Abstract
The functional architecture of sensory brain regions reflects an ingenious biological solution to the competing demands of a continually changing sensory environment. While they are malleable, they have the constancy necessary to support a stable sensory percept. How does the functional organization of sensory brain regions contend with these antithetical demands? Here we describe the functional organization of auditory and multisensory (i.e., auditory-visual) information processing in three sensory brain structures: (1) a low-level unisensory cortical region, the primary auditory cortex (A1); (2) a higher-order multisensory cortical region, the anterior ectosylvian sulcus (AES); and (3) a multisensory subcortical structure, the superior colliculus (SC), We then present a body of work that characterizes the ontogenic expression of experience-dependent influences on the operations performed by the functional circuits contained within these regions. We will present data to support the hypothesis that the competing demands for plasticity and stability are addressed through a developmental transition in operational properties of functional circuits from an initially labile mode in the early stages of postnatal development to a more stable mode in the mature brain that retains the capacity for plasticity under specific experiential conditions. Finally, we discuss parallels between the central tenets of functional organization and plasticity of sensory brain structures drawn from animal studies and a growing literature on human brain plasticity and the potential applicability of these principles to the audiology clinic.
Keywords: Animal model, central auditory system, development, plasticity, remediation
In this tutorial, we provide a review of the recent neuroscience literature pertaining to the organization and plasticity of auditory and audiovisual sensory representations in midbrain and forebrain nuclei. Throughout this process, we build an organizational framework to facilitate an understanding of how sensory representations are progressively shaped during a period of early postnatal development and how the patterns of expression—and their putative mechanisms—change in accordance with sensory experience into adulthood, We begin by discussing the organization and lifelong plasticity of the auditory cortex and then proceed to relate these principles to brain structures specialized to integrate inputs across auditory and visual modalities. We conclude by outlining a series of arguments for how insights from neural plasticity research, which has been conducted most extensively in animal models, have direct relevance to members of the audiology community interested in age-dependent encoding of acoustic and linguistic signals and their potential for remediation via the use of hearing aids, neuroprosthetic implants, computer-based training, or manipulations of the listening environment.
FUNCTIONAL ORGANIZATION AND PLASTICITY OF A1
Selection of Experimental Methodology and Animal Model
Experience exerts a powerful influence on the functional organization of primary auditory cortex (A1). Much of this work has been done in the laboratory rat, Rattus norvegicus, and has focused on neural tuning for sound frequency. Neurons in A1 are well tuned to sound frequency and typically respond to a single tone at threshold intensity (this defines the neuron’s characteristic frequency [CF]). The spatial organization of CF can be derived from microelectrode mapping procedures that sample from the full extent of A1. Mapping A1 in this manner with high spatial density (100–200 μm between microelectrode penetrations) reveals an orderly map of CF that typically mirrors the frequency selectivity of the basilar membrane with low frequencies (~1 kHz) positioned in the posterior edge and high frequencies (~32 kHz) positioned at the anterior edge of A1 (Kelly and Sally, 1988). The spatial distribution of CF across A1 can then be interpolated in two dimensions to generate a complete map of represented frequencies (i.e., a tonotopic map; Fig 1a). The tonotopic organization of A1 can be illustrated graphically using a Voronoi tessellation (e.g., Fig. 1), in which the cortical area corresponding to a single penetration is represented by the area of an individual polygon and the color of the polygon represents the CF for that site.
Figure 1.
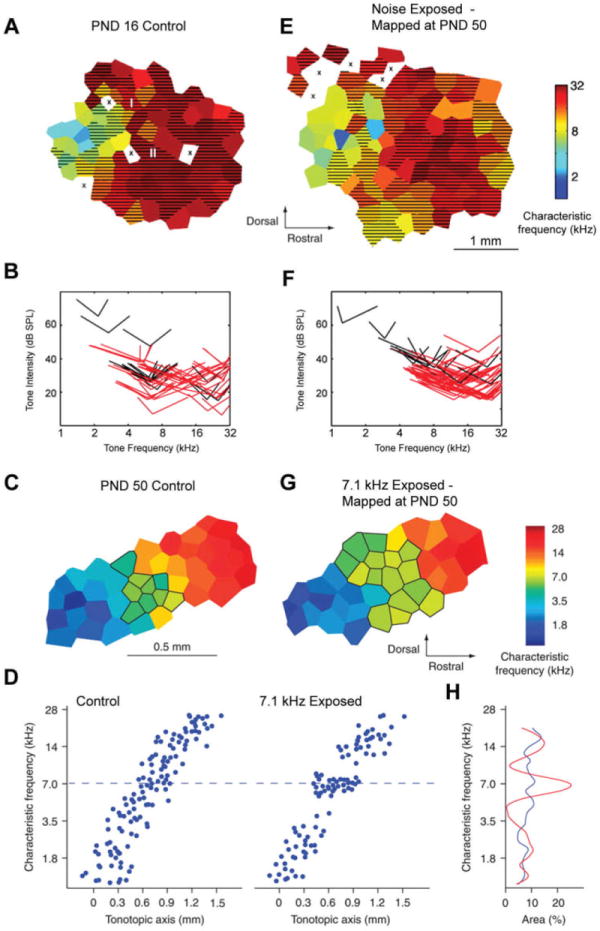
Tonotopic map development in normal and abnormal auditory environments. Representative characteristic frequency (CF) maps from auditory cortex of a PND 16 rat (A), a naïve adult (C), a PND 50 young adult reared in continuous white noise (E), and a young adult rat reared in the presence of pulsed 7.1 kHz tones (G). Neurons sampled from the hatched areas had frequency tuning bandwidths measured 20 dB above threshold (BW20s) greater than 1.5 octaves (A and E) Neurons in outlined areas had characteristic frequencies in a range of 7.1 kHz ± 0.2 octaves (C and G). X = unresponsive sites. Note difference in scale bars between top and bottom pairs of maps. B and F: Receptive fields recorded from maps shown in A and E. Distribution of BW10 tuning curve tips from each map, illustrating the CF threshold, and BW10s recorded at each penetration. Red tips denote BW10s greater than 1.5 octaves, D: Characteristic frequency distribution along the tonotopic axis in control and 7.1 kHz-exposed groups. Note the clustering of characteristic frequencies near 7.1 kHz in the 7.1 kHz-exposed animals. H: Percent primary auditory cortex area representing frequencies in a 0.4 octave frequency band. The representations of 7.1 kHz ± 0.2 octaves were significantly larger in tone-exposed animals (red) than they were in control animals (blue). The data shown in panels A, E, B, and F are from Chang and Merzenich (2003). The data shown in panels C, G , D, and H are from Han et al (2007).
One of the principal advantages of the rat model for developmental studies is that the cochlea does not begin to transduce airborne sound until postnatal day (PND) 9, and behavioral reactions to intense sounds are not documented until PND 10–12 (Harrison and Warr, 1962; Crowley and Hepp-Raymond, 1996). This enables researchers to document the functional organization of A1 from a time before hearing begins until well into adulthood (after PND 80). A second related advantage of the rat model is that an entire housing enclosure can be placed within a small sound-attenuating booth outfitted with a speaker, allowing the researcher to control the acoustic environment (with the exception of self-generated sounds) at any point during development through the presentation of customized sounds.
Development of Frequency Selectivity under Normal Sensory Conditions
Mapping experiments performed shortly after the onset of hearing demonstrate that frequency tuning in A1 is broad and centered on frequencies >7 kHz, producing an irregular and incomplete tonotopic gradient (Zhang et al, 2001) (Figs, 1a–b). Over the course of the following several weeks, the low frequency region of the A1 map emerges, and frequency tuning becomes progressively narrower, reaching its adult form between the first and second month of life (Chang et al, 2005) (Figs. 1c–d). Thus, the tonotopic organization of the auditory cortex matures during the first month of hearing from a large tone-responsive region (~5 mm2) comprised of broadly tuned high frequency responses to the adult representation characterized by an orderly and compact (~1,75 mm2) progression of CFs that span the range of audible frequencies.
Development of Frequency Selectivity under Abnormal Sensory Conditions
The normal postnatal developmental progression can be derailed through exposure to abnormal sound environments during the first several weeks of hearing. The extreme variants of this manipulation entail rearing rats in sound environments consisting of broadband sound spectra versus sound spectra restricted to a single frequency. Prolonged exposure to pulsed broadband noise (i.e., a click) or continuous white noise arrests the normal tonotopic development of A1 (Zhang et al, 2002; Chang and Merzenich, 2003). For example, rats reared in continuous white noise until PND 50 exhibit a primitively organized tonotopic map characterized by a large, poorly organized tone-responsive area composed of nonselective high frequency-tuned neurons (Figs. 1e–f). Based on all response parameters tested, frequency selectivity in young adult rats reared in noise was indistinguishable from infant rat pups (Figs, 1a–b vs. 1e–f).
Tonotopic maps obtained from young adult rats reared in the presence of pulsed tones at a fixed frequency, by contrast, appear similar to age-matched controls in terms of A1 area and sharpness of frequency tuning. The tonotopic gradient, however, is distorted in such a way as to overrepresent CFs near the exposure frequency (Zhang et al, 2001; de Villers-Sidani et al, 2007; Han et al, 2007) (Fig. 1g). The overrepresentation reflects a zero-sum system, such that the expansion of map regions corresponding to the exposure frequency occurs at the expense of a diminished representational area for map regions flanking the exposure frequency (Figs. 1d and h). By contrast, raising animals in enriched acoustic environments improves the functional selectivity of A1 circuits with little obvious cost. Thus, the responses of A1 neurons recorded in rats reared in enriched acoustic environments are more robust, more sensitive, and more narrowly tuned for sound frequency when compared with rats reared in normal cage environments (Engineer et al, 2004).
