-desaturase (D5D), delta-6-desaturase (D6D) and atherogenic pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). In the present study, we have investigated the effect of dietary flaxseed oil and fish oil on serum lipids and fatty acid profiles in streptozotocin–nicotinamide (STZ-NIC) diabetic rats. Additionally, we have also analyzed the effects of these diets on hepatic expression of transcription factors and genes involved in fatty acid metabolism. We have attempted to evaluate the metabolic alterations and to address the mechanisms through which dietary flaxseed oil and fish oil can prove beneficial in diabetes.
Materials and methods
Animals
All the procedures and techniques related to the use, care of animals for research and the experimental design were approved by the Institutional Animal Ethics Committee (IAEC) of the Poona College of Pharmacy, Pune, India, established under The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Approval No.: CPCSEA/45/2010. Thirty-six male Wistar rats weighing 180–200 g were used in the study, which were obtained from National Biosciences, Pune, India. The animals were maintained at 23–25 °C with a 12-h light/12-h dark cycle. The rats received standard chow diet and water ad libitum during the experimental period.
Flaxseed oil and fish oil diets
Flaxseed oil diet contained flaxseed oil, obtained from Ramkrishna oil mills, Pune, India. The standard rodent chow pellets were powdered and mixed with flaxseed oil and re-pelleted so as to contain 10 % w/w flaxseed oil. Fish oil diet contained fish oil, obtained from John Baker, Inc. Colorado, USA. The standard rodent chow pellets were powdered and mixed with fish oil and re-pelleted so as to contain 10 % w/w fish oil. The feeds were prepared weekly and packed in individual plastic sealed bags in quantities sufficient for 1 day’s feed. The plastic bags were flushed with nitrogen, sealed and stored at −20 °C. The feed which was not consumed by the animals was discarded daily.
Experimental design
The male Wistar rats were randomly divided into six groups consisting six rats each. Out of the six groups, three were non-diabetic, while three were diabetic. The non-diabetic groups were designated as follows: Group C (control rats on standard diet), Group CFL (control rats on 10 % w/w flaxseed oil diet) and Group CF (control rats on 10 % w/w fish oil diet). The diabetic groups were termed as follows: Group DC (diabetic rats on standard diet), Group DFL (diabetic rats on 10 % w/w flaxseed oil diet) and Group DF (diabetic rats on 10 % w/w fish oil diet). Group C and Group DC received standard rodent chow diet through the length of the study. Group CFL and Group DFL received 10 % w/w flaxseed oil diet after confirmation of induction of diabetes for 35 days. Group CF and Group DF received 10 % w/w fish oil diet after confirmation of induction of diabetes for 35 days. The design of the experiment is illustrated in Table 1.
Table 1.
Experimental design employed in the study
| Days | C | DC | DFL | DF | CFL | CF |
|---|---|---|---|---|---|---|
| 1a | I.p. injection of saline solution and citrate buffer | I.p injection of nicotinamide (110 mg/kg) and STZ (65 mg/kg) | I.p injection of nicotinamide (110 mg/kg) and STZ (65 mg/kg) | I.p injection of nicotinamide (110 mg/kg) and STZ (65 mg/kg) | I.p. injection of saline solution and citrate buffer | I.p. injection of saline solution and citrate buffer |
| 15a | – | Confirmation of stable hyperglycemia | Confirmation of stable hyperglycemia | Confirmation of stable hyperglycemia | – | – |
| 16 | Regular feed for 35 days | Regular feed for 35 days | 10 % w/w flaxseed oil feed for 35 days | 10 % w/w fish oil feed for 35 days | 10 % w/w flaxseed oil feed for 35 days | 10 % w/w fish oil feed for 35 days |
| 51 | Blood samples were collected, animals were killed and liver tissue harvested | |||||
C Control, DC diabetes control, DFL diabetes + flaxseed oil, DF diabetes + fish oil, CFL control + flaxseed oil, CF control + fish oil
aAll the groups were fed regular diet from day 1 until day 15
Experimental induction of diabetes
Animals of the diabetic groups were administered nicotinamide (100 mg/kg), whereas animals of non-diabetic groups received saline solution by a single intra-peritoneal (i.p.) injection. Fifteen minutes later, animals of the diabetic groups received a single i.p. injection of STZ (65 mg/kg) dissolved in 10 mM sodium citrate buffer (pH 4.0). Animals of the non-diabetic groups received a single i.p. injection of the buffer alone. Diabetic rats were supplied with 5 % glucose solution for 12 h in order to prevent hypoglycemia. After 15 days of the administration of STZ, blood glucose levels were measured to confirm stable hyperglycemia. Animals with blood glucose levels above 250 mg/dl were selected for the study.
Collection of blood and tissues
Animals were anesthetized at the end of the experiment, and blood was withdrawn by the retro-orbital puncture technique. After the withdrawal of blood, the animals were killed, and their livers were removed. The livers were flash-frozen immediately in liquid nitrogen and stored at −80 °C.
Lipid profile
Serum total cholesterol (TC), TG and HDL-C were measured by using reagents and kits available from Accurex Biomedical Pvt. Ltd., Mumbai. The amounts of low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL-C) were calculated with the help of the equation of Friedwald (Friedewald et al. 1972):
LDL-C = TC−HDL-C−(TG/5)
VLDL-C = TG/5
Plasma and RBC fatty acid analysis
Plasma and RBC fatty acids were analyzed as methyl esters by using the method described here (Manku et al. 1983). Briefly, methanol-HCl was used for transesterification of the phospholipid fraction. A PerkinElmer gas chromatograph with a SD 2330 30-m capillary column (Supelco, PA, USA) was used to separate and quantify the methyl esters. The carrier gas used was Helium at 1 ml/min. The oven temperature was maintained at 150 °C for 10 min and was programmed to increase from 150 to 220 °C at 10 °C/min and to steady at 220 °C for 10 min. Detector temperature was maintained at 275 °C, while the injector temperature was maintained at 240 °C. The retention times and peak areas were computed automatically. The peaks of standard fatty acid methyl esters (Sigma, USA) were used to identify individual fatty acids by comparison.
Feed-fatty acid analysis
The fatty acid content of the three types of feeds: regular feed, 10 % w/w flaxseed oil feed and 10 % w/w fish oil feed were analyzed as methyl esters by following procedures similar to those described above (Table 2).
Table 2.
