Abstract
Three-dimensional macromolecular structures shed critical light on biological mechanism and facilitate development of small molecule inhibitors. Clinical success of raltegravir, a potent inhibitor of HIV-1 integrase, demonstrated the utility of this viral DNA recombinase as an antiviral target. A variety of partial integrase structures reported in the past 16 years have been instrumental and very informative to the field. Nonetheless, because integrase protein fragments are unable to functionally engage the viral DNA substrate critical for strand transfer inhibitor binding, the early structures did little to materially impact drug development efforts. However, recent results based on prototype foamy virus integrase have fully reversed this trend, as a number of X-ray crystal structures of active integrase-DNA complexes revealed key mechanistic details and moreover established the foundation of HIV-1 IN strand transfer inhibitor action. In this review we discuss the landmarks in the progress of IN structural biology during the past 17 years.
Keywords: HIV-1, Prototype foamy virus, Integrase, Integration, AIDS, Integrase strand transfer inhibitor, Raltegravir, Structural biology, X-ray crystallography
Introduction
The integration of the linear viral DNA (vDNA) made during reverse transcription into a cell chromosome is one of many essential steps in the retroviral lifecycle. Integration is orchestrated by the viral integrase (IN) protein, which recognizes and acts upon the vDNA ends, catalyzing two sequential endonucleolytic reactions. Initially, IN hydrolyzes a phosphodiester at one or both 3′ ends adjacent to invariant CA sequences to unveil reactive adenosine 3′-OH groups. Then, after finding a suitable target site on chromatin in the cell nucleus, IN carries out DNA strand transfer by using the 3′-hydroxyls to cut phosphodiester bonds on opposing strands of target DNA (tDNA) across the major groove with defined spacing, which at the same time joins the vDNA ends to the chromosome. The resulting DNA recombination intermediate, with unjoined vDNA 5′ ends abutting single stranded tDNA gaps, is repaired by host cell machinery to yield the integrated provirus flanked by the sequence duplication of the double stranded tDNA cut. See Engelman (2010) for a recent overview of retroviral DNA integration.
Seminal work in the late 1980s - early 1990s revealed recombinant IN proteins possess divalent metal ion- (Mn2+ or Mg2+) dependent 3′ processing and DNA strand transfer activities in vitro (Bushman et al., 1990; Craigie et al., 1990; Katz et al., 1990; Katzman et al., 1989; Pahl and Flugel, 1993; Sherman and Fyfe, 1990). From this onset it was evident the 288-residue HIV-1 IN was refractory to structural biology approaches due to relatively poor protein solubility, limited at ~1 mg/ml (Hickman et al., 1994; Jenkins et al., 1996). In work designed to test if HIV-1 IN worked as an enzyme, Chow et al. (1992) discovered a novel in vitro function, disintegration, whereby substrates modeling the DNA strand transfer reaction product could be separated into viral and tDNA components. Although disintegration activity is probably not relevant to virus infection, it was a boon for dissecting IN functionality.
Retroviral IN proteins contain three or four sub-domains of variable evolutionary conservation (Fig. 1). The catalytic core domain (CCD) harbors a D,D-35-E amino acid sequence motif conserved among retroviral and retrotransposon INs as well as some bacterial transposase proteins (Engelman and Craigie, 1992; Kulkosky et al., 1992), and the invariant Asp and Glu residues (Asp64, Asp116, and Glu152 for HIV-1) were critical for catalysis of 3′ processing, DNA strand transfer (Drelich et al., 1992; Kulkosky et al., 1992), and disintegration activities (Engelman and Craigie, 1992; Leavitt et al., 1993; van Gent et al., 1992). Isolated CCDs from HIV-1 (Bushman et al., 1993) and avian sarcoma-leukosis virus (ASLV) (Kulkosky et al., 1995) IN proteins lacked appreciable 3’ processing and DNA strand transfer activities, yet importantly were proficient at disintegration. Mixtures of certain defective HIV IN N-terminal domain (NTD) and C-terminal domain (CTD) deletion mutant proteins moreover supported 3′ processing and DNA strand transfer activities, suggesting that the protein likely functioned as a multimer and that individual IN chains could share their domains within the functional complex (Ellison et al., 1995; Engelman et al., 1993; van Gent et al., 1993). Additional protein mixing experiments yielded overall similar domain organizations for Gammaretrovirus (Jonsson et al., 1996) and Spumavirus (Pahl and Flügel, 1995) INs (Fig. 1). Despite frustrations with full-length INs, these studies established the validity of structural approaches of isolated protein domains.
Fig. 1.