Exposure-Based Plasticity Effects Are Constrained to a Sensitive Period of Development
Myriad examples from the visual, somatosensory, and auditory systems have demonstrated that the profound effects of sensory experience on functional circuit operations are limited to an early period of postnatal development (for review see Hensch, 2004; Keuroghlian and Knudsen, 2007). Thus, the effects of broadband noise exposure and fixed frequency exposure described above are restricted to an early period of postnatal development. For example, fixed frequency exposure initiated after PND 13 has no demonstrable effect on the tonotopic order in A1 (Zhang et al, 2001; de Villers-Sidani et al, 2007). Similarly, broadband noise exposure has no impact on frequency tuning or the tonotopic gradient when exposure is initiated later than PND 29 (Zhang et al, 2002). Collectively, these results suggest that functional circuits in A1 operate in a “sampling mode” during the sensitive period, during which time the overall statistics of the auditory environment can powerfully shape the order and overall size of tonotopic maps as well as the frequency tuning bandwidth among the constituent neurons that make up the map. Once the statistics of the environment are adequately represented in the functional organization of A1, the sensitive period ends and the system enters a more stable state in which passive exposure to an abnormal sensory environment can no longer induce functional reorganization.
Interestingly, the closing of the sensitive period is not strictly determined by age. Recordings performed in the auditory and visual cortices of animals reared in sensory environments lacking salient patterned spatiotemporal inputs (e.g., animals reared in the presence of continuous white noise or absolute darkness) are characterized by the endurance of an immature organizational status that persists well past sexual maturity (Cynader et al, 1980; Mower et al, 1981; White et al, 2001; Chang and Merzenich, 2003), Accordingly, experience with restricted spatiotemporal inputs (e.g., fixed tone rearing or monocular viewing) in these animals later in life can induce significant functional map reorganization despite the fact that their chronological age places them well outside of the classically defined sensitive period. These data suggest that the emergence of the adultlike organizational state may in itself act to halt the constitutive release of “plasticity enabling” factors such as neuromodulators and neurotrophic factors that may exist at high levels when the system is in its sensitive period. A series of elegant studies conducted in the visual system have indeed confirmed that GABAergic inhibitory signaling within the cortical network serves to halt the constitutive release of neurotrophic factors such as brain derived neurotrophic factor (BDNF), thereby closing the sensitive period window and ushering in a period of response stability during which time abnormal sensory experience is no longer able to drive persistent functional reorganization (Castren et al, 1992; Hanover et al, 1999; Hensch et al, 1998).
Engaging Plasticity in Adult A1
While the functional circuit operations in adult A1 generally are refractory to change, this description must be made with one important caveat. Sensory experience can still induce plasticity in adult A1, provided that the experience is behaviorally relevant. Although passive exposure to abnormal sensory environments (e.g., fixed-frequency tones or broadband noise) does not lead to persistent reorganization in adult A1, these functional circuits will express significant plasticity if auditory stimuli are paired with behavioral reinforcement (e.g., reward or punishment). In this sense it is more appropriate to describe the two epochs separated by the closing of the sensitive period as “exposure-based plasticity versus reinforcement-based plasticity” rather than “plastic period versus stable period.”
The behavioral and neural factors that govern adult plasticity have been explored in a series of studies using Pavlovian and instrumental conditioning paradigms, In one type of Pavlovian conditioning experiment, a tone of fixed frequency (CS+) is presented to the adult animal prior to the onset of aversive reinforcement (e.g., a mild foot shock), whereas a tone of a different frequency never precedes the onset of aversive reinforcement (CS−). Neuronal tuning functions derived from A1 neurons before and after conditioning reveal a significant increase in the evoked spike rate to the CS+ and a decrease to the CS− (Bakin and Weinberger, 1990; Ohl and Scheich, 1997; Gao and Suga, 2000). These results demonstrate that adult A1 neurons are capable of shifting their preferred tuning toward tone frequencies that convey information about behaviorally relevant environmental events. By contrast, presentation of the same stimulus schedule without reinforcement does not produce any systematic differences in frequency tuning. Interestingly, the conditioned change in firing rate to the CS+ and CS− emerges within a few dozen conditioning trials (Edeline et al, 1993; Blake et al, 2006), demonstrating that functional circuits in adult A1 express rapid and lasting plasticity provided that the additional requirement for behavioral salience is met.
Similarly, animals trained in instrumental conditioning paradigms, which reward them for making a discriminative behavioral response shortly after the presentation of a “cue” frequency amid a set of repeating “standard” frequency tones, also exhibit enhanced cortical responses to the cue frequency and suppressed responses to the standard frequency (Bakin et al, 1996; Blake et al, 2002; Fritz et al, 2003; Fritz et al, 2005). Moreover, A1 mapping studies have demonstrated that the cue frequency is over-represented in the tonotopic map of animals trained in frequency discrimination paradigms, similar to the overrepresentation of the exposure frequency observed in infant rats reared in fixed-frequency sound environments (Recanzone et al, 1993; Rutkowski and Weinberger, 2005; Polley et al, 2006). Again, however, plasticity of the adult tonotopic map is not observed if animals are passively exposed to the training stimuli. In fact, not only is plasticity of the frequency representations within the adult tonotopic map specific to stimuli that predict behaviorally reinforcing events, the degree to which the cue frequency is overrepresented in the tonotopic map is positively correlated with the animal’s learned proficiency in the frequency discrimination task (Polley et al, 2006).
Functional Organization and Plasticity in A1: Beyond the Tonotopic Map
Functional circuits in A1 encode many auditory parameters other than sound frequency. Studies in cats and nonhuman primates have demonstrated that the complete functional architecture of A1 is more accurately described as a lattice of independent maps that are organized according to stimulus features (e.g., sound frequency, sound level) and response features (e.g., response latency, tuning bandwidth, and binaural interaction type) (for review see Schreiner, 1998). Of those features, CF is the only spatially organized response characteristic that is mapped across the entire spatial extent of A1 in an orderly and complete fashion. The spatial organization of other features, such as tuning bandwidth, binaural interaction type, and preferred intensity, assume organizations best described as spatially contiguous “patches” or “modules” (Imig and Adrian, 1977; Middlebrooks et al, 1980; Kelly and Sally, 1988; Heil et al, 1994; Recanzone et al, 1999; Imaizumi et al, 2004; Nakamoto et al, 2004; Philibert et al, 2005), Despite its small physical scale when compared with primate and cat, rat A1 shows a similar organizational structure, exhibiting nonrandom (Fig. 2a) and mutually independent (Fig. 2b) modules for sharpness of sound frequency tuning (i.e., Q14), stimulus-evoked onset latency, and sharpness of sound intensity tuning (i.e., monotonicity) Each of these features has been observed to coexist alongside CF in the functional architecture of A1 (Polley et al, 2007).
Figure 2.
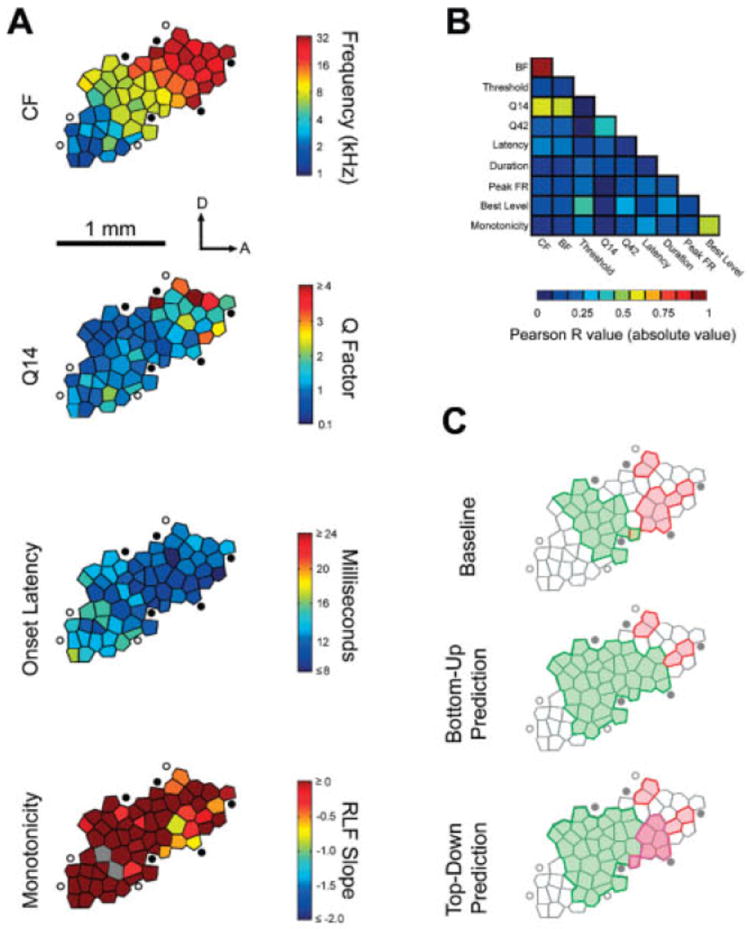
Spatial organization of multiple response features within A1. A: Representative A1 map from a naïve adult. The color of each polygon corresponds to the color scale bar for each response feature shown to the right. D and A correspond to dorsal and anterior, respectively. Filled circles indicate unresponsive sites. Open circles represent sound-responsive sites outside A1. Gray polygons indicate sites for which the response parameter of interest could not be calculated. Q14 = the quality factor (CF/bandwidth) measured 14 dB above minimum response threshold. RLF slope = the slope of the rate-level function; more negative values correspond to sites with nonmonotonic intensity-tuned response functions, B: Mutual correlation matrix for ten response features measured in A1 indicating: the absolute value of the Pearson R correlation coefficient for all unique pairs of variables. Note that related response characteristics (e.g., BF and CF or best level and monotonidty) covaried among themselves but not with the other groupings of response characteristics. C: Schematic of the A1 map shown in A illustrating cortical areas preferring midfrequency tones (green polygons) and low intensity tones (red polygons). Relative the pretraining baseline, an adult rat trained o t respond selectively to midfrequency tones would exhibit an expansion of the midfrequency CF representation. The maps below depict the possibility that intensity characteristics of midfrequency tones, though irrelevant to the task, would also be reflected i n the pattern of reorganization (bottom-up prediction), Alternatively, the plasticity could be restricted only to the stimulus dimension that is relevant to the demands of the task (top-down prediction). The data in panels A and B are from Polley et al, 2007.
