Summary
Neuromyelitis optica (NMO) is an autoimmune inflammatory disorder of the central nervous system. In most NMO patients, autoantibodies to the water channel protein Aquaporin 4 (AQP4) are present at high levels and are thought to drive pathology by mediating complement-dependent destruction of astrocytes. Here we apply recently developed chemical library screening technology to identify a synthetic peptoid that binds anti-AQP4 antibodies in the serum of NMO patients. This finding validates, in a well-defined human disease, that synthetic, unnatural ligands for the antigen-binding site of a disease-linked antibody can be isolated by high-throughput screening.
Introduction
Neuromyelitis optica (NMO) is a rare, autoimmune demyelinating disease that can result in blindness and paralysis(Wingerchuk et al., 2006). A major breakthrough in the understanding of NMO was the discovery that most NMO patients have high levels of circulating IgG autoantibodies against a water channel protein aquaporin 4 (AQP4)(Lennon et al., 2004) expressed on the surface of astrocytes in the central nervous system (CNS). There is evidence that these autoantibodies fix complement on the surface of certain AQP4-expressing cells(Crane et al., 2011), resulting in tissue injury (Papadopoulos and Verkman, 2012). Currently, anti-AQP4 autoantibodies may be detected by a variety of methods: ELISA against recombinant AQP4 protein, tissue-based immunofluorescence, AQP4-transfected cell-based assays, fluorescence immunoprecipitation assays, and flow cytometric assays(Hayakawa et al., 2008; Kalluri et al., 2010; Waters and Vincent, 2008; Waters et al., 2012). The target epitopes recognized by AQP4 autoantibodies in these assays include determinants on the three extracellular loops (Iorio et al., 2012; Pisani et al., 2011); however, the sequence and conformational determinants remain unresolved due to the use of polyclonal patient serum and the limited characterization of the AQP4 protein target.
Despite the high diagnostic specificity of these multiple assays, approximately 25%(Waters et al., 2012) of patients with clinical NMO(Wingerchuk et al., 2006) lack readily detectable anti-AQP4 antibodies. These patients may have low-titer, low affinity anti-AQP4 antibodies, or may produce autoantibodies against alternative CNS targets (Lalive et al., 2006). Misdiagnosis of these patients may lead to unnecessary diagnostic studies and inappropriate therapy and highlights the need for further work on the discovery of biomarkers for the disease.
We have reported chemical library screening-based technology for the discovery of diagnostically useful antibodies(Reddy et al., 2011). In this method, several thousand peptoids(Simon et al., 1992) (oligo-N-substituted glycines) are arrayed on chemically modified glass slides. These peptoid arrays are then exposed to serum from case and control individuals, followed by a labeled anti-human IgG antibody to visualize the binding pattern of the serum IgGs. Peptoids that capture high levels of antibody from the case samples, but not the controls, are taken as ligands for candidate biomarker antibodies. Note that this is not a “fingerprinting” analysis of complex patterns of thousands of spots on the array(Halperin et al., 2011; Restrepo et al., 2011), but rather a chemical screen to identify a few ligands for individual IgG antibody biomarkers of high diagnostic value. Moreover, it is, to our knowledge, the only approach to antibody biomarker discovery that makes use of unnatural libraries of chemical compounds rather than attempting to screen libraries of candidate autoantigens such as peptides or proteins (Nagele et al., 2011; Robinson et al., 2002; Wang et al., 2005). We previously validated this approach using a mouse model for multiple sclerosis and also identified candidate biomarkers for human Alzheimer’s disease(Reddy et al., 2011), which are currently undergoing more extensive testing.
Here we apply this technology to NMO. From the perspective of validation of this novel method for biomarker discovery in a human disease, NMO is an attractive system. One would expect to isolate peptoids that bind to anti-AQP4 autoantibodies, thus providing a clear validation of the approach. We show here that a screen of 100,000 peptoids using a second-generation bead-based screening approach indeed yielded several peptoid ligands for the antigen-binding site of anti-AQP4 antibodies. We show in a small preliminary study that the use of a small panel of these peptoids allows one to distinguish between NMO patient serum and serum from healthy controls or patients with MS, Alzheimer’s Disease, narcolepsy and lupus with high accuracy.
Results
Screening Bead-Displayed Peptoid Libraries For Antibody Ligands
Our previous report(Reddy et al., 2011) employed comparative screening of several thousand peptoids arrayed on a modified glass slide against case and control serum samples. In order to substantially increase the number of compounds that could be employed in such a screen, we first developed a protocol that allowed one bead one compound (OBOC) libraries synthesized on hydrophilic TentaGel beads to be employed directly in the screening step. Libraries of hundreds of thousands or even millions of peptoids are easily prepared in this format by split and pool solid phase synthesis (Alluri et al., 2003; Figliozzi et al., 1996; Lam et al., 1991) and these libraries can be employed productively in screening experiments using recombinant proteins (Lim et al., 2007; Xiao et al., 2007) or cells (Lau et al., 2002; Mikawa et al., 2004; Udugamasooriya et al., 2008) as targets. The strategy that we envisioned is shown in Fig. 1A. The bead library would first be incubated with a pool of control serum samples followed, after washing, by a red quantum dot-labeled secondary antibody to “light up” beads that retain significant amounts of these uninteresting antibodies or bind directly to the secondary antibody. After removal of these beads, the denuded library would then be exposed to a pool of NMO serum samples and the labeled secondary antibody. Hits from this screen would be collected as possible ligands for NMO-specific antibodies and analyzed further (see Methods and Supplemental for details).