Domain organization and secondary structural elements of representative IN proteins. Interdomain linker and C-terminal tail region lengths are indicated above lines; the terminal 38 residues of the ASLV Gag-Pol precursor (parentheses) is cleaved during virus morphogenesis to yield the mature 286-residue IN, though small amounts of the 323-residue protein are detected in virions (Alexander et al., 1987). Secondary structural elements (line, alpha helix; arrow, beta strand) from the HIV-1 intasome model (Krishnan et al., 2010) and ASLV CCD-CTD (Yang et al., 2000) and PFV intasome (Hare et al., 2010a) crystal structures shown underneath each drawing are numbered according to domain (NED, N-terminal extension domain; NTD, N-terminal domain; CCD, catalytic core domain; CTD, C-terminal domain). Domains are color-coded for simplicity; residues conserved among retroviral INs are coded based on chemical property: electronegative, red; electropositive, blue; sulfur-containing, yellow. Proteins alignments based on start of NTD sequences (Jaskolski et al., 2009).
Early analyses of HIV-1 IN activities tended not to distinguish DNA strand transfer reaction products that formed from the integration of a single vDNA end into one strand of tDNA versus the concerted integration of a pair of vDNAs ends into opposing tDNA strands with defined spacing, as occurs during virus infection (Bushman and Craigie, 1991). Reaction modifications that included relatively long vDNA substrates significantly improved concerted HIV-1 IN activity (Faure et al., 2005; Li and Craigie, 2005; Sinha and Grandgenett, 2005; Sinha et al., 2002), important advances for addressing physiologically relevant IN-to-vDNA stoichiometry. The following nomenclature adopts terminology from the bacteriophage Mu transposition field, where earlier work with MuA transposase established DNA cutting and joining reactions analogous to those that occur during retroviral integration and salient nucleoprotein reaction intermediates (reviewed in Chaconas, 1999). A tetramer of IN engages two vDNA ends in an initial stable synaptic complex (SSC), which is converted to the cleaved donor complex (CDC) by 3′ processing. Subsequent tDNA binding yields the target capture complex (TCC), which morphs into the strand transfer complex (STC) after vDNA 3′ end joining (Bera et al., 2009; Kotova et al., 2010; Li et al., 2006; Pandey et al., 2007). These data, combined with prior solution-based measurements of ASLV IN functionality (Bao et al., 2003), helped clarify that an IN tetramer bound to two vDNA ends comprised the functional unit of retroviral integration. This basic nucleoprotein complex is referred to as the intasome (Chen et al., 1999; Hare et al., 2010a; Wei et al., 1997).
To date one HIV-1 IN inhibitor, raltegravir (RAL), has been licensed for patient use (Summa et al., 2008). Because RAL and similar compounds selectively block DNA strand transfer activity, the drugs are collectively known as IN strand transfer inhibitors (INSTIs) (McColl and Chen, 2010). Pipeline INSTIs were shown to bind IN–vDNA complexes with significantly higher affinity than free HIV-1 IN protein (Espeseth et al., 2000; Grobler et al., 2002), indicating that a vDNA-dependent IN conformational change (s) was critical for high affinity binding or the drugs interacted directly with a vDNA component (Alian et al., 2009; McColl and Chen, 2010). Elegant dissection of individual sequence elements highlighted roles for the vDNA end and in particular, the 3′ adenosine, in INSTI binding (Dicker et al., 2007; Langley et al., 2008).
Like any other step of viral replication, integration seems to depend on a plethora of cellular co-factors (reviewed in Turlure et al., 2004 and Van Maele et al., 2006). One HIV-1 IN-binding protein in particular, lens epithelium-derived growth factor (LEDGF) (Cherepanov et al., 2003), has proven central for preferential targeting of lentiviral integration to active transcription units (reviewed in Engelman and Cherepanov, 2008 and Poeschla, 2008). LEDGF interacts with HIV-1 IN via a small evolutionarily-conserved IN binding domain (IBD) (Cherepanov et al., 2004; Vanegas et al., 2005). On the IN side, the CCD is essential and minimally sufficient to bind the host factor, although the NTD is required for high affinity interaction (Maertens et al., 2003). Recombinant LEDGF protein furthermore displayed favorable solubility at isotonic salt concentration and because LEDGF–HIV-1 IN complex solubility mimicked that of LEDGF (P. Cherepanov and A. Engelman, unpublished observations; Busschots et al., 2005), the host factor has become a valuable lentiviral IN structural biology tool (Cherepanov et al., 2005a; Gupta et al., 2010; Hare et al., 2009a; Hare et al., 2009b; Michel et al., 2009).