Fatty acid analysis of diets used in the study
| Fatty acids (g/100 g fatty acids) | Regular feed | 10 % (w/w) Flaxseed oil feed | 10 % (w/w) Fish oil feed |
|---|---|---|---|
| Myristic acid | 03.76 | 00.04 | 00.04 |
| Palmitic acid | 13.50 | 05.44 | 10.35 |
| Stearic acid | 02.73 | 04.09 | 01.71 |
| Oleic acid | 24.27 | 16.55 | 47.79 |
| Linoleic acid | 50.79 | 21.38 | 20.50 |
| ALA | 04.74 | 42.94 | 01.96 |
| ARA | 00.22 | 00.17 | 00.49 |
| EPA | – | – | 01.60 |
| DHA | – | – | 02.14 |
| Omega-3 | 04.74 | 42.94 | 5.7 |
| Omega-6 | 51.01 | 21.55 | 20.99 |
| n − 3: n − 6 | 00.09 | 01.99 | 00.27 |
| Saturated fatty acids | 44.26 | 26.12 | 59.89 |
qPCR
For qPCR analysis, the total RNA from the liver tissues was extracted using TRIZOL reagent (Invitrogen CA, USA) with PureLink RNA mini kit (Invitrogen CA, USA). Quality of the isolated RNA was determined using denaturing agarose gel electrophoresis. Then, the RNA was quantified by determining its UV absorbance at 260 nm. The cDNA was synthesized from 1 μg of total RNA using the SuperScript first-strand synthesis system for quantitative real-time PCR (Invitrogen CA, USA). The qPCR analysis was performed with the help of a StepOne real-time PCR system (Applied Biosystems, CA, USA) along with TaqMan gene expression assays (Applied Biosystems, CA, USA) and TaqMan gene expression master mix (Applied Biosystems, CA, USA). The cycling conditions used were 50 °C for 2 min; 95 °C for 10 min; and 40 cycles of 95 °C for 15 s, 60 °C for 1 min. The Taqman gene expression assays that were used in this study were PPAR-α (Ppara; Rn00566193_m1), SREBP-1 (Srebf1; Rn01495769_m1), D5D (Fads1; Rn00584915_m1), D6D (Fads2; Rn00580220_m1), Liver–fatty acid binding protein (L-FABP) (Fabp1; Rn00664587_m1), adipocyte–fatty acid binding protein (A-FABP) (Fabp4; Rn00670361_m1), TNF-α (Tnf; Rn00562055_m1) and IL-6 (Il6; Rn01410330_m1). The relative amounts of the RNA was normalized to the amount of the two endogenous controls, that is, β-actin (Actb; Rn00667869_m1) and GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) (Gapdh; Rn01775763_g1) using StepOne software version 2.2.2 (Applied Biosystems, CA, USA), DataAssist version 3.0 (Applied Biosystems, CA, USA) and the ΔΔ Ct method (Schmittgen and Livak 2008). The two endogenous controls were combined using the DataAssist version 3.0, and the mean was used for normalization of RNA amounts. Here, as two endogenous control genes were selected for normalization, the DataAssist software calculated the mean of the two endogenous control genes to use as a normalizer (normalization factor), on a per sample basis.
Statistical analysis
Statistical analyses were carried out by using GraphPad Prism software version 5.0 (GraphPad Software Inc., CA, USA). The data were expressed as mean ± SEM, and statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison test. Differences were considered significant if the P value was <0.05.
Results
Food intake
The food intake of animals between different groups was not significantly different. The food consumed by animals (n = 6) of the various groups was the following: Group C: 18.40 ± 00.29 g/rat/day, Group DC: 18.07 ± 00.14 g/rat/day, Group DFL: 18.03 ± 00.30 g/rat/day, Group DF: 18.27 ± 00.19 g/rat/day, Group CFL: 18.23 ± 00.18 g/rat/day, Group CF: 18.33 ± 00.35 g/rat/day.
Effect of flaxseed oil diet or fish oil diet on lipid profile of STZ-NIC diabetic rats
Serum TC levels
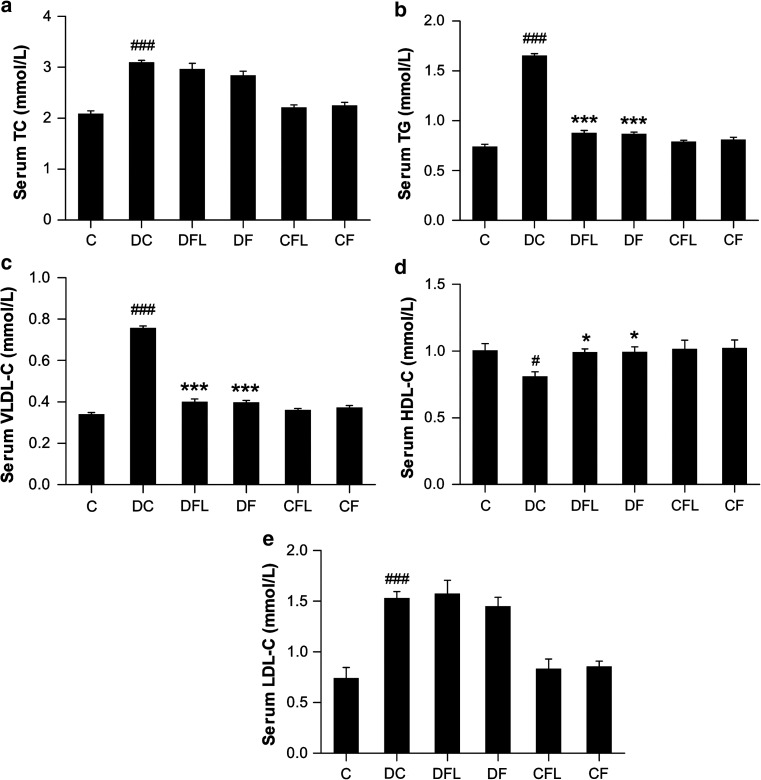
Diabetic control rats showed significantly higher (P < 0.001) serum TC levels than control rats (2.085 ± 0.0656 vs. 3.099 ± 0.0432 mmol/L). While there was a slight reduction in the serum TC levels in diabetic rats receiving flaxseed oil diet or fish oil diet, the reduction was not found to be significant (2.967 ± 0.1124 and 2.843 ± 0.0855 mmol/L, respectively), whereas supplementation of flaxseed or fish oil diets did not have any effect in control groups (Fig. 1a).
Fig. 1.
Effect of flaxseed oil diet or fish oil diet on a Serum TC levels, b Serum TG levels, c Serum VLDL-C levels, d Serum HDL-C levels and e Serum LDL-C levels in STZ-NIC-induced diabetic rats. All the values are expressed as mean ± SEM (n = 6). Comparisons were done between the control group and each individual group by Dunnet’s multiple comparison test. (#P < 0.05, ###P < 0.001, when compared against control group, *P < 0.05, ***P < 0.001, when compared against diabetes control group) C control, DC diabetes control, DFL diabetes + flaxseed oil, DF diabetes + fish oil, CFL control + flaxseed oil, CF control + fish oil
Serum TG levels
Also, serum TG level in STZ-NIC rats on control diet was increased (P < 0.001) as compared to control rats (0.742 ± 0.0204 vs. 1.654 ± 0.0188 mmol/L). Both flaxseed oil diet and fish oil diet caused significant decrease (P < 0.001) in serum TG levels as compared to STZ-NIC rats on control diet (0.877 ± 0.0276 and 0.868 ± 0.0195 mmol/L, respectively). Further, both flaxseed and fish oil diets did not modify serum TG level in control groups (Fig. 1b).
Serum VLDL-C levels
Similarly, in diabetic rats with no intervention, the serum VLDL-C levels were elevated (P < 0.001) as compared to control rats (0.340 ± 0.0093 vs. 0.758 ± 0.0086 mmol/L). The serum VLDL-C levels in diabetic rats receiving flaxseed oil diet or fish oil diet were significantly (P < 0.001) lowered (0.402 ± 0.0126 and 0.398 ± 0.0089 mmol/L, respectively) while no effect was seen on VLDL-C levels in non-diabetic control groups (Fig. 1c).