Fig. 1. Screening a combinatorial peptoid library for ligands to NMO-specific antibodies.
A. Schematic depiction of the screening strategy. An OBOC library was pre-screened with control serum to eliminate the beads that can bind to the antibodies present at high levels in control (non-NMO) sera. The denuded library was then screened against a pool of NMO serum high in anti-AQP4 antibodies. In both the pre-screen and the NMO screen, antibody-binding beads were visualized using a red quantum dot-conjugated secondary antibody. The hits were identified by tandem mass spectrometry. B. General structure of the 8-mer peptoid in the library used for the screening of NMO sera. The invariant linker is shown in black and the variable region in blue with side chains substituents (from the amines) in red. C. List of amines used in the solid-phase synthesis of the library. D. A representative photomicrograph of the library under the fluorescent microscope after incubation with the serum followed by hybridization of anti-human secondary antibody conjugated with Qdot 655. The beads were irradiated through a DAPI filter. The beads with the red halo are the hits.
A library of peptoids containing five variable positions after an invariant linker of four residues was constructed using the sub-monomer synthetic method(Zuckermann et al., 1992). Ten amines were used in the synthesis of the library (Fig. 1C), providing a theoretical diversity of 100,000 compounds. The linker (Fig. 1B) contained a C-terminal methionine residue to facilitate cyanogen bromide-mediated release of the compound from the bead after screening, a furan-containing residue to facilitate post-screening labeling of the compound and two lysine-like peptoid residues (Nlys), which are charged at neutral pH and should aid in the presentation of the peptoid in aqueous solution.
As described above, the library (≈ 100,000 beads) was first exposed to a pool of six serum samples obtained from control individuals that do not have NMO, followed by fluorescently labeled anti-human IgG secondary antibody. Beads that exhibited an obvious fluorescent halo under a low power fluorescence microscope were removed using a micropipette. The remainder of the library was washed several times with buffer and then incubated with a pool of six serum samples obtained from NMO patients whose serum tested positive for complement-mediated cytotoxicity using AQP4-transfected HEK293 cells, followed by labeled secondary antibody. Beads that displayed above background binding of antibodies as evidenced by the red halo (Fig. 1D) were picked. To verify that the beads visualized at this stage are indeed candidate hits, the binding experiment was redone. After stripping the beads with 1% SDS, washing extensively to remove the SDS and re-equilibrating the beads, the NMO serum pool was re-applied. After this step, a total of 43 beads were deemed potential hits. These were segregated into the wells of a microtiter plate. The peptoids were released into solution by cleavage with CNBr and sequenced by tandem mass spectrometry (Supplementary Figure S1).
Initial Characterization of Screening Hits
Due to a variety of complexities with bead-based screening technology, it is often the case that compounds that appear to be hits at the bead stage fail to validate in subsequent assays(Chen et al., 2009). Moreover, in this screen, it is possible that one of the pooled NMO samples may have had very high levels of an antibody idiosyncratic to that patient and peptoid ligands of poor diagnostic utility might be isolated. Therefore, it is imperative to assess the ability of the peptoid hits to distinguish several individual case and control serum samples on a different analytical platform. Ten (Supplementary Table 1) of the 43 hits that corresponded to the brightest beads at the screening stage were re-synthesized and spotted onto chemically modified glass slides (Lesaicherre et al., 2002; Reddy and Kodadek, 2005). We also synthesized a fluorescein-labeled derivative of one of these peptoids NMOP6 (NMO Peptoid 6), and employed it as a control to ensure that the spotting process proceeded efficiently. A peptoid that was found to bind directly to the secondary antibody, NMOP8, was also spotted onto the array as an internal control. A derivative of dinitrophenol (DNP), a small molecule that is recognized by antibodies present in most people, was also spotted as another control.