The start: Individual IN domain structures
Though it became clear the CCD was a more tractable target than full-length HIV-1 IN (Hickman et al., 1994), the isolated domain was relatively insoluble. In an elegant approach, Jenkins et al. discovered the F185K mutation, which dramatically increased solubility (Jenkins et al., 1995) and in turn enabled crystallization and the determination of the X-ray structure of the HIV-1 CCD (Dyda et al., 1994) (Fig. 2A). This landmark study revealed retroviral INs belonged to the large superfamily of polynucleotidyl transferases, typified by RNase H, and established that the invariant carboxylates of the IN D,D-35-E motif indeed comprise the active site (Dyda et al., 1994). The crystallographic asymmetric unit harbored a dimer with an extensive interface, and similar dimeric interfaces have been observed in subsequent crystal structures that contain a retroviral IN CCD (see Table 1 for a comprehensive list of IN structures). The active sites within the dimer were separated from one another by approximately 35 Å, a distance seemingly incompatible with integration across a major groove in tDNA (Dyda et al., 1994), suggesting the active site arrangement in the CCD dimer may not account for the concerted integration of two HIV-1 DNA ends. Recent crystallographic analysis of tetrameric prototype foamy virus (PFV) IN assembled on a pair of vDNA ends confirms this early contention (Hare et al., 2010a) (see below). The polynucleotidyl transferase superfamily has since expanded to include a variety of nucleic acid metabolizing enzymes such as DNA transposase proteins and Argonaute, the nuclease component of the RISC mRNA silencing complex (Nowotny, 2009). Despite its utility to structural biology, the F185K change rendered HIV-1 nonviable due to defects at integration, particle assembly, and reverse transcription (Jenkins et al., 1996). However, substituting Phe185 for His also yielded a crystallizable HIV-1 IN CCD construct (Bujacz et al., 1996a) without associated lethal defects in viral replication (Engelman et al., 1997). Highlighting the importance of Phe185 alterations for structural biology, all CCD-containing HIV-1 IN crystal structures harbor either the F185K or F185H change (Table 1).
Fig. 2.
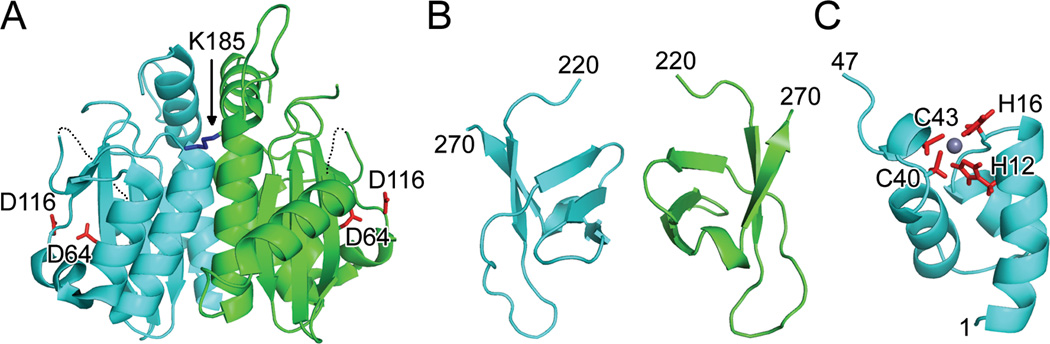
Structures of individual HIV-1 IN domains. (A) X-ray crystal structure (PDB code 1THG) highlighting the common CCD dimer and active site residues Asp64 and Asp116 (red sticks); the third member of the DDE catalytic triad, Glu152, was undecipherable in this initial structure. Only one of two Lys185 solubility-enhancing substitutions (blue stick) is visible in this orientation. Dashed lines indicate disordered gaps in polypeptide chains. (B) CTD NMR structure (PDB code 1IHV) revealed a dimer, with each monomer folded into a five-stranded beta barrel. (C) HIV-1 NTD monomer from PDB code 1WJC. Highlighted are Zn-coordinating residues His12, His16, Cys 40, and Cys43 as well as the bound Zn2+ ion (grey sphere). Visualized IN residue termini are indicated in panels B and C.
Table 1.