Plasticity in adult A1 resulting from instrumental conditioning protocols is similarly multifaceted. Animals conditioned to discriminate changes in pitch-modulated stimuli exhibit plasticity in the frequency domain (see above); animals trained to make discriminations in envelope modulation rate express plasticity in temporal response properties (Beitel et al, 2003; Bao et al, 2004); and animals trained to discriminate variations in loudness express plasticity in the neural encoding of sound intensity (Polley et al, 2004). Thus, the functional architecture of A1 consists of spatially organized representations of multiple response features all embedded within the same neural circuitry. Furthermore, training animals in conditioning protocols that emphasize perceptual acuity in one sound domain is accompanied by plasticity of the corresponding feature representation within A1.
Bottom-Up versus Top-Down Control over Functional Circuit Plasticity in Adult A1
The most parsimonious mechanism for plasticity in adult A1, known as the bottom-up hypothesis, holds that the presentation of an auditory stimulus that has been repeatedly paired with behavioral reinforcement activates subcortical neuromodulatory nuclei along with the concomitant activation of the dedicated sensory neurons along the auditory neuroaxis. In this view, increased neuromodulator release within the cortex just before or during the arrival of the auditory stimulus trace in A1 would temporarily reinstate a sensitive-period-like process and allow the statistics of the conditioned auditory stimulus to reshape the neural selectivity. According to this hypothesis, neuromodulators act as a gating mechanism to permit or deny plasticity in adult cortical networks. During development, neuromodulatory tone in the cortex is consistently high, and the statistical properties of the auditory environment can continuously shape functional circuits in A1. Once the sensitive period has ended, brief elevations of neuromodulatory tone in the cortex (lasting from tens of milliseconds to minutes) elicited by presentation of conditioned auditory stimuli could serve to temporarily open the gate, thereby reinstating a brief sensitive-period-like process.
The bottom-up hypothesis has a great deal of experimental support. For example, it is well established that dopamine-, acetylcholine-, and serotonin-releasing neurons in subcortical structures including the substantia nigra pars compacts, basolateral nucleus of the amygdala, dorsal raphe nucleus, and nucleus basalis are initially activated by the presence of the behavioral reinforcement but, over the course of several stimulus-reward pairings, also become activated by the sensory stimulus that predicts behavioral reinforcement (for review see Schultz, 2000; Schultz and Dickinson, 2000; Seitz and Watanabe, 2005). Furthermore, pairing an auditory stimulus with either exogenous application of the neuromodulator directly to the cortex (Metherate and Weinberger, 1989; Metherate and Weinberger, 1990; Manunta and Edeline, 1997; Manunta and Edeline, 1998; Ji and Suga, 2007) or electrical stimulation of the cholinergic basal forebrain (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; Bao et al, 2001) or the dopaminergic ventral tegmental area (Bao et al, 2001) induces lasting shifts in the tuning profile of A1 neurons. It has been argued, therefore, that elevated levels of neuromodulators such as acetylcholine, noradrenaline, dopamine, and serotonin within A1, when occurring in the presence of a conditioned auditory stimulus, are sufficient to account for the essential role of behavioral relevance in adult A1 plasticity.
One of the principal challenges to the bottom-up hypothesis is that it cannot account for observations from the perceptual learning literature that improved perceptual discrimination, thresholds are specific to stimulus features to which subjects are asked to attend (Ahissar and Hochstein, 1993). If the presence of a behavioral reinforcer strengthens the cortical representation of the conditioned auditory stimulus, it should, according to the bottom-up hypothesis, strengthen the representation for all aspects of the conditioned stimulus, even those that are irrelevant to the task demands. For example, if the cue tones in a frequency discrimination task are also presented at high intensity, the bottom-up hypothesis makes the dual prediction that the cortical area allocated to the cued frequencies will expand and the cortical area allocated to low intensity sound levels will contract (Fig. 2c [middle image]). The alternative explanation, the top-down hypothesis, states that plasticity is restricted to the encoding of stimulus properties that are relevant to the task demands. Therefore, although the tone preceding the reward was characterized by both its frequency and intensity, only frequency was relevant to the task demands, so the territory for the cued tones would expand, but the territory for sound intensity would not be affected (Fig. 2c [bottom image]).
These mutually exclusive hypotheses were tested in a recent experiment in which rats were trained to attend to independent aspects of the stimulus, either frequency or intensity, while an identical set of auditory stimuli were delivered (Polley et al, 2006). Such a design allows top-down task demands to vary while holding the bottom-up sensory input statistics constant. Rats trained in the frequency recognition task were rewarded for responding to the presentation of a 5 kHz tone, irrespective of its intensity. Conversely, rats trained in the loudness recognition task were rewarded for responding to a 35 dB tone, irrespective of its frequency. Despite the fact that the statistics of the conditioned auditory stimuli were identical, A1 map plasticity was only observed in the stimulus dimension to which the rat attended (Fig. 3).
Figure 3.
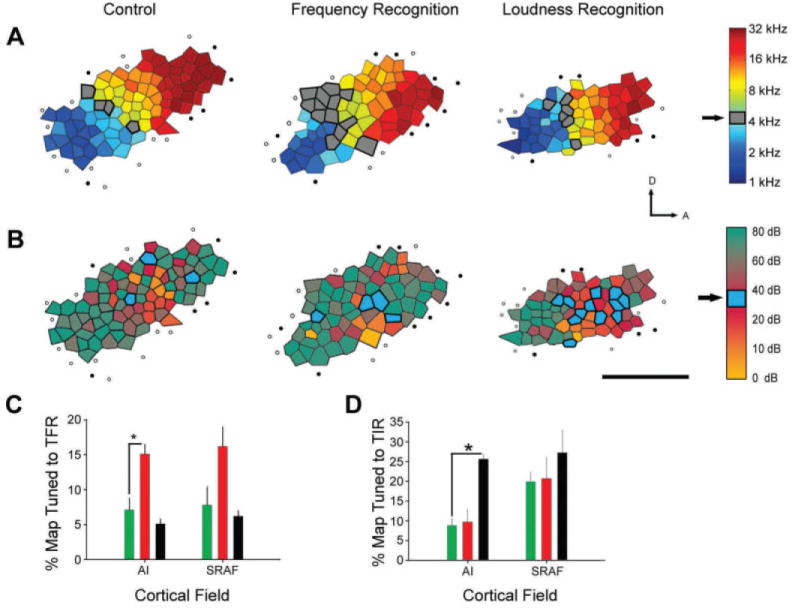
Task-specific functional reorganization in adult rat A1. Maps for CF (A) and best level (B) are presented for a representative naïve control (left column), a representative rat trained in a frequency recognition task (middle column), and a representative rat trained in a loudness recognition task (right column). A: Gray shaded polygons indicate recording sites with CF values within the trained frequency range (5 kHz ± 0.375 octaves). Filled circles indicate unresponsive sites. Open circles represent sites with sound-driven responses that did not meet the criteria for inclusion in A1. Scale bar = 1 mm. The arrows indicate dorsal (D) and anterior (A) orientations, respectively. B: The same conventions as in A, except that the color within each polygon indicates the best level for neurons at that recording site, rather than CF. Empty polygons indicate recording sites where a best level could not be determined. Blue shaded polygons indicate recording sites with best-level values in the trained intensity rage (35 ± 5 dB SPL). C: Percent of map area within A1 and a secondary auditory field, SRAF, allocated to the trained frequency range (TFR) in naïve control rats (green), rats trained in the frequency recognition task (red), and rats trained in the loudness recognition task (black), D: Percent of map area within A1 and SRAF allocated to the trained intensity range (TIR). Color conventions are the same as C. Asterisks indicate statistically significant differences between groups (p < 0.05). The data are from Polley et al (2006).
In summary, these data demonstrate that A1 retains the capacity to express experience-dependent plasticity into adulthood but that the “rules” for engaging plasticity in the mature A1 are quite different than those that operate in early postnatal development. Exposure-based plasticity occurring within the sensitive period can be described as a statistically driven process in which the acoustic properties of signals that occur with high probability are preferentially encoded in A1 functional circuits. Experience-dependent plasticity following closure of the sensitive period can be termed “reinforcement-based plasticity,” in that the changes in A1 functional circuits are restricted to auditory stimuli that predict behaviorally meaningful events. Furthermore, these experience-driven changes in adult A1 circuits require input from subcortical neuromodulatory sources. It is important to note that the increased presence of these neuromodulators does not temporarily reinstate a sensitive-period-like process in which the functional organization of the adult sensory cortex can once again be shaped by the overall sensory input statistics. Plasticity in adult cortical networks that accompanies auditory conditioning protocols is likely to be shaped by an interaction between sensory inputs, neuromodulator release, and top-down influences from higher sensory areas or association cortices. Additional work is required to identify how the behavioral demands of the sensory conditioning tasks can be manipulated to emphasize the contribution of bottom-up and top-down factors. Furthermore, additional work is needed to determine whether the behavioral and experiential factors that govern plasticity across ontogeny in A1 apply to “downstream” cortical regions that process more abstract features of the auditory environment or synthesize auditory signals with inputs from other modalities.
FUNCTIONAL ORGANIZATION AND PLASTICITY OF MULTISENSORY REPRESENTATIONS
An Introduction to Multisensory Processes
We live in a rich sensory world in which we are continually bombarded with information from each of our different senses. Although much of this “multisensory” information is unrelated (and consequently must be processed as such), some is attributable to the same event and must be “bound” together in order to form a unified and coherent perceptual representation of the external world. As a result of this challenge, the brain has created a processing architecture that not only deals with information within the individual sensory systems but that also combines and synthesizes information from the different senses in order to create our perceptual gestalt.
Although it is a process of which we are largely unaware, numerous psychophysical examples serve to highlight how the combination of information from different senses can improve our behaviors and perceptions (for reviews see Stein and Meredith, 1993; Calvert et al, 2004). Examples of the benefits attributable to such “multisensory integration” are improvements in target detection and localization when cues from more than a single sense are present, and gains in speech intelligibility associated with being able to see the moving mouth of the speaker. In addition, a host of perceptual illusions further illustrate the power of multisensory interactions to shape our perceptions. Two of the more familiar (and compelling) of these are the ventriloquist effect, in which the perceived location of an auditory sound source (i.e., the ventriloquist’s voice) is altered by conflicting visual cues (i.e., movements of the dummy’s mouth and head), and the McGurk effect, in which a novel speech percept is created by the fusion of discordant visual and auditory cues. Despite the ubiquity and power of these multisensory interactions, and the fact that they have been described since Aristotelian times, our understanding of the brain bases for these effects has lagged well behind our understanding of how information within the individual sensory modalities is encoded.