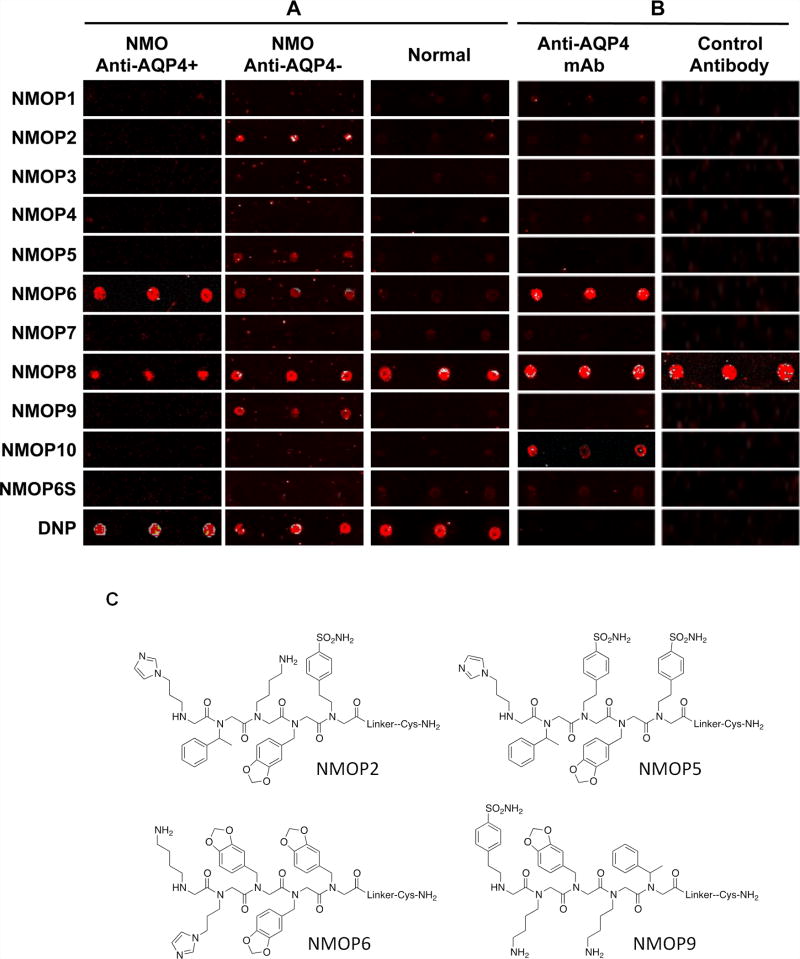
The slides were then incubated with individual serum samples (see methods) and, after washing, fluorescently labeled secondary antibody. As shown in Fig. 2A, when the array was exposed to serum from a control patient, not suffering from NMO, there was little signal observed on any of the arrayed peptoids, except, of course, NMOP8. DNP, as expected, also registered a strong signal. A strong signal was observed in the fluorescein channel for the labeled peptoid, confirming that spotting had proceeded efficiently (not shown). When the experiment was repeated with a serum sample obtained from an NMO patient whose serum tested positive in an assay that scores the ability of serum antibodies to drive complement-mediated killing that express recombinant AQP4 (Phuan et al., 2012), indicating the presence of anti-AQP4 antibodies, strong signals were observed on NMOP6. When the experiment was conducted with a sample from an NMO patient that tested negative for anti-AQP4 antibodies by the cell-killing assay, significant intensities were observed on NMOP6, NMOP2, NMOP5 and NMOP9. Note that a negative result in the cell-killing assay does not mean that the serum does not contain anti-AQP4 antibodies. They could be present at a level that is insufficient to trigger efficient cell killing in this assay or they could be variants that do not readily fix complement. Nonetheless, since the intensity of the NMOP6 signal was markedly lower than was the case for the serum sample that was clearly positive in this assay, it is possible that this peptoid may be a ligand for anti-AQP4 antibodies. The data for the analysis of an additional six individual NMO serum samples, half of which tested positive in the cell-killing assay and half of which did not, are shown in Supplementary Figure S2. NMOP6 showed strong signals for the NMO sera that tested positive for cell killing and lower signals for two of the four NMO samples that tested negative for cell killing. NMOP4, NMOP5 and NMOP9 also retained significant amounts of antibodies from certain NMO samples, but were dark on others. The chemical structures of these microarray-validated hits are shown in Fig. 2C. NMOP1, NMOP3, NMOP7 and NMOP10 failed to provide robust signals in any of the samples. The structures of all of these peptoids are shown in Supplementary Table 1.
Fig. 2. Validation of bead screening hits by microarray analysis.
Results of hybridizing serum samples or purified antibodies to the array, followed by washing and subsequent addition of a red-labeled secondary antibody (see Methods). The screen shots from the microarray scanner are shown. A. Analysis of serum samples from a control individual who did not have NMO (Normal) and NMO patients that tested either positive (Anti-AQP4+) or negative (Anti-AQP4−) for complement-mediated cell killing of cells expressing AQP4. As stated in the main text, this does not necessarily mean that the “anti-AQP4 Ab−“ samples are devoid of anti-AQP4 antibodies. B. Analysis of purified monoclonal anti-AQP4 antibody and control antibody. NMOP8 binds the secondary antibody directly. DNP = dinitrophenol. C. Chemical structures of the peptoids that showed significant affinity for NMO-specific antibodies in one of the samples. The structure of the linker is shown in Fig. 1.
Peptoid NMOP6 is a ligand for anti-AQP4 antibodies
To test the hypothesis that NMOP6 is a ligand for anti-AQP4 antibodies, the array was exposed to a monoclonal antibody isolated from a patient that binds the full-length M1 isoform of human AQP4 (Bennett et al., 2009) or to a control antibody, called rAb-2B4 that binds the measles virus nucleocapsid and is of the same isotype (IgG1) as the anti-AQP4 antibody. As shown in Fig. 2B, NMOP6 retained the anti-AQP4 antibody efficiently, but not the control antibody. This appears to be a specific interaction because a scrambled version of NMOP6 (NMOP6s, see Supplementary Table 1) was also spotted onto the array. This peptoid has the same chemical functionality as NMOP6, but the order of the side chains is scrambled. NMOP6S did not bind the anti-AQP4 monoclonal antibody, or antibodies from the serum of NMO patients (Fig. 2 and Supplementary Figure S3).