Retroviral IN structures reported in the RCSB Protein Data Bank
| Virusa | Domain (s); mutations (s)b | Ligand (s)c | Res.d | PDB codee | Ref.f |
|---|---|---|---|---|---|
| HIV-1 | CCD; F185K | cacodylate | 2.3 | 1ITG | 1 |
| HIV-1 | CTD | NA | 1IHV | 2 | |
| HIV-1 | CCD; F185H | 2.6 | 2ITG | 3 | |
| HIV-1 | NTD | Zn | NA | 1WJC, 1WJD | 4 |
| HIV-1 | NTD | Zn | NA | 1WJA, 1WJB | 4 |
| HIV-1 | NTD; H12C | Cd | NA | 1WJE, 1WJF | 5 |
| HIV-1 | CCD; W131E/F185K | 1.95 | 1BIS | 6 | |
| HIV-1 | CCD; W131E/F185K | Mg | 2.5 | 1BIU | 6 |
| HIV-1 | CCD; C56S/F185K | cacodylate | 1.95 | 1BIZ | 6 |
| HIV-1 | CCD; F185H | Mg | 2.0 | 1BL3 | 7 |
| HIV-1 | CCD; F185H | cacodylate | 2.2 | 1BHL | 7 |
| HIV-1 | CCD; F185H | 2.5 | 1BI4 | 7 | |
| HIV-1 | CCD; F185K | cacodylate | 1.7 | 1B9D | 8 |
| HIV-1 | CCD; G149A/F185K | cacodylate | 2.02 | 1B92 | 8 |
| HIV-1 | CCD; G140A/G149A/F185K | cacodylate | 1.7 | 1B9F | 8 |
| HIV-1 | CCD; W131E/F185K | Mg/5CITEP | 2.1 | 1QS4 | 9 |
| HIV-1 | CTD | NA | 1QMC | 10 | |
| HIV-1 | CCD; C56S/W131D/F139D/F185K | Cd | 1.6 | 1EXQ | 11 |
| HIV-1 | CCD/CTD; C56S/W131D/F139D/F185K/C280S | 2.8 | 1EX4 | 11 | |
| HIV-1 | NTD/CCD; W131D/F139D/F185K | Zn | 2.4 | 1K6Y | 12 |
| HIV-1 | CCD; F185K | TTA | 1.7 | 1HYV | 13 |
| HIV-1 | CCD; F185K | TTO | 2.3 | 1HYZ | 13 |
| HIV-1 | CCD; F185K | LEDGF | 2.02 | 2B4J | 14 |
| HIV-1 | CCD; F185K | Ca/723 | 2.0 | 3LPT | 15 |
| HIV-1 | CCD; F185K | Ca/976 | 1.95 | 3LPU | 15 |
| HIV-1 | CCD; C56S/W131D/W139D/F185H | 1.4 | 3L3U | 16 | |
| HIV-1 | CCD; C56S/W131D/W139D/F185H | Cd/sucrose | 2.0 | 3L3V | 16 |
| HIV-2 | NTD | Zn | NA | 1EOE | 17 |
| HIV-2 | NTD/CCD | Zn/LEDGF/Mg | 3.2 | 3F9K | 18 |
| SIV | CCD/CTD; F185H | 3.0 | 1C6V | 19 | |
| MVV | NTD/CCD | Zn/LEDGF | 3.28 | 3HPG | 20 |
| MVV | NTD/CCD | Zn/LEDGF | 2.64 | 3HPH | 20 |
| BIV | CCD | 2.45 | 3KKR | 21 | |
| BIV | CCD | 2.2 | 3KKS | 21 | |
| ASLV | CCD | HEPES | 1.7 | 1ASU | 22 |
| ASLV | CCD | 2.2 | 1ASLV | 22 | |
| ASLV | CCD | HEPES | 1.8 | 1ASW | 22 |
| ASLV | CCD | Mg/HEPES | 1.7 | 1VSD | 23 |
| ASLV | CCD | HEPES | 2.2 | 1VSE | 23 |
| ASLV | CCD | Mn/HEPES | 2.05 | 1VSF | 23 |
| ASLV | CCD | Zn/HEPES | 1.95 | 1VSH | 24 |
| ASLV | CCD | Ca/HEPES | 2.2 | 1VSI | 24 |
| ASLV | CCD | Cd/HEPES | 2.1 | 1VSJ | 24 |
| ASLV | CCD | Mn/Y3 | 1.9 | 1A5V | 25 |
| ASLV | CCD | Y3 | 2.0 | 1A5W | 25 |
| ASLV | CCD | Y3 | 1.9 | 1A5X | 25 |
| ASLV | CCD; D64N | 2.2 | 1VSK | 26 | |
| ASLV | CCD; D64N | Zn | 2.2 | 1VSL | 26 |
| ASLV | CCD | 2.15 | 1VSM | 26 | |
| ASLV | CCD | HEPES | 1.02 | 1CXQ | 27 |
| ASLV | CCD | 1.42 | 1CXU | 27 | |
| ASLV | CCD; D64N | 1.2 | 1CZ9 | 27 | |
| ASLV | CCD | HEPES | 1.06 | 1CZB | 27 |
| ASLV | CCD/CTD; F199K | 2.53 | 1C0M | 28 | |