Serum HDL-C levels
On the other hand, serum HDL-C levels in STZ-NIC control rats were lower (P < 0.05) as compared to control rats (0.811 ± 0.0332 vs. 1.004 ± 0.0513 mmol/L). In STZ-NIC rats receiving flaxseed oil diet or fish oil diet, the serum HDL-C levels were significantly (P < 0.05) raised (0.992 ± 0.0241 and 0.994 ± 0.0384 mmol/L, respectively) (Fig. 1d).
Serum LDL-C levels
Serum LDL-C levels in diabetic rats on control diet were higher (P < 0.001) than that in control rats (0.741 ± 0.1052 vs. 1.529 ± 0.0643 mmol/L). There was no effect of flaxseed oil diet on LDL-C levels in diabetic rats (1.573 ± 0.1336 mmol/L). Although fish oil diet slightly reduced LDL-C levels in diabetic rats, the reduction was not significant (1.450 ± 0.0857 mmol/L) (Fig. 1e).
Effect of flaxseed oil diet or fish oil diet on fatty acid profiles of STZ-NIC diabetic rats
Plasma fatty acids
Plasma arachidonic acid (ARA) levels in STZ-NIC rats (01.16 ± 00.28 g/100 g fatty acids) were lower (P < 0.001) when compared to control rats (04.81 ± 00.53 g/100 g fatty acids). The levels of plasma ARA among all other groups were not found to be significantly different.
EPA levels in plasma of diabetic control rats (05.25 ± 00.16 g/100 g fatty acids) were lower (P < 0.05) as compared to those in control rats on standard diet (08.01 ± 00.84 g/100 g fatty acids). Both flaxseed oil diet (08.41 ± 00.53 g/100 g fatty acids) and fish oil diet (08.39 ± 00.58 g/100 g fatty acids) increased (P < 0.05) plasma EPA levels in diabetic rats. Control rats receiving flaxseed oil or fish oil diets did not show any significant difference in plasma EPA levels when compared against control rats on standard diet.
In diabetic rats on standard diet (04.33 ± 00.67 g/100 g fatty acids), plasma DHA levels were reduced (P < 0.001) when compared with control rats (10.05 ± 01.01 g/100 g fatty acids). Plasma DHA levels in diabetic rats on flaxseed oil (11.53 ± 00.84 g/100 g fatty acids) or fish oil (10.50 ± 01.09 g/100 g fatty acids) diets were higher (P < 0.001) than that in diabetic rats on standard diet, while control rats fed flaxseed oil or fish oil diets did not show any significant changes. Here, flaxseed oil diet showed a slightly higher increase in plasma DHA levels than that shown by fish oil diet in diabetic rats.
Plasma linoleic acid, ALA levels and the n − 3 to n − 6 ratio were not significantly different among rats of different groups (Table 3).
Table 3.
Effect of flaxseed oil or fish oil diets on plasma fatty acid profile of STZ-NIC-induced diabetic rats
| Fatty acids (g/100 g fatty acids) | C | DC | DFL | DF | CFL | CF |
|---|---|---|---|---|---|---|
| Linoleic acid | 02.56 ± 00.44 | 01.89 ± 00.41 | 02.81 ± 00.39 | 02.45 ± 00.40 | 02.24 ± 00.34 | 02.23 ± 00.51 |
| ALA | 03.40 ± 00.45 | – | 07.53 ± 01.34 | – | 01.11 ± 00.33 | – |
| ARA | 04.81 ± 00.53 | 01.16 ± 00.28### | 02.83 ± 00.27 | 01.80 ± 00.41 | 03.25 ± 00.57 | 03.12 ± 00.69 |
| EPA | 08.01 ± 00.84 | 05.25 ± 00.16# | 08.41 ± 00.53* | 08.39 ± 00.58* | 08.42 ± 00.80 | 08.25 ± 00.92 |
| DHA | 10.05 ± 01.01 | 04.33 ± 00.67### | 11.53 ± 00.84*** | 10.50 ± 01.09*** | 11.43 ± 00.99 | 11.50 ± 01.03 |
| Omega-3 | 21.46 ± 01.24 | 09.58 ± 00.63### | 27.48 ± 01.01*** | 18.89 ± 01.50*** | 20.96 ± 01.40 | 19.75 ± 01.69 |
| Omega-6 | 07.37 ± 00.74 | 03.06 ± 00.67### | 05.65 ± 00.21* | 04.25 ± 00.68 | 05.49 ± 00.34 | 05.35 ± 00.67 |
| n − 3: n − 6 | 03.06 ± 00.33 | 03.69 ± 00.68 | 04.91 ± 00.30 | 05.39 ± 01.51 | 03.99 ± 00.56 | 03.94 ± 00.43 |
All values are expressed as mean ± SEM (n = 6). Comparisons were done between the control group and each individual group by Dunnet’s multiple comparison test. (#P < 0.05, ###P < 0.001, when compared against control group, * P < 0.05, *** P < 0.001, when compared against diabetic control group)
C Control, DC diabetes control, DFL diabetes + flaxseed oil, DF diabetes + fish oil, CFL control + flaxseed oil, CF control + fish oil
RBC fatty acids
Linoleic acid levels in RBCs of diabetic rats (00.38 ± 00.09 g/100 g fatty acids) were lower (P < 0.05) as compared to control rats (02.28 ± 00.44 g/100 g fatty acids). In diabetic rats receiving flaxseed oil diet or fish oil diet, the RBC linoleic acid levels were not significantly different as compared to diabetic control rats. RBC linoleic acid levels in control rats on flaxseed oil (05.21 ± 00.76 g/100 g fatty acids) or fish oil (05.62 ± 00.78 g/100 g fatty acids) diets were higher (P < 0.001) when compared to control rats.
ARA levels in RBCs of diabetic control rats and control rats did not differ significantly. RBC ARA levels in diabetic rats on flaxseed oil diet (06.29 ± 00.75 g/100 g fatty acids) or fish oil diet (10.28 ± 00.96 g/100 g fatty acids) were higher (P < 0.001) when compared to diabetic control rats (01.61 ± 00.52 g/100 g fatty acids). ARA levels in control rats fed flaxseed oil (06.59 ± 00.86 g/100 g fatty acids) or fish oil (06.91 ± 00.82 g/100 g fatty acids) diets were higher (P < 0.01) when compared to control rats (02.32 ± 00.38 g/100 g fatty acids). Here, fish oil diet increased RBC ARA levels to greater extent than flaxseed oil diet in diabetic rats.
Levels of EPA in RBCs of STZ-NIC control rats (02.55 ± 00.27 g/100 g fatty acids) were lower (P < 0.001) when compared to control rats (08.15 ± 00.87 g/100 g fatty acids). Flaxseed oil diet (08.11 ± 00.75 g/100 g fatty acids) and fish oil diet (05.33 ± 00.43 g/100 g fatty acids) increased (P < 0.05) RBC EPA levels in STZ-NIC rats as compared to control STZ-NIC rats. Here, flaxseed oil diet showed higher RBC EPA levels that fish oil diet in diabetic rats.
In diabetic control rats (02.69 ± 00.41 g/100 g fatty acids), the DHA levels in RBCs were lower (P < 0.001) as compared to control rats (11.57 ± 01.15 g/100 g fatty acids). Flaxseed oil diet (10.88 ± 01.15 g/100 g fatty acids) or fish oil diet (06.23 ± 00.35 g/100 g fatty acids) significantly increased (P < 0.05) DHA levels in RBCs of diabetic rats as compared to control diabetic rats. Here, flaxseed oil diet increased RBC DHA levels to greater extent than fish oil diet in diabetic rats (Table 4).