NMOP10 also showed a weak signal for the anti-AQP4 Ab, indicating that it is likely a very low affinity ligand for this antibody, but since this peptoid was not of utility in serum screening, this issue was not pursued further.
To ask in a different way if NMOP6 indeed recognizes anti-AQP4 antibodies, the serum from an NMO patient was passed over a column of immobilized recombinant AQP4 or, as a control, bovine serum albumin (BSA). The depleted serum was then applied to the array. As shown in Fig. 3, the robust signal observed on NMOP6 for the NMO sample was abolished when the anti-AQP4 antibodies were removed from it, but the signal was unaffected by passage over a BSA column. Moreover, the signal on the DNP control spot was unaffected by passage of the serum over either column, proving that there was no general depletion of antibodies during the procedure. The microarray images of the binding pattern of the sera before and after depletion experiments are shown in Supplementary Figure S3. Based on these data and the experiment using the monoclonal antibody, we conclude that NMOP6 binds anti-AQP4 antibodies.
Fig. 3. Peptoid NMOP6 is an AQP4 antigen surrogate.
The graph depicts the level of signal on an array for the peptoid and serum samples indicated. MS = multiple sclerosis. The serum samples indicated were either applied to the array directly or after being incubated with immobilized AQP4 or, as a control, BSA. NMOP6S is a scrambled version of NMOP6. Note that the terms “anti-AQP4 Ab+” and “anti-AQP4 Ab−“ connote serum samples that tested positive and negative, respectively, in the assay for complement-mediated killing of AQP4-expressing cells. As stated in the main text, this does not necessarily mean that the “anti-AQP4 Ab−“ samples are devoid of anti-AQP4 antibodies.
Peptoids NMOP2, NMOP5 and NMOP9 did not retain significant amounts of antibodies from the serum sample employed for the above depletion experiment. But this does not necessarily mean that they are ligands for antibodies that recognize antigens other than AQP4. The anti-AQP4 antibody spectrum in any patient is polyclonal (Iorio et al., 2012). Given that a small molecule like a peptoid is likely to recognize only a fraction of this polyclonal population, it is possible that NMOP2, NMOP5 and NMOP9 are ligands for anti-AQP4 antibodies whose epitope selectivity is different than the antibodies recognized by NMOP6. Therefore, we repeated the depletion experiment with another serum sample obtained from an NMO patient that tested negative for anti-AQP4 antibodies by the cell-killing assay, but which showed readily detectable signals on these peptoids. This is the same serum sample used in the Fig. 2 experiment (second column). As shown in Fig. 3, the signals on NMOP2, NMOP5 and NMOP9 were almost completely abolished by passage of the serum over immobilized AQP4 protein, similar to NMOP6. However, approximately 50% of the signal on these three peptoids was lost when the serum was passed over immobilized BSA. A reasonable interpretation of these results is that NMOP2, NMOP5 and NMOP9 are indeed ligands for anti-AQP4 antibodies that recognize different epitopes than the antibodies bound by NMOP6, but that these antibodies are “stickier” or more promiscuous in their binding selectivity. However, this issue will require more experimentation to address unequivocally.
Utility of the Peptoids As Diagnostic Reagents
NMO patients are sometimes misdiagnosed as MS patients, with potentially serious adverse consequences (Uzawa et al., 2010). Since MS patients lack antibodies to AQP4, we would predict that the peptoids isolated from the screen would not cross-react with antibodies from MS patients. As shown in Fig. 3, when serum from three MS patients was hybridized to the array, little or no signal was observed on any of the peptoids, though the DNP signal was strong, showing that the peptoids may be useful in distinguishing these sometimes symptomatically similar diseases from one another.
Since MS patients are not known to have high levels of autoantibodies, we also challenged the peptoid probes with sera from diseases where autoantibodies have been detected or are thought to exist. For example, lupus patients are known to have unusually high levels of autoantibodies. We also examined sera from patients with narcolepsy, which is believed to be an autoimmune condition (Hallmayer et al., 2009), and Alzheimer’s Disease (AD), where disease-related antibodies have been reported (Nagele et al., 2011; Reddy et al., 2011). A total of 12 serum samples from these patients and six additional samples from normal control individuals were used. Each individual sample was applied to the array under the same conditions. None of the peptoids, with the exception of the NMOP8 control, showed significant signal with any of these samples above background, though it should be noted that the background was quite high with two of the lupus samples (Fig. S4). Peptoid ADP3, which was used as a control since it was previously reported to bind antibodies associated with AD, showed clear binding for some of the AD samples (Fig. S4). These data indicate that the peptoids identified in this study are indeed selective ligands for NMO antibodies.
To determine the ability of the peptoids to accurately diagnose NMO more generally, we conducted an internally double-blinded study of 15 serum samples. The samples were applied to the array and called as NMO (N in Fig. 4) or controls (C in Fig. 4) based on the intensities observed at each feature. A positive call entailed observing a signal on any of the peptoids, other than NMOP8, that was above zero relative intensity or had a mean intensity greater than 50% of the intensity of NMOP8. The relative intensity was calculated by subtracting the mean intensity of each compound by the overall median intensity. The calls were then reported to the second individual who then obtained the key from the first technician to check the accuracy of the calls.
Fig. 4. Blinded analysis of 15 serum samples.