| ASLV | CCD/CTD; F199K | 3.1 | 1C1A | 28 | |
| PFV | CCD | Mg | 2.2 | 3DLR | 29 |
| PFV | CCD; I127M/I227M/L253M | Mn | 2.06 | 2X6N | 30 |
| PFV | CCD; I127M/I227M/L253M | Mg | 2.29 | 2X6S | 30 |
| PFV | CCD; I127M/I227M/L253M | 2.34 | 2X74 | 30 | |
| PFV | CCD | 2.0 | 2X78 | 30 | |
| PFV | Full-length | Zn/vDNA | 3.25 | 3L2Q | 31 |
| PFV | Full-length | Zn/vDNA/Mg | 2.88 | 3L2R | 31 |
| PFV | Full-length | Zn/vDNA/Mn | 2.55 | 3OY9 | 31, 32 |
| PFV | Full-length | Zn/vDNA/Mg/RAL | 2.65 | 3OYA | 31, 32 |
| PFV | Full-length | Zn/vDNA/Mg/EVG | 3.15 | 3L2U | 31 |
| PFV | Full-length | Zn/vDNA/Mn/RAL | 3.2 | 3L2V | 31 |
| PFV | Full-length | Zn/vDNA/Mn/EVG | 3.2 | 3L2W | 31 |
| PFV | Full-length | Zn/vDNA/Mg/tDNA | 2.81 | 3OS0 | 33 |
| PFV | Full-length | Zn/vDNA/Mg/tDNA | 2.97 | 3OS1 | 33 |
| PFV | Full-length | Zn/vDNA/Mg/tDNA | 3.32 | 3OS2 | 33 |
| PFV | Full-length | Zn/vDNA/Mg/MK-2048 | 2.54 | 3OYB | 32 |
| PFV | Full-length | Zn/vDNA/Mg/PICA | 2.66 | 3OYC | 32 |
| PFV | Full-length | Zn/vDNA/Mg/GS-9160 | 2.54 | 3OYD | 32 |
| PFV | Full-length | Zn/vDNA/Mg/compound2 | 2.74 | 3OYE | 32 |
| PFV | Full-length | Zn/vDNA/Mg/L,870810 | 2.51 | 3OYF | 32 |
| PFV | Full-length | Zn/vDNA/Mg/compound1 | 2.56 | 3OYG | 32 |
| PFV | Full-length | Zn/vDNA/Mg/MK-0536 | 2.74 | 3OYH | 32 |
| PFV | Full-length; S217Q | Zn/vDNA/Mn | 2.72 | 3OYI | 32 |
| PFV | Full-length; S217Q | Zn/vDNA/Mg/MK-2048 | 2.68 | 3OYJ | 32 |
| PFV | Full-length; S217H | Zn/vDNA/Mn | 2.72 | 3OYK | 32 |
| PFV | Full-length; S217H | Zn/vDNA/Mn/MK-2048 | 2.54 | 3OYL | 32 |
| PFV | Full-length; N224H | Zn/vDNA/Mn | 2.02 | 3OYM | 32 |
| PFV | Full-length; N224H | Zn/vDNA/Mg/MK-2048 | 2.68 | 3OYN | 32 |
HIV-1, human immunodeficiency virus type 1; HIV-2, human immunodeficiency virus type 2; SIV, simian immunodeficiency virus; MVV, maedi-visna virus; BIV, bovine immunodeficiency virus; ASLV, avian sarcoma-leukosis virus; PFV, prototype foamy virus.
CCD, catalytic core domain; NTD, N-terminal domain; CTD, C-terminal domain.
5CITEP, 1-(5-chloroindol-3-yl)-3-hydroxy-3-(2h-tetrazol- 5-yl)-propenone; TTA, tetraphenyl-arsonium; TTO, (3,4-dihydroxy-phenyl)-triphenyl-arsonium; Y3, 4-acetylamino-5-hydroxynaphthalene-2,7-disulfonic acid; 723, (6-chloro-2-oxo-4-phenyl-1,2-dihydroquinolin- 3-yl)acetic acid; 976, (2S)-2-(6-chloro-2-methyl-4-phenylquinolin- 3-yl)pentanoic acid; LEDGF, lens epithelium-derived growth factor; vDNA, viral DNA; RAL, raltegravir; EVG, elvitagravir; tDNA, target DNA.
Res, resolution in Å. NA, not applicable, as structure was solved by NMR.
PDB, protein data base. Two codes are listed for studies that reported collective and minimized average NMR structures.