Functional Organization of Multisensory Subcortical and Cortical Circuits
A great deal of recent work has been directed toward filling this void in our knowledge, by characterizing the functional organization of multisensory interactions in both subcortical and cortical brain regions. The best-studied model to date has been a subcortical structure, the superior colliculus (SC) (for reviews see Sparks, 1986; Stein and Meredith, 1991; Stein and Meredith, 1993; Sparks and Groh, 1995; King, 2004). The SC receives convergent visual, auditory, and somatosensory (i.e., tactile) inputs, thus creating a large population of individual neurons that are responsive to or influenced by stimuli in two or more sensory modalities (i.e., multisensory neurons). Neurons in the SC have distinct receptive fields—discrete areas of sensory space to which they are tuned. Furthermore, these neurons are arranged in such a way as to create a two-dimensional spatiotopic map of the sensory world, with neighboring neurons representing neighboring regions of the sensory space. These sensory maps are coregistered with a similarly ordered motor map, creating a striking sensorimotor correspondence that appears to represent an efficient means of transforming sensory signals related to stimulus location into motor commands that orient the eyes, ears, and head toward a stimulus of interest. The large population of multisensory neurons in the SC coupled with its well-defined behavioral role has made it an excellent model for furthering our understanding of multisensory processes.
Individual multisensory SC neurons do far more than passively respond to stimuli from multiple sensory modalities (Fig. 4). Rather, these neurons actively integrate these inputs to give rise to responses that are far different from the responses to either of the individual stimuli, and that frequently are far different from what would be predicted by a linear sum of each of the responses (Meredith and Stein, 1983; Meredith and Stein, 1985; Meredith and Stein, 1986b; Wallace et al, 1993, 1998; Wallace et al, 1996). Such multisensory integration can be divided into two broad functional categories; response enhancements and response depressions. The sign (i.e., enhancement or depression) and magnitude of these integrated multisensory responses have been shown to be critically dependent on the spatial and temporal relationships of the paired stimuli, as well as on their relative effectiveness in eliciting a response (Meredith et al, 1986a, 1986b; Meredith et al, 1987; Meredith and Stein, 1996). As a general rule, two unisensory (e.g., an auditory stimulus and a visual stimulus) stimuli that are weakly effective individually, when presented in close spatial and temporal proximity, result in significant response enhancements (i.e., increases in activity) in the SC. Conversely, stimuli that are spatially and/or temporally disparate, or that are highly effective when presented alone, often result in response depressions (i.e., decreases in activity). Reinforcing the strong sensorimotor role of the SC, these same integrative principles that were first defined at the neurophysiological level in the sensory responses of individual neurons apply equally well to SC-mediated orientation behaviors (Stein et al, 1988; Stein et al, 1989). Thus, multisensory stimulus pairings that result in enhancements in individual SC neurons also result in improvements in behavioral performance, whereas those that give rise to depressions in neuronal response typically result in behavioral impairments.
Figure 4.
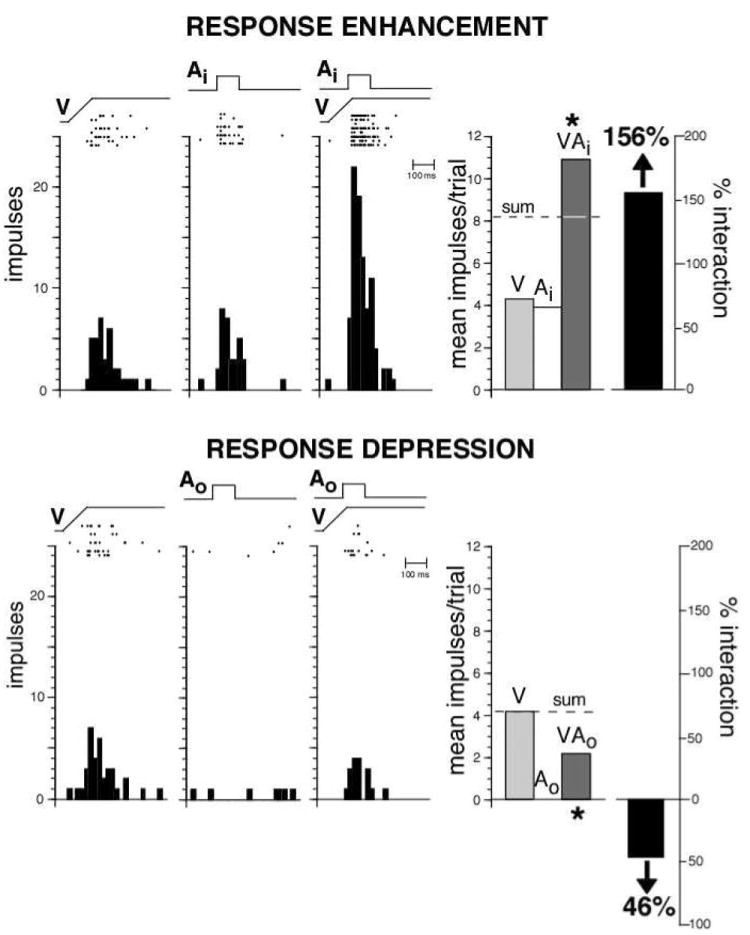
Multisensory integration can be divided into two broad operational categories. Response enhancement (top) represents a gain (i.e., increase) in response upon presentation of stimuli in multiple modalities. Rasters and peristimulus time histograms on the left depict the responses of a single multisensory neuron to a visual stimulus alone (V), to an auditory stimulus alone (Ai), and to the combined presentation of these stimuli. Ramps and square waves show the relative timing of the stimuli. Summary bar graphs on the right plot this neuron’s mean response to each of these conditions, as well as the relative gain in response (i.e., 156%) to the multisensory combination. Note that the percent interaction is calculated in this example as [(VAi − V)/V] × 100. In addition, note that, the multisensory response exceeds that predicted by a simple addition of the component unisensory responses. Response depression (bottom) represents a decline in response relative to the best unisensory condition. Here we see that the presentation of an auditory stimulus outside of its receptive field (Ao—contrast this with the Ai condition shown above) results in a significant decline in the visual response.
More recent work has extended these studies beyond the SC and into cortical domains that are likely to play a more important role in the generation of our perceptual gestalt. One of the best characterized of these areas has been a region of “association” cortex in the cat, the anterior ectosylvian sulcus (AES). In addition to being subdivided into separate auditory, visual, and somatosensory domains, the AES also contains a large population of multisensory neurons that appear to be clustered at the borders between the major unisensory domains (Wallace et al, 1992; Jiang et al, 1994a, 1994b; Benedek et al, 2004; Meredith et al, 2006; Wallace et al, 2006). Moreover, AES is known to project to SC, and has been shown to play an important modulatory or gating role over the multisensory interactions characterized within the SC (Wallace et al, 1993; Wallace and Stein, 1994; Jiang et al, 2001; Jiang and Stein, 2003). Although not as extensively studied as their counterparts in the SC, all evidence suggests that these cortical multisensory neurons abide by a very similar set of principles when integrating cues from multiple modalities. It has been suggested that such commonalities may represent an effective means of linking subcortical multisensory networks responsible for overt behaviors with the cortical networks responsible for the perceptual correlates of these stimuli/behaviors (Stein and Wallace, 1996). To date, the contributions of AES toward multisensory perception have not been identified. However, converging evidence suggests that it is likely to play a role(s) in motion perception/binding, coordinate transformations, and eye movements (Tamai et al, 1989; Scannell et al, 1995; Scannell et al, 1996; Nagy et al, 2003; Benedek et al, 2004).
Ongoing Search for Multisensory Brain Regions: What Constitutes a Multisensory Area?
With the increasing interest in multisensory processes has come an equivalent growth in the number of studies focused on the identification and characterization of other multisensory brain regions. Perhaps not surprisingly, such studies have resulted in an ever-increasing catalog of regions with demonstrable multisensory processing capabilities. Many of these areas are well known to receive convergent input from multiple unisensory domains, and these are typically situated fairly high in the sensory processing hierarchies. Although beyond the scope of the current discussion, these “higher-order” multisensory regions have been proposed to play a variety of roles in crossmodal perceptual processing (for a recent review see Ghazanfar and Schroeder, 2006).
More germane to the current discussion are the number of recent findings that have extended the definition of “multisensory” into areas of the brain traditionally considered the domain of a single sensory modality. Most relevant here are human neuroimaging (i.e., fMRI [functional magnetic resonance imaging] and EKP [event related potentials]) studies that have demonstrated the penetrance of sensory information from other modalities into regions classically denoted as regions of auditory cortex. The first evidence for this came from work showing that silent lipreading in human subjects resulted in activation of regions of auditory cortex (Calvert et al, 1997). This was soon followed by studies that not only extended this finding of visual modulation of auditory cortical (Giard and Peronnet, 1999; Molholm et al, 2002; Besle et al, 2004; Brosch et al, 2005; Ghazanfar et al, 2005; Pekkola et al, 2005; van Wassenhove et al, 2005) but which also showed strong tactile (somatosensory) influences on neuronal responses in auditory cortex (Schroeder et al, 2001; Foxe et al, 2002; Lutkenhoner et al, 2002; Fu et al, 2003; Gobbele et al, 2003; Kayser et al, 2005). These findings have revolutionized contemporary thinking about sensory processing by highlighting the links across the sensory systems, even at some of the earliest stages of cortical processing.
Development of Multisensory Brain Circuits
Despite this burgeoning interest in multisensory processes, our knowledge of the developmental antecedents of mature multisensory brain circuits has been slow to evolve. Although there has been a great deal of discussion and debate among developmental psychologists as to the (multi)sensory state of the newborn brain, little consensus has been reached as to the neural underpinnings that enable (or fail to enable) crossmodal processes early in life. Whereas some schools of thought argue that the newborn brain is an obligate multisensory organ (and that newborn perceptions are highly synesthetic), others put forth that multisensory circuits come online only with the progressive appearance and maturation of the individual sensory systems (for a review of these issues see Lewkowicz, 1994). The wealth of the evidence that has been ushered forth for both views has come from perceptual studies in human infants, studies that are unfortunately fraught with interpretational limitations and caveats.