15 serum samples were analyzed in a blinded fashion on arrays of the type shown in Fig. 2. A heat map representing the intensities observed on the arrays is shown. The samples were called NMO if any of the peptoids (other than NMOP8, which binds the secondary antibody directly) displayed a clear signal above background. The calls, made prior to unblinding, are shown in red at the bottom of the heat map and the identities of the samples are shown in black below the calls. C = control, N+ = NMO serum that tested positive in the complement-mediated cell-killing assay, N− = NMO serum that tested negative in the cell-killing assay.
The data are shown in Fig. 4 in the form of a heat map. Beneath the map are the calls and the identities of the samples revealed after unblinding, including if the NMO serum samples tested positive or negative by the cell-killing assay (N+ or N−). The results showed that the panel of peptoids provides an accurate diagnostic test for NMO. 14 of the 15 samples were called correctly. Sample 15 was the exception. It was obtained from an NMO patient whose serum tested negative in the cell-killing assay. While peptoids NMOP2, NMOP4 and NMOP6 showed slight signals, these were very weak and did not pass the criteria for calling the sample NMO. In the other 14 cases, there was a very clear distinction between cases and controls, including all four of the other samples that were obtained from NMO patients whose antibodies did not mediate killing of AQP4-expressing cells. Indeed, NMOP6 alone correctly predicted 13 of the 15 samples (two false negatives).
Discussion
We previously reported a novel technology for the discovery of serum biomarkers that involved comparative screens of the total complement of circulating IgG antibodies from case and control samples against a collection of synthetic, unnatural compounds (peptoids) (Reddy et al., 2011). The goal was to identify compounds that retain antibodies that are present in much higher amounts in the serum of patients or animals with a particular disease and thus would serve as useful serum biomarkers for diagnosis. We validated this approach in EAE (experimental autoimmune encephalomyelitis), an animal model for MS induced by immunization with a self-antigen peptide derived from myelin oligodendrocyte glycoprotein (MOG). As anticipated, peptoids that bound anti-MOG peptide antibodies were identified (Reddy et al., 2011).
However, validation of this approach in a simple animal model does not equate with efficacy in the far more complicated arena of human disease. To address this issue, we performed a study on sera from patients with Alzheimer’s disease (AD). In a preliminary study of 54 patients, the same screening approach identified peptoids that captured antibodies found only in AD patients, not age-matched healthy controls or patients with Parkinson’s disease (Reddy et al., 2011). However, because these putative AD biomarker antibodies are novel the results cannot be taken as clear evidence of successful biomarker discovery until more extensive validation trials are completed. These are in progress.
NMO provides an excellent opportunity to identify disease-specific peptoid biomarkers in the context of an autoimmune disorder with a known antigenic target, AQP4. There is a strong expectation that application of this screening technology to NMO should identify peptoids that bind to anti-AQP4 antibodies. This was indeed the case. Peptoid NMOP6, one of several hits, was clearly identified as a ligand for anti-AQP4 antibodies based on its ability to bind a monoclonal anti-AQP4 antibody (Fig. 2B) and the ablation of signal from a serum sample that had been depleted of anti-AQP4 antibodies by passage over immobilized AQP4 (Fig. 3).
Note that peptoid NMOP6 cannot possibly mimic a native AQP4 epitope. In a peptoid, the side chains protrude from the sp2-hybridized nitrogen, rather than the sp3-hybridized α-carbon, of the main chain of the backbone. Moreover, most of the side chains in NMOP6 do not correspond to those found in proteins. Nonetheless, this relatively small molecule must recognize some conserved feature of the antigen-binding sites in some fraction of the anti-AQP4 polyclonal spectrum, though in the absence of structural data it is impossible to speculate on the detailed mode of binding.
From a preliminary blinded study (Fig. 4), it appears that peptoid NMOP6 and some of the other peptoid hits will be useful as diagnostic reagents. NMOP6 alone was able to call 13 of the 15 samples correctly. The two misses were NMO samples that tested negative in the cell-killing assay and thus might have low levels of anti-AQP4 antibodies, though we cannot rule out other explanations, as stated above. These were called as false negatives. Sample 15, as discussed above, showed a very low signal and sample 10 essentially showed only background signal. However, a moderate signal was observed on NMOP5 for sample 10, allowing it to be called correctly. This was the only peptoid in the group that captured significant amounts of antibody from sample 10. Indeed, only peptoids NMOP5 and NMOP6 would have been necessary to achieve the 14/15 accuracy obtained in this initial study. NMOP2 and NMOP9 also displayed significant signals for several of the samples. Since these peptoids apparently bind different antibodies than NMOP6, it is likely that in larger studies the use of a four-peptoid panel might be advantageous. None of the peptoids exhibited significant signals when presented to 18 other samples, consisting of healthy controls and patients with lupus, MS, narcolepsy and Alzheimer’s disease (Fig S4). Lupus patients, in particular, are known to have high levels of several circulating autoantibodies, so this result supports the contention that the peptoids identified in this study are indeed selective ligands for NMO-related antibodies. Considering all of the samples analyzed in this study, the diagnostic sensitivity of the assay is 93% and the diagnostic specificity 100%. Note that for all of the samples, the signal obtained on the scrambled NMOP6s peptoid was negligible and the signal on DNP was high, providing useful negative and positive controls, respectively.