Ref, references: 1 – Dyda et al., 1994; 2 – Lodi et al., 1995; 3 – Bujacz et al., 1996; 4 – Cai et al., 1997; 5 – Cai et al., 1998; 6 – Goldgur et al., 1998; 7 – Maignan et al., 1998; 8 – Greenwald et al., 1999; 9 – Goldgur et al., 1999; 10 – Eijkelenboom et al., 1999; 11 – Chen, J.C. et al., 2000; 12 – Wang et al., 2001; 13 – Molteni et al., 2001; 14 – Cherepanov et al., 2005a; 15 – Christ et al., 2010; 16 – Wielens et al., 2010; 17 – Eijkelenboom et al., 2000; 18 – Hare et al., 2009b; 19 – Chen, Z. et al., 2000; 20 – Hare et al., 2000a; 21 – Yao et al., 2010; 22 – Bujacz et al., 1995; 23 – Bujacz et al., 1996; 24 – Bujacz et al., 1997; 25 – Lubkowski et al., 1998b; 26 – Lubkowski et al., 1998a; 27 – Lubkowski et al., 1999; 28 – Yang et al., 2000; 29 – Valkov et al., 2009; 30 – Réty et al., 2010; 31 – Hare et al., 2010a; 32 – Hare et al., 2010b; 33 – Maertens et al., 2010.
Work conducted at around the same time elucidated the X-ray structure of the ASLV IN CCD at 1.7 Å resolution (Bujacz et al., 1995) (Table 1). Importantly, all three active site D,D-35-E carboxylates were ordered in this structure. A flurry of additional HIV-1 (Goldgur et al., 1999; Goldgur et al., 1998; Greenwald et al., 1999; Maignan et al., 1998; Molteni et al., 2001) and ASLV (Bujacz et al., 1997; Bujacz et al., 1996b; Lubkowski et al., 1999; Lubkowski et al., 1998a; Lubkowski et al., 1998b) CCD structures quickly followed suit, which in some instances improved resolution and suggested binding sites for preclinical catalytic (Goldgur et al., 1999; Lubkowski et al., 1998b) or allosteric (Molteni et al., 2001) IN inhibitors and/or catalytic metal ion co-factors (Goldgur et al., 1999; Goldgur et al., 1998; Maignan et al., 1998). Though important advances, these studies nonetheless fell short of achieving complete active site pictures because they lacked a critical component, the vDNA end substrate. Crystal structures of PFV (Réty et al., 2010; Valkov et al., 2009) and bovine immunodeficiency virus (BIV) (Yao et al., 2010) IN CCDs have been reported more recently (Table 1).
NTD and CTD structures were initially solved using NMR spectroscopy. The HIV-1 CTD folds into a 5-stranded beta barrel that interestingly resembles a Src homology 3 (SH3) domain (Eijkelenboom et al., 1995; Eijkelenboom et al., 1999; Lodi et al., 1995) (Fig. 2B). The CTD was initially described as a likely DNA-binding domain (Engelman et al., 1994; Vink et al., 1993; Woerner et al., 1992; Woerner and Marcus-Sekura, 1993) and although SH3 domains most often interact with Pro-rich regions in proteins (Kaneko et al., 2008), some, such as Sulfolobus solfatarius Sso7d, are indeed known to bind DNA (Baumann et al., 1994). Solution structures of the NTDs from HIV-1 (Cai et al., 1997) and HIV-2 (Eijkelenboom et al., 1997; Eijkelenboom et al., 2000) INs revealed 3-helical bundles stabilized through binding a Zn2+ ion (Fig. 2C). The metal had been shown to be an important IN co-factor (Lee and Han, 1996; Lee et al., 1997; Zheng et al., 1996) and the structures confirmed that the invariant His and Cys residues (Fig. 1), previously implicated in Zn2+ binding (Burke et al., 1992; Bushman et al., 1993), serve to tetrahedrally coordinate the ion (Fig. 2C). Although the HIV-1 NTD and CTD constructs were dimeric (Cai et al., 1997; Eijkelenboom et al., 1995; Eijkelenboom et al., 1999; Lodi et al., 1995), the dimer interfaces have not been observed in later crystal structures (Chen et al., 2000a; Chen et al., 2000b; Hare et al., 2010a; Yang et al., 2000). Hence, the relevance of such homomeric dimerization among CTD or NTD constructs is not clear.