In an effort to provide a more brain-baaed view into this issue, recent work in animal models has begun to explore the maturation of brain regions known to be multisensory in the adult. As highlighted above, two of the preeminent models for multisensory research are the SC and AES, and these structures have been the focus of this developmental line of inquiry. Evidence from these two brain regions strongly suggests that multisensory circuits are quite immature at birth and develop over a fairly protracted period of postnatal life.
In the best-studied model species, the cat, the sensory chronology in both the SC and AES is very similar (Wallace and Stein, 1997; Wallace, 2004a; Wallace et al, 2006). Somatosensory neurons appear first and are soon followed by auditory neurons. Once these two modalities are represented, the first multisensory (i.e., somatosensory-auditory) neurons appear. Neurons with visual responses are the last to appear, and as soon as they are present, visually responsive multisensory neurons are observed. With time, the multisensory population in both the SC and AES gradually increases, until by approximately four to five months after birth, the incidence of multisensory neurons is at adult levels (Fig. 5). Consistent with general timetables for cortical and subcortical development, the maturation of multisensory responses in AES lags behind SC by several weeks.
Figure 5.
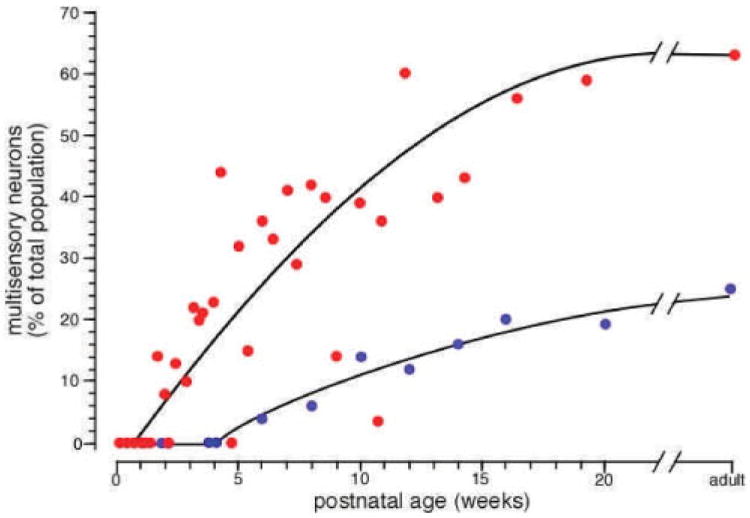
Multisensory neurons in the cat SC and AES mature over the first four to five months of postnatal life. Graph plots the incidence of multisensory neurons as a percentage of the total neuronal populations in the SC and AES as a function of postnatal age. Note for both structures the absence of multisensory neurons immediately after birth and the gradual rise in their incidence with time. Note also the delay in cortical multisensory maturation as opposed to subcortical maturation. Data adapted from Wallace and Stein, 1997, and Wallace et al, 2006.
Early multisensory neurons are far different from those found at later developmental stages. They have extremely large receptive fields, and most notably, they lack the ability to integrate their different sensory inputs, Thus, and despite the fact that they are now capable of responding to cues from two or more senses, these early multisensory neurons respond to stimulus combinations in ways that differ little from the constituent unisensory responses (Fig. 6A). However, as developmental progresses, a striking change is noted in these neurons, with a steadily increasing proportion of them acquiring the capacity to integrate their different sensory inputs, consequently generating responses that are far different from the unisensory responses (Fig, 6B). Once again, by about four to five months after birth, the system reaches maturity, with 70–80% of the multisensory neurons demonstrating integrative capabilities.
Figure 6.
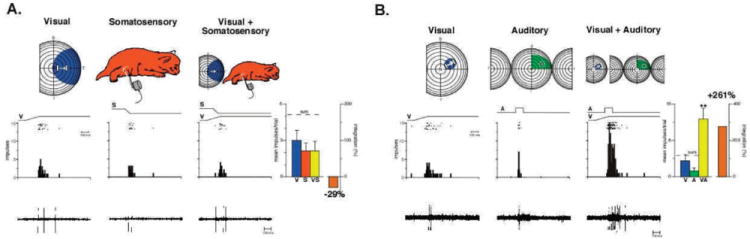
Multisensory integration is absent in the earliest multisensory neurons and appears during postnatal development. Shown is representative data from two multisensory SC neurons at two different developmental ages: 20 days postnatal (A, left) and 30 days postnatal (B, right), At the top of each are shown the receptive fields (shading) and locations of the stimuli used in sensory testing (icons). Middle panels show Tasters, histograms, and summary bar graphs depicting the responses to both of the unisensory stimuli and to the multisensory combination. The lines at the top show the relative timing of the stimuli, and the bar graph on the far right in each shows the magnitude of the multisensory interaction (i.e., % integration). At the bottom are shown representative oscillographic traces for a single trial to each of the unisensory stimuli and to the multisensory combination. Note that whereas the neuron in the 20-day-old animal responds to the multisensory combination in a manner virtually identical to its response to the unisensory stimuli, the neuron from the older animal shows a large response enhancement to the stimulus combination, and that this gain exceeds that predicted from the simple summation of the unisensory responses. Data adapted from Wallace and Stein, 1997.
The protracted maturation timetable by which multisensory circuits abide strongly suggests that the experiences gained during postnatal life in the utilization of crossmodal cues plays a critical role in this developmental process. Although suggestive, proof of the importance of sensory experience in multisensory maturation awaited experiments in which crossmodal experiences could be eliminated or selectively altered.
Developmental Plasticity in Multisensory Brain Circuits: The Role of Visual Experience
The most straightforward way to assess the importance of early sensory experience for the normal maturation of multisensory circuits was to eliminate these experiences and examine its impact. This was accomplished by raising animals in an environment devoid of visual cues (i.e., dark rearing). Once animals reached adulthood, neurophysiological recordings targeted multisensory neurons in both the SC and AES (Wallace, 2004b). In both structures, the effects of dark rearing were profound, In the SC, despite the retention of many neurons with visual responses, multisensory integration was abolished, with neurons responding to stimulus combinations in ways that were indistinguishable from their responses to the individual unisensory stimuli, very much resembling the responses seen early in postnatal life. In AES the effects were a bit different, with many neurons showing depressive interactions to stimulus combination that typically result in response enhancements. Whether elimination of patterned auditory and/or soma to sensory inputs would exert a similar influence remains to be determined, and each represent a less tractable problem because of the difficulties inherent in trying to eliminate experiences in these modalities in a reversible manner.
Developmental Plasticity in Multisensory Brain Circuits: Alterations in Cross modal Experience
Although the aforementioned studies have established the necessity of visual experience for the development of normal multisensory circuits in both the SC and AES, they have provided only limited insight into how sensory experience sculpts these networks. In order to gain a better handle on this question it was necessary to alter, rather than eliminate, multisensory experiences. Given that it has been well established that the spatial and temporal relationships of multisensory stimulus pairs are key elements in how they are integrated, animals were reared in circumstances in which these relationships were systematically altered.
In the first of these experiments, animals were raised in an environment in which visual and auditory stimulus pairs were presented concurrently in time, but at a fixed spatial disparity of 30° (Wallace and Stein, 2007). This was accomplished by two different means, each of which had similar effects. In one method, animals were raised in an environment in which the only visual cues presented were always paired with auditory cues separated in space by 30° (the remainder of their experience was in the dark). In the other, animals wore prismatic spectacles that served to deviate the visual world by 30° (consequently misaligning the visual and auditory representations). When examined as adults, multisensory neurons in both the SC and AES of these spatial-disparity-reared animals exhibit a predictable change in their receptive field architecture that reflects the altered sensory experiences (i.e., their spatial alignment was shifted by approximately 30°). Even more dramatic was the change induced in the spatial architecture of multisensory processing in these neurons. As opposed to exhibiting response enhancements to spatially-coincident stimulus combinations (as is seen in “normal” animals), these neurons were found to exhibit these enhancements to spatially-disparate combinations that mimicked their early sensory experiences (Fig, 7A). In contrast, spatially-coincident combinations resulted in no integration.
Figure 7.
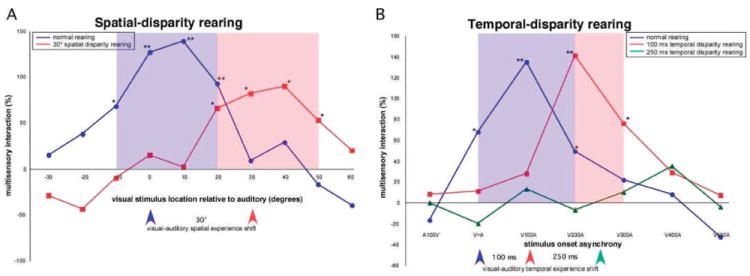
The development of multisensory integration is highly plastic and can be shaped by manipulating early postnatal sensory experience. A: Multisensory interactions as a function of the spatial location of paired visual and auditory stimuli are plotted for two representative SC neurons—one in an animal raised under normal sensory conditions and the other from an animal raised in an environment in which the visual and auditory stimuli were always displaced by 30°. In this representation, the neuron’ responses to the multisensory (i.e., auditory-visual) condition were compared to its responses to the most effective unisensoiy stimulus in order to determine the multi sensory interaction using the formula (M − Umax/Umax) × 100 where M is the multisensory response and Umax is the largest unisensory response (see Stein and Meredith, 1993, for additional detail). For the neuron from the normally reared animal (blue), note that the largest multisensory interactions are seen for pairings in which the stimuli are in close spatial proximity (i.e., 0° and 10°). In contrast, for the animal raised in the spatially disparate environment, the largest interactions are seen for disparities close to those that reflect the early sensory experiences (i.e., 30°, red). Blue and red shading show the spatial “window” for multisensory interactions in these two neurons, in which the stimulus combination results in a significant change in activity. B: Multisensory interactions as a function of the temporal interval between paired visual and auditory stimuli are plotted for three representative SC neurons—one in an animal raised under normal sensory conditions (blue), one from an animal raised in an environment in which the visual and auditory stimuli were always temporally offset by 100 msec (red), and one from an animal raised in an environment in which the visual and auditory stimuli were always temporally offset by 250 msec (green). Note that in the normally reared animal the largest multisensory interactions are seen when the visual stimulus precedes the auditory stimulus by 100 msec. In the neuron from the 100 msec temporal disparity reared animal this interval is increased to 200 msec. In comparison, in the 250 msec temporal disparity reared animal no multisensory interactions are seen.