Table 4.
Effect of flaxseed oil or fish oil diets on RBC fatty acid profile of STZ-NIC-induced diabetic rats
| Fatty acids (g/100 g fatty acids) | C | DC | DFL | DF | CFL | CF |
|---|---|---|---|---|---|---|
| Linoleic acid | 02.28 ± 00.44 | 00.38 ± 00.09# | 01.31 ± 00.21 | 00.82 ± 00.08 | 05.21 ± 00.76### | 05.62 ± 00.78### |
| ARA | 02.32 ± 00.38 | 01.61 ± 00.52 | 06.29 ± 00.75*** | 10.28 ± 00.96*** | 06.59 ± 00.86## | 06.91 ± 00.82### |
| EPA | 08.15 ± 00.87 | 02.55 ± 00.27### | 08.11 ± 00.75*** | 05.33 ± 00.43* | 08.12 ± 00.83 | 08.27 ± 00.65 |
| DHA | 11.57 ± 01.15 | 02.69 ± 00.41### | 10.88 ± 01.15*** | 06.23 ± 00.35* | 10.39 ± 01.29 | 10.54 ± 00.61 |
| Omega-3 | 19.72 ± 01.41 | 05.25 ± 00.49### | 18.99 ± 01.28*** | 11.57 ± 00.68** | 18.51 ± 01.78 | 18.81 ± 00.34 |
| Omega-6 | 04.60 ± 00.59 | 01.99 ± 00.51 | 07.60 ± 00.72** | 11.10 ± 00.95*** | 11.81 ± 01.55### | 12.54 ± 00.96### |
| n − 3: n − 6 | 04.75 ± 00.75 | 03.91 ± 01.20 | 02.69 ± 00.44 | 01.09 ± 00.13* | 01.66 ± 00.20## | 01.55 ± 00.14## |
All values are expressed as mean ± SEM (n = 6). Comparisons were done between the control group and each individual group by Dunnet’s multiple comparison test. (#P < 0.05, ##P < 0.01, ###P < 0.001, when compared against control group, * P < 0.05, ** P < 0.01, *** P < 0.001, when compared against diabetic control group)
C Control, DC diabetes control, DFL diabetes + flaxseed oil, DF diabetes + fish oil, CFL control + flaxseed oil, CF control + fish oil
Effect of flaxseed oil diet or fish oil diet on hepatic gene expression in STZ-NIC diabetic rats
Transcription factors regulating TG levels
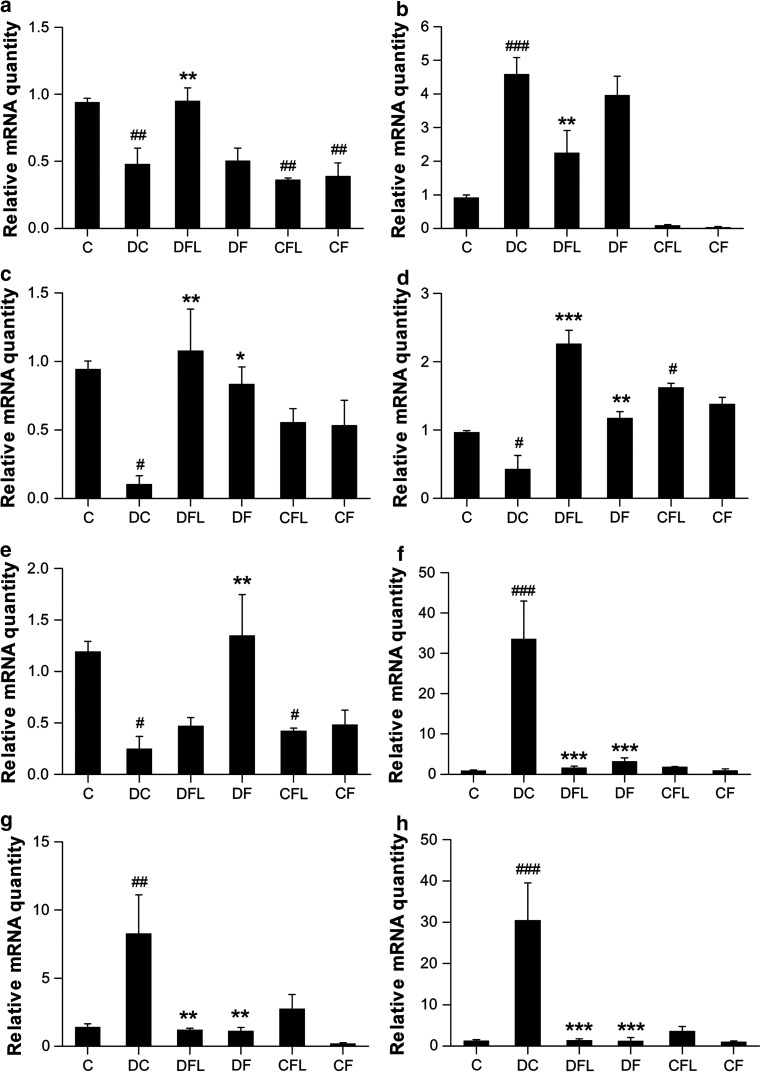
In STZ-NIC rats, the hepatic expression of PPAR-α was down-regulated (P < 0.01) by ~2.0-fold when compared to control rats. In STZ-NIC rats receiving flaxseed oil diet, the hepatic PPAR-α expression was up-regulated (P < 0.01) by ~2.0-fold, thus bringing it back to normal levels. The hepatic PPAR-α expression was not affected by fish oil diet and was almost similar to that in STZ-NIC rats. In control rats fed flaxseed oil or fish oil, the PPAR-α expression was lowered (P < 0.01) by ~2.5-fold and ~2.4-fold, respectively, when compared to control rats on standard chow. Here, flaxseed oil diet had better effect on PPAR-α expression as it increased its expression in diabetic rats while fish oil had no effect (Fig. 2a).
Fig. 2.
Effect of flaxseed oil diet or fish oil diet on hepatic expression of a PPAR-α, b SREBP-1, c D5D, d D6D, e L-FABP, f A-FABP, g TNF-α and h IL-6 in STZ-NIC-induced diabetic rats. All the values are expressed as mean ± SEM (n = 3). Comparisons were done between the control group and each individual group by Dunnet’s multiple comparison test. (#P < 0.05, ##P < 0.01, ###P < 0.001, when compared against control group, *P < 0.05, **P < 0.01, ***P < 0.001, when compared against diabetic control group) C control, DC diabetes control, DFL diabetes + flaxseed oil, DF diabetes + fish oil, CFL control + flaxseed oil, CF control + fish oil
Hepatic SREBP-1 expression was ~5.0-fold higher (P < 0.001) in STZ-NIC rats on control diet when compared to control rats. Flaxseed oil diet decreased (P < 0.01) the hepatic SREBP-1 expression by ~2.0-fold in STZ-NIC rats. On the other hand, fish oil diet lowered SREBP-1 expression by ~1.1-fold in STZ-NIC rats, but the difference was not statistically significant. Thus, flaxseed oil diet proved to be better at decreasing SREBP-1 expression than fish oil diet (Fig. 2b).