Structures of 2-domain IN fragments hint at multimer functionality
Having solved the structure of each canonical IN domain in isolation, it behooved investigators to determine how they meshed together within active IN complexes (see Chiu and Davies, 2004 and Jaskolski et al., 2009 for recent reviews). The combination of five solubility-enhancing mutations (C56S, W131D, F139D, F185K, and C280S) enabled full-length HIV-1 IN to be concentrated to >12 mg/ml but did not yield high-quality crystals (Chen, J.C. et al., 2000). Crystals of a CCD-CTD construct containing the aforesaid mutations diffracted to 2.8 Å resolution, and the resulting structure revealed asymmetric alpha helices connecting CTDs to the canonical CCD dimer (Chen, J.C. et al., 2000) (Fig. 3A). Reports of two other CCD-CTD structures at about the same time interestingly revealed strikingly different arrangements among the protein domains. For simian immunodeficiency virus (SIV), a sole CTD could be observed in closer proximity to the CCD dimer (Chen, Z. et al., 2000) (Fig. 3B); due to the lack of CCD-CTD interdomain linker electron density in these crystals, alternative spatial interpretations were possible (Wang et al., 2001). In the case of ASLV CCD-CTD crystals, variable linker connections yielded still different CTD positions (Yang et al., 2000) (Fig. 3C). Comparing the results, it became clear that crystal packing interactions likely impacted interdomain linker flexibility of these construct. Hence, none of the resulting relative domain orientations could be generalized. Consistent with this notion, Arg199, which as part of α6 aligns just 3 residues from the CCD terminus (Fig. 1 and Fig. 3A), became hypersensitive to chemical modification when full-length HIV-1 IN bound vDNA (Zhao et al., 2008). In hindsight, interdomain linker changes that occur upon substrate binding (Hare et al., 2010a; Zhao et al., 2008) likely limited the use of CCD-CTD structures to predict vDNA binding platforms.
Fig. 3.
Two-domain X-ray crystal structures revealed variable CCD-CTD arrangements among retroviral INs. (A) The HIV-1 structure (PDB code 1EX4) unveiled CTDs extending from each monomer within the canonical CCD dimer. Arg199 side chains are indicated by blue stick. (B) SIV structure (PDB code 1C6V) showing a single CTD in closer proximity to its CCD dimer as compared to the HIV-1 structure in panel A. (C) The ASLV 2-domain structure revealed two CTDs off kilter from the 2-fold CCD dimer symmetry axis. The drawing is scaled for similar CCD dimer sizes, each positioned as in Fig. 2A. Red sticks indicate catalytic DDE triads; dashed lines, disordered gaps.
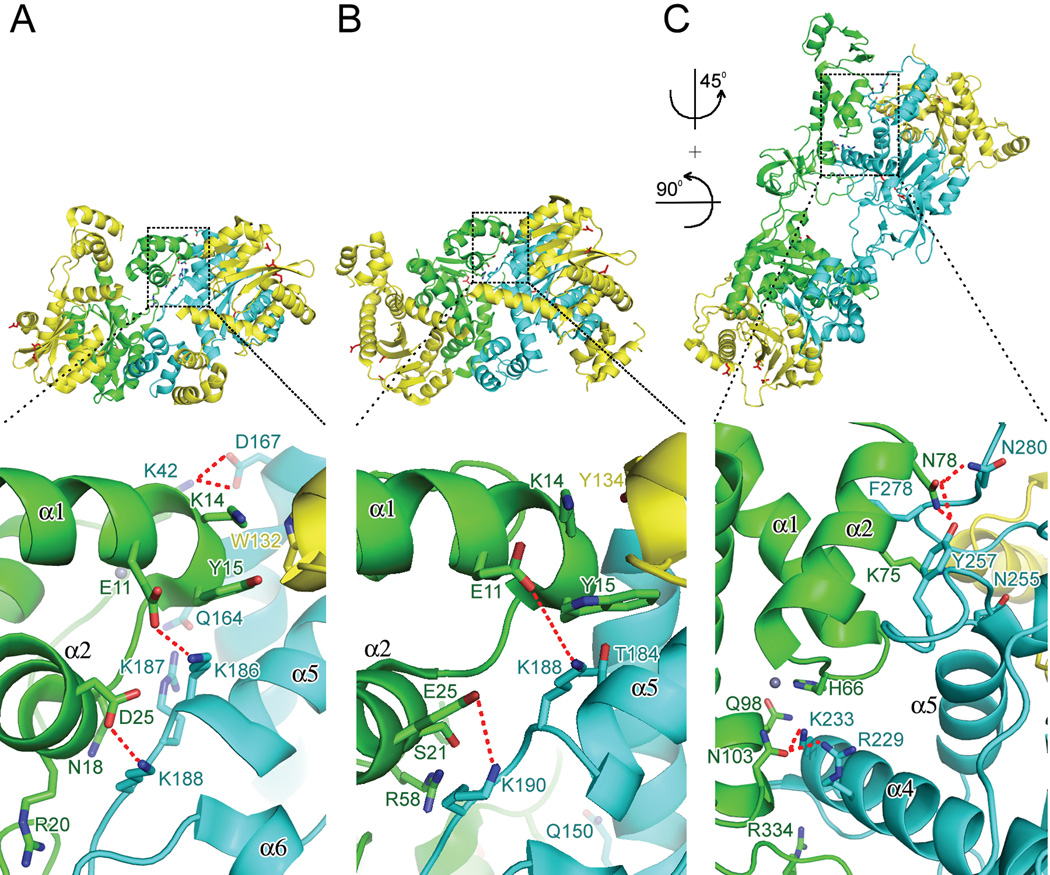
Three solubilizing mutations (W131D, F139D and F185K) enabled NTD-CCD fragment crystallization and structure refinement to 2.4 Å resolution (Fig. 4A) (Wang et al., 2001). Considering growing evidence for the relevance of an IN tetramer, the NTD-CCD crystallographic asymmetric unit interestingly harbored a dimer of dimers (Fig. 4A, green/yellow and cyan/yellow). Due to disorder of the NTD-CCD linkers and packing in these crystals, it was impossible to unambiguously assign which NTD belonged to which CCD (Hare et al., 2009b; Jaskolski et al., 2009). However, the interface between interacting dimers included a pair of NTDs, and the assignment of these NTDs was confirmed by later studies with divergent retroviral INs (Hare et al., 2009a; Hare et al., 2010a). When describing the IN tetramer, it helps to differentiate between two types of structurally and functionally distinct subunits. The inner subunits (colored green and cyan