NMOP2, NMOP5 and NMOP9 may also bind to anti-AQP4 antibodies based on the serum depletion experiment (Fig. 3), but, if so, apparently to a different part of the polyclonal spectrum of this antibody population. A detailed characterization of the antibody-binding properties of these peptoids will require additional study.
Finally, it is noteworthy that this study employed a second generation screening technology different than the approach we have published previously, which employed peptoids displayed on microarrays as the primary screening platform (Reddy et al., 2011). Here we employed bead-displayed peptoids made by solid-phase split and pool synthesis (Figliozzi et al., 1996). Whereas the microarray technology limits the number of molecules that can be used in the primary screen to a few thousand, libraries of a few million compounds can be made on beads (Alluri et al., 2003). Studies of peptide libraries have demonstrated that larger libraries are more likely to contain high affinity ligands (Wilson et al., 2001), so this technological advance may be useful in the future for the discovery of superior antibody capture agents. This type of screening protocol has been applied by us and others to identify ligands for individual protein targets, but not for serum screening. A potential downside of this approach is that it involves the sequential screening of pooled serum samples (first control, then diseased), whereas the primary microarray screens involved exposing several microarrays to individual serum samples, both cases and controls, and identifying peptoids that “lit up” when exposed to the diseased samples, but not the controls. The danger of the pooling strategy is that one or more, but not all, of the diseased serum samples might contain high levels of a antibodies that are idiosyncratic to those individuals, but not be related to the disease of interest. In theory, if such antibodies were present at very high levels in even a single person in the pool one could identify peptoid ligands to it that would be mistaken as capture agents for disease-specific antibodies. Thus, it is critical to validate the hits from the bead screen with several individual serum samples, which we did here using the array platform. This might explain why several of the peptoids identified as strong hits in the bead screen did not capture significant amounts of peptoids from any of the NMO serum samples. Of course, there could be technical reasons for this as well, for example some type of binding mode that is facilitated by the bead surface, but not the glass slide.
Significance
The discovery of diagnostically useful serum biomarkers is of great interest in translational science. In many diseases, it is likely that an adaptive immune response produces disease-specific antibodies that would be excellent candidates for such biomarkers. However, a major impediment to mining the immune system for useful biomarkers is the assumption that only the native antigen will bind to disease-specific antibodies with sufficient affinity and selectivity to retain them from the serum and allow their levels to be measured. The discovery of such antigens has proven difficult in most diseases. We previously introduced a different approach that involved the discovery of synthetic “antigen surrogates” by comparative screening of peptoid libraries against case and control sera (Reddy et al., 2011). The utility of this approach was validated in a simple mouse model for multiple sclerosis and some preliminary data was presented indicating that this technique might also be applicable to the discovery of antibody biomarkers for Alzheimer’s disease in humans. In this study we took advantage of the well-characterized autoimmune response against Aquaporin 4 (AQP4) in neuromyelitis optica (NMO) patients to validate that this technology is clearly applicable to human disease. A screen of 100,000 peptoids using a bead-based, sequential strategy yielded several peptoids that bind antibodies present in NMO patients, but not healthy controls or patients with MS, lupus, Alzheimer’s disease or narcolepsy. At least one of these peptoids binds to anti-AQP4 antibodies. These results confirm the utility of this technology for the identification of serum antibody biomarkers in a human disease.
Experimental Procedures
Serum Screening Protocol Using A One Bead One Compound Peptoid Library
The beads were swelled in DMF overnight, then washed several times with water and kept in water with gentle shaking for 12–15 hour followed by equilibrating in TBST for at least 5 hours before screening.
To pre-screen the library, serum samples from healthy people were used as the control serum to pre-screen the library to eliminate the beads binding to the antibodies present in the healthy serum samples. Six different normal control sera (NC) were pooled in TBST. Enough buffer was added to achieve a final total protein concentration of 100 μg/mL. The beads (200 mg, pre-processed as described above) were incubated with 4 mL of NC serum sample (100 μg/mL) at 4 °C overnight with gentle shaking. The beads were washed three times with TBST and incubated with anti-human secondary antibody conjugated with quantum dot (Qdot 655, Invitrogen; 20 μL in 4 mL TBST) at RT for 2 hours. The beads were washed again three times with TBST and the red beads were removed under a fluorescent microscope to get the denuded library. The remaining beads were washed several times with TBST to screen against the disease serum.
The pre-screened library was incubated with a pool of sera obtained from 6 NMO patients with high levels of anti-AQP4 Ab. The total protein concentration was adjusted to 50 μg/mL total protein in 50 % PBS Starting Block Buffer (Thermo Scientific). The beads were incubated at 4 °C overnight by gentle shaking. After the incubation step the beads were washed three times with TBST and treated with quantum dot-conjugated anti-human secondary antibody (Qdot 655; 20 μL in 4 mL TBST) at RT for 2 hour. The beads were washed again with TBST three times and the beads with a red halo were collected under a fluorescent microscope.