Desaturases
The hepatic expression of both D5D and D6D was lowered (P < 0.05) by ~8.8- and ~2.2-fold, respectively, in diabetic control rats as compared to control rats. Hepatic D5D expression was up-regulated by ~10.1- or ~7.8-fold by flaxseed oil diet (P < 0.01) or fish oil diet (P < 0.05), respectively, in diabetic rats. While flaxseed oil diet up-regulated (P < 0.001) hepatic D6D expression by ~5.2-fold, fish oil diet up-regulated (P < 0.01) the hepatic D6D by ~2.7-fold in diabetic control rats. In control rats receiving flaxseed oil diet, the D6D expression was up-regulated (P < 0.05) by ~1.7-fold when compared to control rats. Here, while both diets increased the expression of desaturase genes in diabetic rats, flaxseed oil diet increased their expression to a greater extent than that by fish oil diet. Moreover, flaxseed oil diet showed up-regulation of D6D gene in control rats, which was not shown by fish oil diet (Fig. 2c, d).
FABPs
In diabetic rats receiving control diet, the expression of L-FABP in the liver was reduced (P < 0.05) by ~4.7-fold as compared to control rats. In diabetic rats receiving fish oil diet, the hepatic L-FABP expression was up-regulated (P < 0.01) by ~5.4-fold as compared to diabetic rats receiving control diet. The hepatic L-FABP expression was slightly increased non-significantly by flaxseed oil diet. L-FABP expression in control rats on flaxseed oil diet was down-regulated (P < 0.05) by ~2.8-fold as compared to control rats. Fish oil diet showed a higher up-regulation of L-FABP expression in diabetic rats than that shown by flaxseed oil diet (Fig. 2e).
Atherogenic cytokines
Hepatic A-FABP expression was increased (P < 0.001) by ~34.0-fold in STZ-NIC control rats as compared to control rats. Flaxseed oil diet significantly (P < 0.001) down-regulated hepatic A-FABP expression by ~19.9-fold in STZ-NIC rats. Whereas fish oil diet down-regulated (P < 0.001) hepatic A-FABP expression by ~10.2-fold in STZ-NIC rats. Here, flaxseed oil diet fared better than fish oil diet in down-regulating A-FABP expression in diabetic rats (Fig. 2f).
In STZ-NIC rats on control diet, both TNF-α and IL-6 were up-regulated (P < 0.01 and P < 0.001) by ~5.8- and ~23-fold, respectively, as compared to control rats. Hepatic TNF-α expression was down-regulated by ~6.6- or ~7.2-fold by flaxseed oil diet (P < 0.01) or fish oil diet (P < 0.01), respectively, in STZ-NIC rats. Flaxseed oil diet down-regulated (P < 0.001) hepatic IL-6 expression by ~21.8-fold, whereas fish oil diet down-regulated (P < 0.001) the hepatic IL-6 by ~24.3-fold in STZ-NIC rats. Here, fish oil diet was better at down-regulating both TNF-α and IL-6 expressions in diabetic rats than flaxseed oil diet (Fig. 2g, h).
Discussion
The administration of STZ along with nicotinamide in rats results in a condition, which is similar to T2DM in humans and is thus a suitable model for studying short as well as long-term effects of drugs and natural compounds on diabetic complications (Masiello et al. 1998; Szkudelski 2012).
Several studies have reported the TG lowering effect of dietary fish oil (Rivellese et al. 1996; Montori et al. 2000). Various cardio protective effects of dietary flaxseed have been reported in animal models as well as in humans (Bassett et al. 2009). Flaxseed oil has shown to have beneficial effects on hepatic cholesterol metabolism in high fat diet rats (Vijaimohan et al. 2006). A recent review on the benefits of omega-3 fatty acids in T2DM in human clinical trials, points out that the effects of ALA (contained in flaxseed oil) in T2DM are not well studied (Hendrich 2010). The aim of the present study is to bridge this gap and to compare effects of flaxseed oil diet and fish oil diet on lipid levels, transcription factor activation and gene expression. One of the objectives was to bring out the differences in pathways through which flaxseed oil and fish oil exert their beneficial effects on lipid metabolism.
Firstly, we evaluated the effect of flaxseed oil diet and fish oil diet in lipid profiles of STZ-NIC and control rats. Flaxseed oil and fish oil have been reported to have a TG lowering effect in STZ-diabetic rats (Kaithwas and Majumdar 2012; Mahmud et al. 2004). Likewise, we report that flaxseed oil diet and fish oil diet reduced serum TG and VLDL-C levels in STZ-NIC rats. Recently, flaxseed oil has been shown to increase HDL-C levels in STZ-diabetic rats (Kaithwas and Majumdar 2012). Similarly, here, we demonstrated that both diets increase HDL-C levels in diabetic rats. Both the diets did not show any effect on serum TC and LDL-C levels in diabetic rats. Also, in control rats, neither flaxseed oil diet nor fish oil diet have any effect on the lipid profile. Thus, both the diets have similar effects on serum lipid profile. Similarly, it has been previously reported that fish oil or flaxseed oil have comparable beneficial effects on tissue lipid profile in high fat fed mice (Riediger et al. 2008).
Further, we analyzed the plasma and RBC fatty acid profile in control and diabetic rats. The increase in n − 3 to n − 6 ratio has been shown to decrease insulin resistance in human subjects (Huang et al. 2010). Thus, an increase in this omega-3 status would be beneficial to curb the progression of T2DM and may also benefit pre-diabetic patients. Interestingly, we found equivalent levels of omega-3 fatty acids in plasma of diabetic rats fed flaxseed oil or fish oil diets. Whereas diabetic rats fed flaxseed oil diet showed higher levels of omega-3 fatty acids in RBCs than that shown by fish oil fed diabetic rats. This was quite unexpected, but could be attributed to the higher levels of expression of desaturase genes in flaxseed fed diabetic rats. It is to be noted that control rats fed with flaxseed oil or fish oil diets have equivalent omega-3 content in plasma and RBCs. In our study, plasma and RBC arachidonic acid (ARA) levels were decreased in diabetic rats possibly due to lower levels of D5D and D6D activity. This is in compliance with a previously reported study where ARA levels were decreased in liver, kidney and brain of STZ-NIC diabetic rats (Murugan and Pari 2007; Pari and Murugan 2007). It is to be noted here that both the diets increased ARA levels in plasma and RBCs of diabetic rats with concomitant increase in D5D and D6D expression.