Although establishing that spatial coincidence is not a prerequisite for the development of multisensory integration, this initial study left open the role that the temporal structure of the paired stimuli play in this process (in these spatial experiments the visual and auditory stimuli were always simultaneously presented). To address this issue, animals were raised in an environment in which visual and auditory stimuli were now presented as spatially-coincident pairs, but in which their temporal relationship was no longer simultaneous. Two temporal rearing conditions were tested—one in which the auditory stimulus always lagged the visual stimulus by 100 msec and the other in which this lag was extended to 250 msec. Although still very preliminary, the results of these temporal-disparity rearing experiments appear to be robust and dramatic. In the 100 msec disparity group, multisensory integration was still a hallmark feature of most neurons, but the temporal delay that produced maximal multisensory interaction was increased by 100 msec relative to normally reared controls (Fig. 7B). In contrast, in the 250 msec disparity group, multisensory integration was abolished. Thus, although there appears to be a temporal plasticity to multisensory maturation that is similar to that seen in the spatial realm, there also appears to be a limit on the temporal interval that can separate two stimuli and in which they can be “bound” into a singular multisensory percept. Such a limit may also exist in the spatial domain, since disparities greater than 30° were not tested. Taken together, the results of these spatial and temporal manipulations highlight the intrinsic malleability of multisensory interactions, and illustrate that the sensory contingencies of the animal’s early sensory environment play a critical role in shaping their multisensory circuits.
Adult Plasticity in Multisensory Brain Circuits: Preliminary Observations and Future Directions
The previously described results illustrate the enormous plastic capacity of the developing brain to be shaped by the statistics of the animal’s early crossmodal environment. In contrast, surprisingly little is known about experience-driven plasticity of multisensory networks in the mature brain. Thus far, we have observed that passive exposure appears to be insufficient to drive substantial changes, a finding in close correspondence to the results from the auditory system discussed earlier. Thus, placing adult animals in darkness for six months (a period equivalent to the time developing animals spent in the dark) induces little change in the integrative abilities of both subcortical and cortical multisensory neurons. Similarly, when adults were subjected to spatially disparate visual-auditory combinations in a passive context (i.e., stimuli were simply delivered from different locations), no change was evident. However, preliminary data from an animal in which spatially altered visual and auditory cues were actively utilized (i.e., by having the animal wear prisms that displace the visual world by 30° and in which the animal is allowed to actively interact with its environment) suggests that the adult SC retains the capacity for significant plasticity—but only if the altered statistics of the multisensory world are of behavioral relevance. In this animal, a number of SC neurons (work in AES is currently ongoing) showed visual and auditory receptive fields that were spatially displaced, and that showed large response enhancements to spatially disparate combinations that mimicked the change in experience. Although much more work needs to be done to bolster and elaborate this conclusion, these early results suggest that the adult brain is indeed capable of substantial crossrnodal plasticity, but only if crossmodal experience is actively used while interacting with the sensory world.
Toward a Unified View of Sensory Plasticity
As can be seen from the previous descriptions, there are remarkable parallels between the characteristics of the plasticity seen within an auditory brain structure and that seen in multisensory systems specialized to process crossmodal cues. Furthermore, the basic features of this plasticity appear to be preserved at various levels of the neuroaxis, ranging from the multisensory midbrain (i.e., the SC) through primary unisensory cortex (i.e., A1) and on into association cortical domains (i.e., AES). Although much work still needs to be done to fully detail the phenomenological characteristics of the plasticity at each level, its mechanistic underpinnings, and its relative time course(s), we can draw several firm conclusions based on the evidence to date. First, developmental plasticity appears to be ubiquitous and is highly dependent on the statistics of the early sensory world. Second, there appears to be a sensitive period during early postnatal life in which the capacity for change is greatest and in which stimulus statistics predominate as the driving force behind this change. Third and finally, adult plasticity is demonstrable in each of the systems examined but requires not only an alteration in the statistical properties of sensory inputs but also that these altered inputs be predictive of behaviorally relevant environmental events. Although perhaps surprising at first, the commonalities in these plastic processes across sensory systems and brain networks make a great deal of intuitive sense, in that they serve to coordinate change across the system in a parsimonious manner, ultimately allowing for the maintenance of a behavioral and perceptual unity even under highly dynamic conditions.
Parallels between Plasticity in Animal and Human Brains and Their Potential Applicability to Audiology
Many of the concepts described in this article can be related to clinical observations in individuals with compromised auditory system function. For example, numerous studies suggest that early identification of hearing loss and initiation of services (e.g., provision of hearing aid[s] and/or cochlear implant[s]) is associated with improved reading abilities and receptive/expressive speech and language skills (Yoshinaga-Itano and Apuzzo, 1998; O’Donoghue et al, 2000; Yoshinaga-Itano et al, 2000; Yoshinaga-Itano, 2003; McConkey Robbins et al, 2004; Zwolan et al, 2004; Harrison et al, 2005; Geers, 2006; Nicholas and Geers, 2006; Miyamoto et al, 2008). Bridging back to concepts discussed earlier, it is likely that improving the statistical salience of the acoustic signal through amplification or implantation in early development can promote normal maturation of central auditory circuits through the developmental exposure-based plasticity mechanisms. Another clear parallel comes from studies describing an age-dependent recovery of function in congenitally deaf children that have undergone cochlear implantation. Analyses of cortical event-related potentials elicited by implant stimulation demonstrate improvements in waveform morphology and reductions in response latency in children who receive cochlear implants before five years of age. Late-implanted children are less likely to exhibit responses similar to those of age-matched normal hearing children (Sharma et al, 2002; Sharma et al, 2005; Dorman et al, 2007). For a review, see Dorman et al, 2007 and Sharma et al, 2007. Findings from position emission tomography (PET) scans complement these studies by showing that the degree of glucose metabolism in auditory cortices of prelingually deafened children is greater the earlier they are implanted (Lee et al, 2005). From the multisensory perspective, it has also been shown that the “recruitment” of auditory cortex by visual information is associated with improved speech perception scores (Lee et al, 2001). Collectively, these findings lend credence to the notion that the functional maturation of auditory and multisensory brain systems are regulated during discrete sensitive periods of development and that therapeutic interventions will have maximal efficacy if they are considered in light of these developmental windows for brain plasticity.
Although these early interventions are likely to be most efficacious, there is good reason to believe that sensory brain regions continue to exhibit experience-dependent plasticity through reinforced sensory training paradigms in adults. There has been a resurgence of research investigating the efficacy of auditory training and rehabilitation in adults with hearing loss using amplification (i.e., hearing aids) and/or cochlear implants. These studies have shown improved auditory performance exceeding that achieved with passive experience alone (Sweetow and Palmer, 2005; Pichora-Fuller and Singh, 2006; Sweetow and Sabes, 2006, 2007; Boothroyd, 2007; Fu and Galvin, 2007, 2008), Both bottom-up and top-down training protocols have been shown to improve auditory skills with and without hearing aids/cochlear implants in humans (Rubinstein and Boothroyd, 1987; Kricos and Holmes, 1996; Rosen et al, 1999; Fu et al, 2005), Indeed, rather than invoking a single mechanism, training-induced improvements in auditory processing likely involve both bottom-up and top-down influences that may be invoked differentially depending on task demands. Finally, transfer of learning and training induced changes in neural activity provide strong evidence of neural plasticity in humans (Tremblay et al, 2003), a finding that is again consistent with the detailed studies of adult brain plasticity in the animal models described above.
In addition to compensatory changes in the auditory system that accompany hearing loss and training, human studies have also shown plastic changes that extend beyond the auditory domain. For example, a longitudinal study tracking word recognition performance based on auditory, visual, or combined audio-visual cues illustrates that individuals with cochlear implants demonstrate better auditory-visual performance than normal hearing subjects (Rouger et al, 2007), Furthermore, these changes can not be entirely explained by superior speech reading (visual only) skills, suggesting an improved neural capacity to integrate auditory and visual cues. Similarly, using the McGurk illusion as an index of audiovisual function, it has recently been shown that cochlear implant patients make greater use of visual cues than normal hearing subjects while reporting on their percepts (Rouger et al, 2008).
Developmental Dyslexia as a Neurological Condition That Can Be Better Understood and Effectively Treated through an Appreciation of Experience-Dependent Plasticity Mechanisms
Developmental dyslexia is a common neurobehavioral disorder affecting as much as 10% of the general population. Dyslexia can present profound and long-lasting social and professional challenges for those who struggle to keep pace in today’s increasingly information-driven society. Although many studies have identified a purely auditory temporal signal processing deficit in dyslexia (see Temple, 2002, for review), increasing evidence suggests that processing changes in multiple sensory systems might also play an important role (Laasonen et al, 2000, 2001, 2002; Hayes et al, 2003). In pursuing this line of inquiry, recent work has focused on an extended temporal window for the binding of visual and auditory stimuli in dyslexic readers when compared with typical readers, an expansion that could ultimately result in reading disability (see Figure 8; Hairston et al, 2005).
Figure 8.