The hit beads were stripped off the antibody by heating in a 1 % SDS solution at 95 °C for 15 minutes and washed extensively with water before validating by rebinding experiments using the same procedure described above
Identification of the Compound
The antibody bound to the hit beads were stripped off by using 1 % SDS solution at 95 °C and the beads were washed extensively with water followed by a 50 % acetonitrile-water mixture. The beads were treated with CNBr (30 mg CNBr in 1 mL cleaving cocktail solution, ACN:H2O:HOAc in the ratio 5:4:1) to cleave the compound from the bead. The structure of the compounds was then determined by tandem MALDI TOF-TOF mass spectrometry.
Antibody Depletion Experiment
Purified AQP4 protein was coupled to an amine-reactive protein immobilization column (MicroLink Protein Coupling Kit, Thermo Scientific) following the manufacturer’s protocol. The protein coupling efficiency was found to be greater than 95 %. Approximately 80–120 μg of protein was immobilized per column. Serum samples were incubated with the AQP4-immobilized column overnight at 4 °C to deplete the anti-AQP4 specific antibodies from the serum. The experiment was repeated twice with the same serum samples to ensure a complete depletion. Similarly BSA immobilized column was used as a control column in this experiment. These depleted serum samples were used in microarray in appropriate dilution.
Microarray Spotting, Hybridization, and Data Analysis
A stock solution (500 μM) of the peptoid was prepared in 50 % DMSO with 50 % PBS, and distributed in 384-well plates. All peptoids were shown to be completely soluble in this spotting solution. Slides were spotted on a NanoPrint LM 60 (TeleChem International Inc., Sunnyvale, CA) with MP946 Micro Spotting Pins. Spots generated were approximately 120-μm in diameter and were printed with a spot-to-spot spacing of 375 μm. The pins were rinsed in between samples using two cycles of wash (for 10s) and sonication (for 10s) in reservoirs containing 10 % ethanol followed by drying under reduced pressure (for 10s). The slides were allowed to stand for at least 2 hours on the printer platform and stored at 4 °C until use. Before incubation with the serum sample, the slides were quenched with 100 mM cysteine in PBS (pH 7.2) for 20 min and washed with deionized water.
The serum sample was diluted in a binding buffer (100 mM phosphate buffer pH 7.2, 150 mM NaCl, 10 mM EDTA) volume of 50 μL containing 0.25 % BSA, 0.01% Tween® 20. The serum sample (4~10 μg/mL in total protein concentration, 50 μL) was applied to the array and this was incubated at room temperature for two hours, washed with 1 × TBST (3 × 10 min), then with deionized water three times and dried by centrifugation. Secondary antibody solution (Alexa Fluor® 647 Goat Anti-Human IgG (H+L), Invitrogen, 1:1500) in TBST with 0.25% BSA, was then applied and the array was incubated at room temperature for 1 hour, washed with 1 × TBST (3 × 10 min), then deionized water three times and dried by centrifugation.
Slides were scanned on a microarray scanner (GenePix 4200AL, Molecular Devices, USA) by using the 488/635 nm laser at 100% power and a 500-photomultiplier-tube gain. All the scanned images were analyzed using GenePix Pro 6.0 (Molecular Devices, USA) software. The experiments were done in triplicate, and each group of three included slides printed in different batches to avoid bias due to batch-to-batch differences in the slides. Local background subtracted mean (F635 Mean - B635) spot intensities were used for further analysis. These signal intensities were used for downstream analysis using Excel software. The same criteria were used to analyze all the test experiments on microarray.
Analysis of Blind Human Serum Samples
The blind human serum samples were analyzed in exactly the same way as described above. Local background subtracted mean (F635 Mean - B635) spot intensities were used as net intensities. For the heat map, net intensities was subtracted by overall F635 Median, and analyzed by TreeView (http://rana.lbl.gov/EisenSoftware.htm) software.
Supplementary Material
Peptoid ligands for anti-AQP4 antibodies that drive the autoimmune disease Neuromyelitis Optica (NMO) have been identified.
Some of these peptoids, when used in combination, provide an accurate blood test for the disease.