Genes involved in fatty acid metabolism
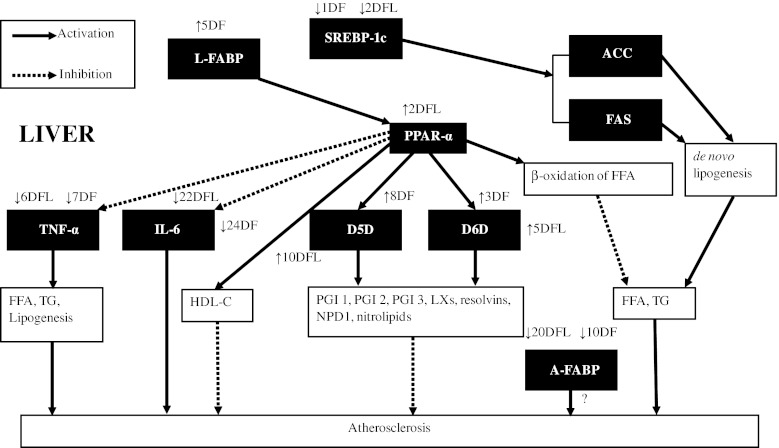
The hepatic expression of the genes studied in this report is depicted here in an articulated manner (Fig. 3). PPAR-α, a member of the nuclear receptor family of PPARs, acts as a transcription factor and plays a key role in maintaining carbohydrate and lipid homeostasis (Keller and Wahli 1993). The activation of PPAR-α has been shown to stimulate beta-oxidation of fatty acids, thereby reducing serum TG level (Schoonjans et al. 1996) (Fig. 3). Additionally, PPAR-α activation caused the down-regulation of expression of atherogenic cytokines- IL-6 and TNF-α in mice liver and microglia, respectively (Mansouri et al. 2008; Jana et al. 2007) + (Fig. 3). Also, PPAR-α agonists have beneficial effects (qualitative and quantitative) on HDL-C levels (Wallace et al. 2005) (Fig. 3). Consequently, PPAR-α activation has a therapeutic value in T2DM patients in order to control diabetic dyslipidemia. Previously, it has been demonstrated that omega-3 fatty acids protect against high-fat-induced hepatic insulin resistance and reduce TG via a mechanism involving PPAR-α (Neschen et al. 2007; Davidson 2006). Similarly, in our study, flaxseed oil diet up-regulated hepatic PPAR-α along with an increase in HDL-C levels and a decrease in TG levels in diabetic rats. Thus, it seems that these effects of flaxseed oil diet are mediated via PPAR-α. Whereas fish oil diet did not show any effect on PPAR-α expression, but could decrease TG and increase HDL-C levels in diabetic rats. Therefore, fish oil seems to have a ‘TG lowering effect’ that is independent of PPAR-α. Previously, fish oil has been shown to have a PPAR-α independent effect on lipid and glucose metabolism in obese mice (Wakutsu et al. 2010).
Fig. 3.
Network diagram representing hepatic transcription factors and genes and their effects on lipid metabolism and the effect of fish oil and flax oil on their hepatic expression as observed in this study. Up arrow Up-regulation, down arrow Down-regulation. DFL effect of flax oil in STZ-NIC rats, DF effect of fish oil in STZ-NIC rats
SREBPs are transcription factors involved in the regulation of fatty acid and cholesterol metabolism in the liver (Horton et al. 2002). Out of the two isoforms of SREBP-1, namely 1a and 1c, SREBP-1c is relatively abundant in the liver. The overexpression of SREBP-1a has been shown to result in the increased production of cholesterol and TG (Shimano et al. 1996), while overexpression of SREBP-1c resulted in the increased production of TG (Shimano et al. 1997). Shimomura et al. (1999) reported increased expression of lipogenic genes, acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) in diabetic mice following the overexpression of SREBP-1c (Fig. 3). Additionally, in humans, hepatic steatosis is associated with increased levels of SREBP-1c (Higuchi et al. 2008). Thus, down-regulation of SREBP-1 in the liver has a therapeutic value in treating diabetic hepatic steatosis and carbohydrate-induced hypertriglyceridemia (Moon et al. 2012). It has been previously demonstrated that, omega-3 fatty acids bring about their hypotriglyceridemic effects by lowering SREBP-1c expression (Davidson 2006). Similarly, in our study, flaxseed oil diet decreased serum TG levels, along with down-regulation of hepatic SREBP-1 in diabetic rats (Fig. 1b). Whereas fish oil diet decreased serum TG levels, with marginal decrease in SREBP-1 expression (non-significant). Thus, the TG lowering effect demonstrated by both the diets seems to be mediated by SREBP-1.
The present data suggest different mechanisms for the hypotriglyceridemic effect of flaxseed oil and fish oil diets. Flaxseed oil diet demonstrated the activation of PPAR-α on one hand, which would enhance β-oxidation and on the other hand suppression of SREBP-1, which would reduce lipogenesis. On the contrary, fish oil diet had no effect on PPAR-α, but reduced SREBP-1c, although non-significantly, in our study.
D5D and D6D are key enzymes involved in the metabolism of PUFAs. A recent review underlines the relevance of D5D and D6D enzyme activities in the development of T2DM (Kroger and Schulze 2012). There are very few studies concerning the significance of these desaturases in T2DM. The dis-regulation of D5D and D6D expression in T2DM would result in lower or abnormal levels of PUFAs. It has been proposed that lower activities of D5D and D6D enzymes would result in lower levels of LCPUFAs like Gamma-linolenic acid (GLA), EPA, DHA, etc. This, in turn, would lower the production of prostaglandin E1 (PGE 1), prostacyclin (PGI 2), PGI 3, lipoxins (LXs), resolvins, neuroprotectin D1 (NPD1) and nitrolipids, which have anti-inflammatory and anti-aggregatory actions, thus resulting in a greater risk of development of atherosclerosis (Das 2007) (Fig. 3). The lowered activity of D5D and D6D in diabetic conditions leads to a presumption that flaxseed oil would have fewer benefits in diabetes. In our study, both diets up-regulated D5D and D6D expression in diabetic rats (Fig. 2c, d). The key finding of this study is the enhanced activation of both these genes by flaxseed oil diet than that by fish oil diet, thus explaining the higher levels of EPA and DHA in RBCs of diabetic rats on flaxseed oil diet. The up-regulation of D6D by flaxseed oil diet in control animals was significant and reiterates the role of flaxseed oil in elevating omega-3 status. It has been previously reported that, in rat liver, the activation of PPAR-α brings about the induction of D5D and D6D enzymes (Song He et al. 2002) (Fig. 3). Thus, the effect of flaxseed oil diet on desaturase expression could be attributed to its activation of PPAR-α.
L-FABP and A-FABP are members of a multigene family of cytoplasmic lipid transport proteins which are potential targets for treatment of dyslipidemia, atherosclerosis and insulin resistance (Boord et al. 2002). Although their exact function is not been elucidated, it has been hypothesized that L-FABP plays a vital role in lipid metabolism during metabolic stress conditions (Newberry et al. 2003). Accordingly, L-FABP-null mice show poor accumulation of TG in liver and this reflects as increased TG in serum (Newberry et al. 2003). Additionally, L-FABP facilitates cytosolic fatty acid oxidation in the liver (Veerkamp and van Moerkerk 1993), which would prove to be beneficial in hyperlipidemic conditions. Moreover, it has been demonstrated that L-FABP acts as a gateway to PPAR-α agonists like, PUFAs and hypolipidemic drugs (Wolfrum et al. 2001) (Fig. 3). Thus, L-FABP up-regulation would enhance the activation of PPAR-α via these hypolipidemic agents. As far as we know, this is the first report on the effect of omega-3 fatty acids on the hepatic expression of L-FABP in diabetic condition. The present study, for the first time indicated that fish oil diet up-regulates L-FABP in diabetic rats, which could potentially be one of the mechanisms underlying its ‘TG lowering’ effect (Fig. 2e). FABPs are also associated with the DHA uptake (Dutta-Roy 2000), which might explain the higher activation of L-FABP in fish oil fed diabetic rats, nevertheless, flaxseed oil diet increases the expression of this gene, although marginally.