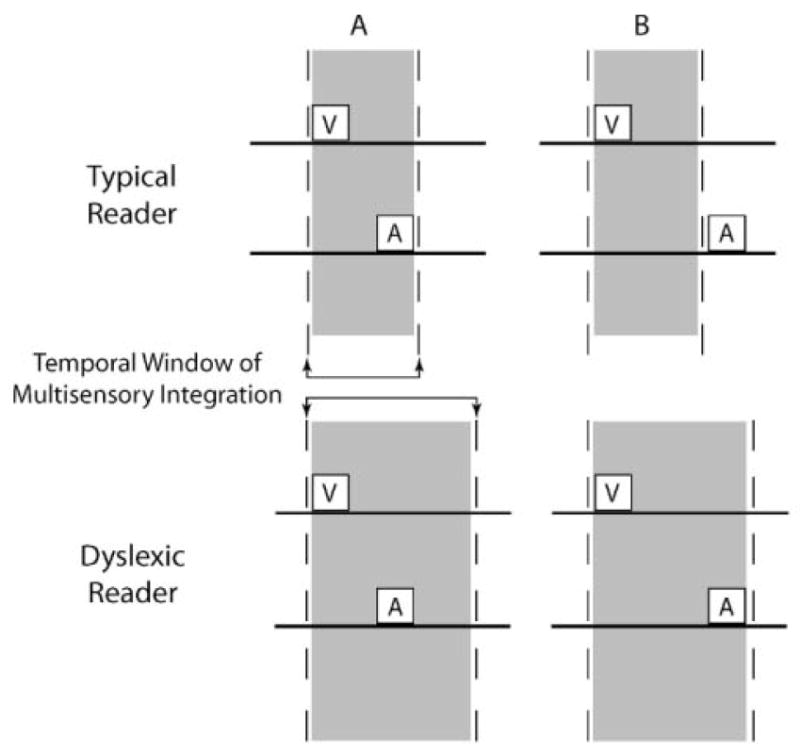
A simple model of the enlargement in the temporal window of multisensory integration in dyslexic readers. Shown at the top in yellow shading is the temporal window of multisensory binding in a typical reader, Note in panel A that if a visual (V) and auditory (A) event occur within this window, they are bound as a single perceptual entity. In contrast, with additional temporal separation (B), they are processed as separate events. At the bottom is shown the extended temporal window proposed for dyslexic readers, and the consequent impact of this window on the binding of visual and auditory events. Adapted from Hairston et al, 2005.
With the establishment of temporal alterations in auditory signal processing and multisensory processes in the dyslexic brain comes the hope that engagement of the plastic mechanisms discussed throughout this paper represent a potential tool for remediation. Encouraging progress is already being made in the remediation of rapid auditory signal processing in dyslexics (Temple et al, 2003; Gaab et al, 2007). Ongoing research is focusing on the malleability possible in the multisensory temporal window, with the goal that narrowing this window through training may result in improvements in the phonological and reading skills that are critically dependent on the integrity of the incoming sensory streams.
Abbreviations
- A1
primary auditory cortex
- AES
anterior ectosylvian sulcus
- CF
center frequency
- PND
postnatal day
- SC
superior colliculus
References
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc Natl Acad Sci U S A. 1993;90(12):5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci. 1996;110(5):905–913. doi: 10.1037//0735-7044.110.5.905. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536(1-2):271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7(9):974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci U S A. 2003;100(19):11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Eordegh G, Chadaide Z, Nagy A. Distributed population coding of multisensory spatial information in the associative cortex. Eur J Neurosci. 2004;20(2):525–529. doi: 10.1111/j.1460-9568.2004.03496.x. [DOI] [PubMed] [Google Scholar]
- Besle J, Fort A, Delpuech C, Giard MH. Bimodal speech: early suppress visual effects in human auditory cortex. Eur J Neurosci. 2004;20(3):2225–2234. doi: 10.1111/j.1460-9568.2004.03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52(2):371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci U S A. 2002;99(15):10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd A. Adult aural rehabilitation: what is it and does it work. Trends Amplif. 2007;11(2):63–71. doi: 10.1177/1084713807301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25(29):6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Woodruff PW, Iversen SD, David AS. Activation of auditory cortex during silent lipreading. Science. 1997;276(5312):593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Spence C, Stein BE, editors. The Handbook of Multisensory Processes. Cambridge, MA; The MIT Press: 2004. [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102(45):16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300(5618):498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Crowley DE, Hepp-Raymond MC. Development of cochlear function in the ear of the infant rat. J Comp Physiol Psychol. 1966;62:427–432. [Google Scholar]
- Cynader M, Timney BN, Mitchell DE. Period of susceptibility of kitten visual cortex to the effects of monocular deprivation extends beyond six months of age. Brain Res. 1980;191(2):545–550. doi: 10.1016/0006-8993(80)91303-7. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27(1):180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Sharma A, Gilley P, Martin K, Roland F. Central auditory development: evidence from CAEP measurements in children fit with cochlear implants. J Commun Disord. 2007;40(4):284–294. doi: 10.1016/j.jcomdis.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107(4):539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92(1):73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Wylie GR, Martinez A, Schroeder CE, Javitt DC, Guilfoyle D, Ritter W, Murray MM. Auditory-somatosensory multisensory processing in auditory association cortex: an fMRI study. J Neurophysiol. 2002;88(1):540–543. doi: 10.1152/jn.2002.88.1.540. [DOI] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci. 2005;25(33):7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6(11):1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Fu KM, Johnston TA, Shah AS, Arnold L, Smiley J, Hackett TA, Garraghty PE, Schroeder CE. Auditory cortical neurons respond to somatosensory stimulation. J Neurosci. 2003;23(20):7510–7515. doi: 10.1523/JNEUROSCI.23-20-07510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ., 3rd Perceptual learning and auditory training in cochlear implant recipients. Trends Amplif. 2007;11(3):193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ., 3rd Maximizing cochlear implant patients’ performance with advanced speech training procedures. Hear Res. 2008;242(1-2):198–208. doi: 10.1016/j.heares.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G, Galvin JJ., 3rd Auditory training with spectrally shifted speech: implications for cochlear implant patient auditory rehabilitation. J Assoc Res Otolaryngol. 2005;6(2):180–189. doi: 10.1007/s10162-005-5061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JD, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory signal processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor Neurol Neurosci. 2007;25:295–310. [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci U S A. 2000;97(14):8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25(20):5004–50l2. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory. Trends Cogn Sci. 2006;10(6):278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci. 1999;11(5):473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- Gobbele R, Schurmann M, Forss N, Juottonen K, Buchner H, Hari R. Activation of the human posterior parietal and temporoparietal cortices during audiotactile interaction. Neuroimage. 2003;20(1):503–511. doi: 10.1016/s1053-8119(03)00312-4. [DOI] [PubMed] [Google Scholar]
- Hairston WD, Burdette JH, Flowers DL, Wood FB, Wallace MT. Altered temporal profile of visual-auditory multisensory interactions in dyslexia. Exp Brain Res. 2005;166:474–480. doi: 10.1007/s00221-005-2387-6. [DOI] [PubMed] [Google Scholar]
- Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10(9):1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JM, Warr WB. A study of the cochlear nuclei and ascending auditory pathways of the medulla. J Comp Neurol. 1962;119:341–379. doi: 10.1002/cne.901190306. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Tiippana K, Nicol TG, Sams M, Kraus N. Integration of heard and seen speech: a factor in learning disabilities in children. Neurosci Lett. 2003;351(1):46–50. doi: 10.1016/s0304-3940(03)00971-6. [DOI] [PubMed] [Google Scholar]
- Heil F, Rajan R, Irvine DR. Topographic representation of tone intensity along the isofrequency axis of cat primary auditory cortex. Hear Res. 1994;76(1-2):138–202. doi: 10.1016/0378-5955(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282(5393):1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K, Priebe NJ, Crum PA, Bedenbaugh PH, Cheung SW, Schreiner CE. Modular functional organization of cat anterior auditory field. J Neurophysiol. 2004;92(1):444–157. doi: 10.1152/jn.01173.2003. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Adrian HO. Binaural columns in the primary field (A1) of cat auditory cortex. Brain Res. 1977;138(2):241–257. doi: 10.1016/0006-8993(77)90743-0. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci. 2007;27(18):4910–918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lepore F, Ptito M, Guillemot JP. Sensory interactions in the anterior ectosylvian cortex of cats. Exp Brain Res. 1994a;101(3):385–396. doi: 10.1007/BF00227332. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lepore F, Ptito M, Guillemot JP. Sensory modality distribution in the anterior ectosylvian cortex (AEC) of cats. Exp Brain Res. 1994b;97(3):404–414. doi: 10.1007/BF00241534. [DOI] [PubMed] [Google Scholar]
- Jiang W, Stein BE. Cortex controls multisensory depression in superior colliculus. J Neurophysiol. 2003;90(4):2l23–2135. doi: 10.1152/jn.00369.2003. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 2001;85(2):506–522. doi: 10.1152/jn.2001.85.2.506. [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Augath M, Logothetis NK. Integration of touch and sound in auditory cortex. Neuron. 2005;48(2):373–384. doi: 10.1016/j.neuron.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Sally SL. Organization of auditory cortex in the albino rat: binaural response properties. J Neurophysiol. 1988;59(6):1756–1769. doi: 10.1152/jn.1988.59.6.1756. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EL. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82(3):109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol. 2004;14(9):R335–338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kricos PB, Holmes AE. Efficacy of audiologic rehabilitation for older adults. J Am Acad Audiol. 1996;7(4):219–229. [PubMed] [Google Scholar]
- Laasonen M, Tomma-Halme J, Lahti-Nuuttila P, Service E, Virsu V. Rate of information segregation in developmentally dyslexic children. Brain Lang. 2000;75(1):66–81. doi: 10.1006/brln.2000.2326. [DOI] [PubMed] [Google Scholar]
- Laasonen M, Service E, Virsu V. Temporal order and processing acuity of visual, auditory, and tactile perception in developmentally dyslexic young adults. Cogn Affect Behav Neurosci. 2001;1(4):394–410. doi: 10.3758/cabn.1.4.394. [DOI] [PubMed] [Google Scholar]
- Laasonen M, Service E, Virsu V. Crossmodal temporal order and processing acuity in developmentally dyslexic young adults. Brain Lang. 2002;80(3):340–354. doi: 10.1006/brln.2001.2593. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kang E, Oh SH, Kang H, Lee DS, Lee MC, Kim CS. Preoperative differences of cerebral metabolism relate to the outcome of cochlear implants in congenitally deaf children. Hear Res. 2005;203(1-2):2–9. doi: 10.1016/j.heares.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Development of intersensory perception in human infants. In: Lewkowicz DJ, Lickliter R, editors. The Development of Intersensory Perception: Comparative Perspectives. Hills-dale, NJ: Lawrence Erlbaum Associates; 1994. [Google Scholar]
- Lutkenhoner B, Lammertmann C, Simoes C, Hari K. Magnetoencephalographic correlates of audiotactile interaction. Neuroimage. 2002;15(3):509–522. doi: 10.1006/nimg.2001.0991. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of rat auditory cortex neurons. Ear J Neurosci. 1997;9(4):S33–847. doi: 10.1111/j.1460-9568.1997.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on rate-level function of auditory cortex neurons: is there a “gating” effect of noradrenaline? Exp Brain Res. 1998;118(3):361–372. doi: 10.1007/s002210050290. [DOI] [PubMed] [Google Scholar]