Acknowledgments
This study was funded by a contract from the NHLBI for the Stanford Proteomics Center (N01-HV-00242) and grants from the Guthy-Jackson Charitable Foundation and the National Multiple Sclerosis Society (RG4320).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alluri PG, Reddy MM, Bacchawat-Sikder K, Olivos HJ, Kodadek T. Isolation of protein ligands from large peptoid libraries. J Amer Chem Soc. 2003;125:13995–14004. doi: 10.1021/ja036417x. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tan PH, Zhang Y, Pei D. On-bead screening of combinatorial libraries: reduction of nonspecific binding by decreasing surface ligand density. Journal of combinatorial chemistry. 2009;11:604–611. doi: 10.1021/cc9000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. The Journal of biological chemistry. 2011;286:16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figliozzi GM, Goldsmith R, Ng SC, Banville SC, Zuckermann RN. Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol. 1996;267:437–447. doi: 10.1016/s0076-6879(96)67027-x. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, Mayer G, Plazzi G, Nevsimalova S, Bourgin P, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nature genetics. 2009;41:708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin RF, Stafford P, Johnston SA. Exploring antibody recognition of sequence space through random-sequence peptide microarrays. Mol Cell Proteomics. 2011;10:M110 000786. doi: 10.1074/mcp.M110.000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S, Mori M, Okuta A, Kamegawa A, Fujiyoshi Y, Yoshiyama Y, Mitsuoka K, Ishibashi K, Sasaki S, Hattori T, et al. Neuromyelitis optica and anti-aquaporin-4 antibodies measured by an enzyme-linked immunosorbent assay. J Neuroimmunol. 2008;196:181–187. doi: 10.1016/j.jneuroim.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Iorio R, Fryer JP, Hinson SR, Fallier-Becker P, Wolburg H, Pittock SJ, Lennon VA. Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays. J Autoimmun. 2012 doi: 10.1016/j.jaut.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri SR, Illes Z, Srivastava R, Cree B, Menge T, Bennett JL, Berthele A, Hemmer B. Quantification and functional characterization of antibodies to native aquaporin 4 in neuromyelitis optica. Archives of neurology. 2010;67:1201–1208. doi: 10.1001/archneurol.2010.269. [DOI] [PubMed] [Google Scholar]
- Lalive PH, Menge T, Barman I, Cree BA, Genain CP. Identification of new serum autoantibodies in neuromyelitis optica using protein microarrays. Neurology. 2006;67:176–177. doi: 10.1212/01.wnl.0000223346.09426.34. [DOI] [PubMed] [Google Scholar]
- Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- Lau DH, Guo L, Liu R, Song A, Shao C, Lam KS. Identifying peptide ligands for cell surface receptors using cell-growth-on-bead assay and one-bead one-compound combinatorial library. Biotechnology letters. 2002;24:497–500. [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Lesaicherre ML, Mahesh U, Chen GYJ, Yao SQ. Developing site-specific immobilization strategies of peptides in a microarray. Bioorg Med Chem Letters. 2002;12:2079–2083. doi: 10.1016/s0960-894x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- Lim HS, Archer CT, Kodadek T. Identification of a peptoid inhibitor of the proteasome 19S regulatory particle. J Amer Chem Soc. 2007;129:7750–7751. doi: 10.1021/ja072027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa M, Wang H, Guo L, Liu R, Marik J, Takada Y, Lam K, Lau D. Novel peptide ligands for integrin alpha 4 beta 1 overexpressed in cancer cells. Mol Cancer Ther. 2004;3:1329–1334. [PubMed] [Google Scholar]
- Nagele E, Han M, Demarshall C, Belinka B, Nagele R. Diagnosis of Alzheimer’s disease based on disease-specific autoantibody profiles in human sera. PLoS One. 2011;6:e23112. doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman A. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11:535–544. doi: 10.1016/S1474-4422(12)70133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuan PW, Ratelade J, Rossi A, Tradtrantip L, Verkman AS. Complement-dependent Cytotoxicity in Neuromyelitis Optica Requires Aquaporin-4 Protein Assembly in Orthogonal Arrays. The Journal of biological chemistry. 2012;287:13829–13839. doi: 10.1074/jbc.M112.344325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani F, Mastrototaro M, Rossi A, Nicchia GP, Tortorella C, Ruggieri M, Trojano M, Frigeri A, Svelto M. Identification of two major conformational aquaporin-4 epitopes for neuromyelitis optica autoantibody binding. The Journal of biological chemistry. 2011;286:9216–9224. doi: 10.1074/jbc.M110.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Kodadek T. Protein “fingerprinting” in complex mixtures with peptoid microarrays. Proc Natl Acad Sci U S A. 2005;102:12672–12677. doi: 10.1073/pnas.0501208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of candidate IgG biomarkers for Alzheimer’s Disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo L, Stafford P, Magee DM, Johnston SA. Application of immunosignatures to the assessment of Alzheimer’s disease. Ann Neurol. 2011;70:286–295. doi: 10.1002/ana.22405. [DOI] [PubMed] [Google Scholar]
- Robinson WH, Steinman L, Utz PJ. Protein and peptide array analysis of autoimmune disease. Biotechniques. 2002 Dec;(Suppl):66–69. [PubMed] [Google Scholar]
- Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Marlowe CK, et al. Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci U S A. 1992;89:9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. A peptoid “antibody surrogate” that antagonizes VEGF receptor 2 activity. J Amer Chem Soc. 2008;130:5744–5752. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- Uzawa A, Mori M, Hayakawa S, Masuda S, Kuwabara S. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol. 2010;17:672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Yu JQ, Sreekumar A, Varambally S, Shen R, Giachero D, Mehra R, Montie JE, Pienta KJ, Sanda MG, et al. Autoantibody signatures in prostate cancer. New England J Med. 2005;355:16–27. [Google Scholar]
- Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. Int MS J. 2008;15:99–105. [PubMed] [Google Scholar]
- Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, Palace J, Mandrekar JN, Vincent A, Bar-Or A, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78:665–671. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Keefe AD, Szostak JW. The use of mRNA display to select high-affinity protein-binding peptides. Proc Natl Acad Sci USA. 2001;98:3750–3755. doi: 10.1073/pnas.061028198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- Xiao X, Yu P, Lim HS, Sikder D, Kodadek T. Design and synthesis of a cell-permeable synthetic transcription factor mimic. Journal of combinatorial chemistry. 2007;9:592–600. doi: 10.1021/cc070023a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient method for the preparation of peptoids (oligo N-substituted glycines) by submonomer solid-phase synthesis. J Amer Chem Soc. 1992;114:10646–10647. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.