It has been shown that the serum levels of A-FABP are associated with non-alcoholic fatty liver disease (NAFLD) in T2DM (Koh et al. 2009) and with abnormalities in glucose metabolism and development of T2DM in Chinese cohort (Tso et al. 2007). Levels of A-FABP are associated with severity of CAD, and its inhibition can be helpful in treatment of atherosclerosis and T2DM (Bao et al. 2011; Furuhashi et al. 2007; Tuncman et al. 2006). Although there are many studies regarding the serum levels of A-FABP and its association with T2DM, none of the studies report hepatic levels of A-FABP or expression of its gene. Here, for the first time, we showed that hepatic mRNA levels of A-FABP to be dramatically up-regulated by ~34-fold in STZ-NIC diabetic rats (Fig. 2f). We hypothesize that such high changes in expression of A-FABP are attributed to an important role in the liver in conditions like T2DM; however, its exact role is yet unclear (Fig. 3). Also as far as we know, there are no studies related to the effects of omega-3 fatty acids on serum levels of A-FABP. In our study, both the diets down-regulated the expression of A-FABP in livers of diabetic rats, while flaxseed oil diet was more effective in down-regulating A-FABP. Various FABPs have been previously shown to be markers of various tissue injuries (Pelsers et al. 2005). Consequently, higher level of A-FABP could act as novel marker for hepatic injury. Further studies are required to decipher the functional role of A-FABP in the liver in health as well as in metabolic disorders.
Hepatic expression of atherogenic cytokines
In 1997, Pickup et al. first suggested diabetes to be an inflammatory condition, after which there have been numerous evidences that have established a co-relation between higher levels of pro-inflammatory factors with insulin resistance and T2DM. Inflammatory cytokines, TNF-α and IL-6 are instrumental in contributing toward pathogenesis of insulin resistance as well as to the risk of development of cardiovascular diseases (CVD) in T2DM due to their pro-atherogenic nature. T2DM patients have higher circulating levels of these cytokines, probably produced by non-circulating cells such as hepatocytes (Pickup et al. 2000). Apart from its role in inflammation, TNF-α has been suggested to have effects on lipid metabolism, such as decreased free fatty acid (FFA) uptake, increased lipogenesis and lipolysis, thus contributing to hyperlipidemia and subsequently to atherogenesis (Chen et al. 2009a). TNF-α contributes in the development of atherosclerotic lesions, insulin resistance, hypertriglyceridemia, lipolysis and increased FFA levels, all of which may result in increased risk of the development of CVD in T2DM (Popa et al. 2007) (Fig. 3). Similarly, IL-6 plays a significant role in the pathogenesis of coronary heart disease (CHD) (Yudkin et al. 2000). Additionally, IL-6 also plays a significant role in the development of early atherosclerosis (Chen et al. 2009b) (Fig. 3). Thus, down-regulation of these cytokines could arrest the progression of atherosclerosis in T2DM. The anti-inflammatory effects of omega-3 fatty acids result in down-regulation of TNF-α and IL-6, thus arresting the progress of various cardiac and vascular disorders (Mori and Beilin 2004). In the present study, flaxseed oil or fish oil diets down-regulated TNF-α and IL-6 in livers of diabetic rats (Fig. 2g, h).
Figure 3 shows a model linking hepatic expression of different genes evaluated in the present study. It depicts how these genes potentially contribute to lipid abnormalities prevailing in diabetic conditions. These lipidic abnormalities eventually lead to atherosclerosis in T2DM. PPAR-α increases beta-oxidation of FFAs, which leads to a decrease in the levels of TG and FFA. It also increases HDL-C levels and up-regulates D5D and D6D genes. On the other hand, PPAR-α decreases the expression of atherogenic cytokines, TNF-α and IL-6. Down-regulation of SREBP-1 reduces ACC and FAS genes, leading to decreased de novo lipogenesis. Both these transcription factors collectively explain observed lowered serum TG levels. Up-regulation of D5D and D6D genes increase the levels of EPA, which in turn has anti-inflammatory and anti-aggregatory properties. L-FABP probably shuttles omega 3 fatty acids and thereby assists in activating PPAR-α. Although there are no reports regarding the expression of A-FABP in liver, the increase in serum levels of A-FABP has shown to be associated with CAD. TNF-α and IL-6 are atherogenic in nature, and their increased expression leads to higher levels of TG, FFA and an increase in lipogenesis.
In conclusion, both diets show a ‘TG lowering’ effect in STZ-NIC diabetic rats. Flaxseed oil diet may have brought about this effect via modulation of PPAR-α and SREBP-1, while fish oil diet showed a ‘TG lowering’ effect via modulation of SREBP-1 and L-FABP. Both diets improved omega-3 fatty acid levels in plasma and RBCs of diabetic rats, probably via up-regulation of D5D and D6D genes. Both flaxseed oil and fish oil diets down-regulated the expression of TNF-α, IL-6 and A-FABP in livers of diabetic rats. The derailed lipid metabolism and inflammation contribute substantially to the pathology of T2DM. The diabetic therapy therefore should focus on these aspects of T2DM as well. Flaxseed oil or fish oil can potentially correct these metabolic issues, and their dietary supplementation has a therapeutic value in preventing irregularities in lipid metabolism in T2DM.
Acknowledgments
The authors would like to acknowledge the Department of Biotechnology (DBT), Government of India, for providing financial support.
Conflict of interest
None.
Ethical standards
The experiments comply with the current laws of India and were approved by the Institutional Animal Ethics Committee (IAEC) of the Poona College of Pharmacy, Pune, India, established under The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Approval No.: CPCSEA/45/2010.