- McConkey Robbins A, Koch DB, Osberger MJ, Zimmerman-Phillips S, Kishon-Rabin L. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg. 2004;130(5):570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Keniston LR, Dehner LR, Clemo HR. Crossmodal projections from somatosensory area SIV to the auditory field of the anterior ectosylvian sulcus (FAES) in Cat: further evidence for subthreshold forms of multisensory processing. Exp Brain Res. 2006;172(4):472–484. doi: 10.1007/s00221-006-0356-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. 1 Temporal factors. J Neurosci. 1987;7:3215–3229. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Descending efferents from the superior colliculus relay integrated multisensory information. Science. 1985;227(4687):657–659. doi: 10.1126/science.3969558. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986a;365:350–354. doi: 10.1016/0006-8993(86)91648-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol. 1986b;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol. 1996;75(5):1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res. 1989;480(1–2):372–377. doi: 10.1016/0006-8993(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6(2):133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Dykes RW, Merzenich MM. Binaural response-specific bands in primary auditory cortex (AI) of the cat: topographical organization orthogonal to isofrequency contours. Brain Res. 1980;181(1):31–48. doi: 10.1016/0006-8993(80)91257-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol. 2008;128(4):373–377. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res. 2002;14(1):115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220(2):255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Nagy AT, Eordegh G, Benedek G. Spatial and temporal visual properties of single neurons in the feline anterior ectosylvian visual area. Exp Brain Res. 2003;151(1):108–114. doi: 10.1007/s00221-003-1488-3. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Zhang J, Kitzes LM. Response patterns along an isofrequency contour in cat primary auditory cortex (AI) to stimuli varying in average and interaural levels. J Neurophysiol. 2004;91(1):118–135. doi: 10.1152/jn.00171.2003. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27(3):286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue GM, Nikolopoulos TP, Archbold SM. Determinants of speech perception in children after cochlear implantation. Lancet. 2000;356(9228):466–468. doi: 10.1016/S0140-6736(00)02555-1. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Orderly cortical representation of vowels based on formant interaction. Proc Natl Acad Sci U S A. 1997;94(17):9440–9444. doi: 10.1073/pnas.94.17.9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkola J, Ojanen V, Autti T, Jaaskelainen IP, Mottonen R, Tarkiainen A, Sams M. Primary auditory cortex activation by visual speech: an fMRI study at 3 T. Neuroreport. 2005;16(2):125–128. doi: 10.1097/00001756-200502080-00010. [DOI] [PubMed] [Google Scholar]
- Philibert B, Beitel RE, Nagarajan SS, Bonham BH, Schreiner CE, Cheung SW. Functional organization and hemispheric comparison of primary auditory cortex in the common marmoset (Callithrix jacchus) J Comp Neurol. 2005;487(4):391–406. doi: 10.1002/cne.20581. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Singh G. Effects of age on auditory and cognitive processing: implications for hearing aid fitting and audiologic rehabilitation. Trends Amplif. 2006;10(1):29–59. doi: 10.1177/108471380601000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci U S A. 2004;101(46):16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97(5):3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26(18):4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol. 1999;415(4):460–481. doi: 10.1002/(sici)1096-9861(19991227)415:4<460::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Rosen S, Faulkner A, Wilkinson L. Adaptation by normal listeners to upward spectral shifts of speech: implications for cochlear implants. J Acoust Soc Am. 1999;106(6):3629–3636. doi: 10.1121/1.428215. [DOI] [PubMed] [Google Scholar]
- Rouger J, Fraysse B, Deguine O, Barone P. McGurk effects in cochlear-implanted deaf subjects. Brain Res. 2008;1188:87–99. doi: 10.1016/j.brainres.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Rouger J, Lagleyre S, Fraysse B, Deneve S, Deguine O, Barone P. Evidence that cochlear-implanted deaf patients are better multisensory integrators. Proc Natl Acad Sci U S A. 2007;104(17):7295–7300. doi: 10.1073/pnas.0609419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein A, Boothroyd A. Effect of two approaches to auditory training on speech recognition by hearing-impaired adults. J Speech Hear Res. 1987;30(2):153–160. doi: 10.1044/jshr.3002.153. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell JW, Blakemore C, Young MP. Analysis of connectivity in the cat cerebral cortex. J Neurosci. 1995;15(2):1463–1483. doi: 10.1523/JNEUROSCI.15-02-01463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell JW, Sengpiel F, Tovee MJ, Benson PJ, Blakemore C, Young MP. Visual motion processing in the anterior ectosylvian sulcus of the cat. J Neurophysiol. 1996;76(2):895–907. doi: 10.1152/jn.1996.76.2.895. [DOI] [PubMed] [Google Scholar]
- Schreiner CE. Spatial distribution of responses to simple and complex sounds in the primary auditory cortex. Audiol Neurootol. 1998;3(2–3):104–122. doi: 10.1159/000013785. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC. Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol. 2001;85(3):1322–1327. doi: 10.1152/jn.2001.85.3.1322. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. A unified model for perceptual learning. Trends Cogn Sci. 2005;9(7):329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46(9):494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986;66(1):118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Groh JM. The superior colliculus: a window for viewing issues in integrative neuroscience. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Stein B, Meredith M, Huneycutt W, McDade L. Behavioral indices of multisensory integration: orientation to visual cues is affected by auditory stimuli. J Cogn Neurosci. 1989;1:12–24. doi: 10.1162/jocn.1989.1.1.12. [DOI] [PubMed] [Google Scholar]
- Stein BE, Huneycutt WS, Meredith MA. Neurons and behavior: the same rules of multisensory integration apply. Brain Res. 1988;448:355–358. doi: 10.1016/0006-8993(88)91276-0. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. Functional organization of the superior colliculus. In: Leventhal AG, editor. The Neural Basis of Visual Function. Hampshire, UK: Macmillan; 1991. [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Stein BE, Wallace MT. Comparisons of cross-modality integration in midbrain and cortex. Prog Brain Res. 1996;112:289–299. doi: 10.1016/s0079-6123(08)63336-1. [DOI] [PubMed] [Google Scholar]
- Sweetow R, Palmer CV. Efficacy of individual auditory training in adults: a systematic review of the evidence. J Am Acad Audiol. 2005;16(7):494–504. doi: 10.3766/jaaa.16.7.9. [DOI] [PubMed] [Google Scholar]
- Sweetow RW, Sabes JH. The need for and development of an adaptive Listening and Communication Enhancement (LACE) Program. J Am Acad Audiol. 2006;17(8):538–558. doi: 10.3766/jaaa.17.8.2. [DOI] [PubMed] [Google Scholar]
- Sweetow RW, Sabes JH. Technologic advances in aural rehabilitation: applications and innovative methods of service delivery. Trends Amplif. 2007;11(2):101–111. doi: 10.1177/1084713807301321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai Y, Miyashita E, Nakai M. Eye movements following cortical stimulation in the ventral bank of the anterior ectosylvian sulcus of the cat. Neurosci Res. 1989;7(2):159–163. doi: 10.1016/0168-0102(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Temple E. Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol. 2002;12(2):178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JD. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci USA. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114(7):1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- van Wassenhove V, Grant KW, Poeppel D. Visual speech speeds up the neural processing of auditory speech. Proc Natl Acad Sci U S A. 2005;102(4):1181–1186. doi: 10.1073/pnas.0408949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT. The development of multisensory integration. In: Calvert GA, Spence C, Stein BE, editors. The Handbook of Multisensory Processes. Cambridge, MA: MIT Press; 2004a. [Google Scholar]
- Wallace MT. The development of multisensory processes. Cogn Process. 2004b;5(2):69–83. [Google Scholar]
- Wallace MT, Carriere BN, Perrault TJ, Jr, Vaughan JW, Stein BE. The development of cortical multisensory integration. J Neurosci. 2006;26(46):11844–11849. doi: 10.1523/JNEUROSCI.3295-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Integration of multiple sensory modalities in cat cortex. Exp Brain Res. 1992;91(3):484–488. doi: 10.1007/BF00227844. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69(6):1797–1809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Multisensory integration in the superior colliculus of the alert cat. J Neurophysiol. 1998;80(2):1006–1010. doi: 10.1152/jn.1998.80.2.1006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol. 1994;71(1):429–432. doi: 10.1152/jn.1994.71.1.429. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17(7):2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97(1):921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Wilkinson LK, Stein BE. Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol. 1996;76(2):1246–1266. doi: 10.1152/jn.1996.76.2.1246. [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature. 2001;411(6841):1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C. Early intervention after universal neonatal hearing screening: impact on outcomes. Ment Retard Dev Disabil Res Rev. 2003;9(4):252–266. doi: 10.1002/mrdd.10088. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Apuzzo ML. The development of deaf and hard of hearing children identified early through the high-risk registry. Am Ann Deaf. 1998;143(5):416–424. doi: 10.1353/aad.2012.0118. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Coulter D, Thomson V. The Colorado Newborn Hearing Screening Project: effects on speech and language development for children with hearing loss. J Perinatol. 2000;20(8, Pt. 2):S132–137. doi: 10.1038/sj.jp.7200438. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4(11):1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci U S A. 2002;99(4):2309–2314. doi: 10.1073/pnas.261707398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan TA, Ashbaugh CM, Alarfaj A, Kileny PR, Arts HA, El-Kashlan HK, Telian SA. Pediatric cochlear implant patient performance as a function of age at implantation. Otol Neurotol. 2004;25(2):112–120. doi: 10.1097/00129492-200403000-00006. [DOI] [PubMed] [Google Scholar]