References
- Bao Y, Lu Z, Zhou M, Li H, Wang Y, Gao M, Wei M, Jia W. Serum levels of adipocyte fatty acid-binding protein are associated with the severity of coronary artery disease in Chinese women. PLoS ONE. 2011;6:e19115. doi: 10.1371/journal.pone.0019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett CM, Rodriguez-Leyva D, Pierce GN. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl Physiol Nutr Metab. 2009;34:965–974. doi: 10.1139/H09-087. [DOI] [PubMed] [Google Scholar]
- Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:121–124. doi: 10.1055/s-2003-39781. [DOI] [PubMed] [Google Scholar]
- Boord JB, Fazio S, Linton MF. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Lipidol. 2002;13:141–147. doi: 10.1097/00041433-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;77:287–293. doi: 10.1016/j.plefa.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Chen L, Frister A, Wang S, Ludwig A, Behr H, Pippig S, Li B, Simm A, Hofmann B, Pilowski C, Koch S, Buerke M, Rose-John S, Werdan K, Loppnow H. Interaction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances IL-6 and MCP-1 production. Am J Physiol Heart Circ Physiol. 2009;296:H987–H996. doi: 10.1152/ajpheart.01158.2008. [DOI] [PubMed] [Google Scholar]
- Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(1 Suppl):171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Das UN. A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;76:251–268. doi: 10.1016/j.plefa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98(4A):27–33. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005;206:103–116. doi: 10.1007/s00232-005-0783-2. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RJ, Worgall TS, Seo T. n-3 Fatty acids and gene expression. Am J Clin Nutr. 2006;83(6 Suppl):1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- Djoussé L, Pankow JS, Eckfeldt JH, Folsom AR, Hopkins PN, Province MA, Hong Y, Ellison RC. Relation between dietary linolenic acid and coronary artery disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2001;74:612–619. doi: 10.1093/ajcn/74.5.612. [DOI] [PubMed] [Google Scholar]
- Dutta-Roy AK. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr. 2000;71(1 Suppl):315S–322S. doi: 10.1093/ajcn/71.1.315s. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45(Suppl 3):S27–S30. doi: 10.2337/diab.45.3.s27. [DOI] [PubMed] [Google Scholar]
- Gougeon R, Pencharz PB, Marliss EB. Effect of NIDDM on the kinetics of whole-body protein metabolism. Diabetes. 1994;43:318–328. doi: 10.2337/diabetes.43.2.318. [DOI] [PubMed] [Google Scholar]
- Hardman WE. Omega-3 fatty acids to augment cancer therapy. J Nutr. 2002;132(11 Suppl):3508S–3512S. doi: 10.1093/jn/132.11.3508S. [DOI] [PubMed] [Google Scholar]
- Hendrich S. (n-3) Fatty acids: clinical trials in people with type 2 diabetes. Adv Nutr. 2010;1:3–7. doi: 10.3945/an.110.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R, Enjoji M. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wahlqvist ML, Xu T, Xu A, Zhang A, Li D. Increased plasma n-3 polyunsaturated fatty acid is associated with improved insulin sensitivity in type 2 diabetes in China. Mol Nutr Food Res. 2010;54(Suppl 1):S112–S119. doi: 10.1002/mnfr.200900189. [DOI] [PubMed] [Google Scholar]
- Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol. 2004;554:27–43. doi: 10.1007/978-1-4757-4242-8_5. [DOI] [PubMed] [Google Scholar]
- Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B α in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaithwas G, Majumdar D. In-vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur J Lipid Sci Technol. 2012 [Google Scholar]
- Keller H, Wahli W. Peroxisome proliferator-activated receptors A link between endocrinology and nutrition? Trends Endocrinol Metab. 1993;4:291–296. doi: 10.1016/1043-2760(93)90048-J. [DOI] [PubMed] [Google Scholar]
- Koh JH, Shin YG, Nam SM, Lee MY, Chung CH, Shin JY. Serum adipocyte fatty acid-binding protein levels are associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Diabetes Care. 2009;32:147–152. doi: 10.2337/dc08-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–1504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ, AHA Nutrition Committee American Heart Association Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.ATV.0000057393.97337.AE. [DOI] [PubMed] [Google Scholar]
- Kroger J, Schulze MB. Recent insights into the relation of Δ5 desaturase and Δ6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol. 2012;23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5. [DOI] [PubMed] [Google Scholar]
- Mahmud I, Hossain A, Hossain S, Hannan A, Ali L, Hashimoto M. Effects of Hilsa ilisa fish oil on the atherogenic lipid profile and glycaemic status of streptozotocin-treated type 1 diabetic rats. Clin Exp Pharmacol Physiol. 2004;31(1–2):76–81. doi: 10.1111/j.1440-1681.2004.03953.x. [DOI] [PubMed] [Google Scholar]
- Manku MS, Horrobin DF, Huang S, Morse N. Fatty acids in plasma and red cell membranes. Lipids. 1983;18:906–908. doi: 10.1007/BF02534572. [DOI] [PubMed] [Google Scholar]
- Mansouri RM, Baugé E, Staels B, Gervois P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-α control of the acute-phase response. Endocrinology. 2008;149:3215–3223. doi: 10.1210/en.2007-1339. [DOI] [PubMed] [Google Scholar]
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–229. doi: 10.2337/diabetes.47.2.224. [DOI] [PubMed] [Google Scholar]
- McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715–728. doi: 10.1592/phco.27.5.715. [DOI] [PubMed] [Google Scholar]
- Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619–624. doi: 10.1172/JCI2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000;23:1407–1415. doi: 10.2337/diacare.23.9.1407. [DOI] [PubMed] [Google Scholar]
- Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- Murugan P, Pari L. Protective role of tetrahydrocurcumin on changes in the fatty acid composition in streptozotocin-nicotinamide induced type 2 diabetic rats. J Appl Biomed. 2007;5:31–38. [Google Scholar]
- Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, Kim JH, Shulman GI. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-α-dependent manner. Diabetes. 2007;56:1034–1041. doi: 10.2337/db06-1206. [DOI] [PubMed] [Google Scholar]
- Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- Pari L, Murugan P. Tetrahydrocurcumin prevents brain lipid peroxidation in streptozotocin-induced diabetic rats. J Med Food. 2007;10:323–329. doi: 10.1089/jmf.2006.058. [DOI] [PubMed] [Google Scholar]
- Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/S0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- Price PT, Nelson CM, Clarke SD. Omega-3 polyunsaturated fatty acid regulation of gene expression. Curr Opin Lipidol. 2000;11:3–7. doi: 10.1097/00041433-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diabetes.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:50–60. doi: 10.1016/j.amjcard.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n-6: n-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr. 2008;47:153–160. doi: 10.1007/s00394-008-0709-8. [DOI] [PubMed] [Google Scholar]
- Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19:1207–1213. doi: 10.2337/diacare.19.11.1207. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Shaikh SR, Sech LA, Yount HR, Stillwell W, Zaloga GP. Omega 3-fatty acids: health benefits and cellular mechanisms of action. Mini Rev Med Chem. 2004;4:859–871. doi: 10.2174/1389557043403431. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- Song He W, Nara TY, Nakamura MT. Delayed induction of delta-6 and delta-5 desaturases by a peroxisome proliferator. Biochem Biophys Res Commun. 2002;299:832–838. doi: 10.1016/S0006-291X(02)02743-2. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- Tkac I, Kimball BP, Lewis G, Uffelman K, Steiner G. The severity of coronary atherosclerosis in type 2 diabetes mellitus is related to the number of circulating triglyceride-rich lipoprotein particles. Arterioscler Thromb Vasc Biol. 1997;17:3633–3638. doi: 10.1161/01.ATV.17.12.3633. [DOI] [PubMed] [Google Scholar]
- Tso AW, Xu A, Sham PC, Wat NM, Wang Y, Fong CH, Cheung BM, Janus ED, Lam KS. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp JH, van Moerkerk HT. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol Cell Biochem. 1993;123:101–106. doi: 10.1007/BF01076480. [DOI] [PubMed] [Google Scholar]
- Vijaimohan K, Jainu M, Sabitha KE, Subramaniyam S, Anandhan C, Shyamala Devi CS. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006;79:448–454. doi: 10.1016/j.lfs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Wakutsu M, Tsunoda N, Shiba S, Muraki E, Kasono K. Peroxisome proliferator-activated receptors (PPARs)-independent functions of fish oil on glucose and lipid metabolism in diet-induced obese mice. Lipids Health Dis. 2010;9:101. doi: 10.1186/1476-511X-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Schwarz M, Coward P, Houze J, Sawyer JK, Kelley KL, Chai A, Rudel LL. Effects of peroxisome proliferator-activated receptor α/delta agonists on HDL-cholesterol in vervet monkeys. J Lipid Res. 2005;46:1009–1016. doi: 10.1194/jlr.M500002-JLR200. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci USA. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/S0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]