in Fig. 4) interact via intermolecular NTD–CCD interfaces. The outer subunits (yellow) do not have a clear role in tetramer formation, and their function has not been clarified to-date. The interaction between inner and outer subunits within each half of the tetramer is mediated by the canonical CCD–CCD dimerization interface. The key contacts in the NTD–CCD interaction, later confirmed to be important for IN tetramerization (Berthoux et al., 2007; Hare et al., 2009a; McKee et al., 2008), included hydrophobic interactions mediated by NTD residue Tyr15 and salt-bridges between NTD residues Glu11 and Asp25 and CCD residues Lys186 and Lys188, respectively (Fig. 4A, lower panel; see below for further discussion).
Fig. 4.
Intermolecular NTD-CCD interactions among different multidomain IN crystal structures. (A) The dimer of dimers observed in the HIV-1 IN NTD-CCD crystallographic asymmetric unit (PDB code 1K6Y) (Wang et al., 2001). Inner monomers are painted green and cyan; outer monomer (yellow) interactions are intradimer with partners connected via canonical CCD interfaces. Interdimer NTC (green)–CCD (cyan) contact residues are shown in the lower panel, with salt bridges between ionic side chains highlighted by red dashes. Interactions for other highlighted residues are mediated through polypeptide backbone atoms. (B) The MVV IN NTDCCD structure from PDB code 3HPH (Hare et al., 2009a), oriented and labeled as in panel A. LEDGF IBDs were omitted for clarity. (C) The Mn-bound PFV intasome (PDB code 3OYA) (Hare et al., 2010a; Hare et al., 2010b) oriented 45° to the right and 90° into the page with respect to panels A and B to highlight interactions between the green NTD and cyan CCD. DNA molecules were omitted for clarity. Side chains of DDE catalytic triads are shown as red sticks in upper panels; secondary elements in lower panels based on Figure 1; grey spheres, Zn atoms.
Co-crystal structures of IN and LEDGF fragments
The initial NMR structure of the LEDGF IBD revealed a dyad helix-loop-helix PHAT (pseudo HEAT repeat analogous topology) fold (Cherepanov et al., 2005b). Co-crystallization with HIV-1 CCD/F185K revealed the essential portion of the virus-host factor interface (Cherepanov et al., 2005a). The tip of the finger-like IBD structure interacted with a small patch formed at the CCD dimer interface. Symmetry of the dimeric CCD construct allowed binding of two IBD molecules, resulting in IN:LEDGF stoichiometry of 1:1. LEDGF residues Ile365, Asp366, Phe406, and Val408, identified earlier as critical for the interaction with HIV-1 IN (Cherepanov et al., 2005b), were involved in contacts with IN. In particular Asp366 a made bidentate H bond to main chain amides of Glu170 and His171. The structure refined at 2.0 Å resolution played an essential role in the recent design of competitive inhibitors of the HIV-1 IN–LEDGF interaction, which bind to the viral protein by mimicking the H bonding and hydrophobic functions of LEDGF Asp366 and Ile365, respectively (Christ et al., 2010).
More recently, co-crystal structures of NTD-CCD constructs from HIV-2 and MVV INs bound to the LEDGF IBD elucidated what is likely the complete lentiviral IN–LEDGF interface (Hare et al., 2009a; Hare et al., 2009b). The NTD contributes electronegative residues situated on its α1, which interact with a positively charged patch on the side of the IBD structure formed by Lys401, Lys402, and Arg405. Although not essential for the HIV-1 IN–LEDGF interaction, the charge-charge interface makes a substantial difference to its apparent affinity (Hare et al., 2009b). The flexibility of the NTD-CCD connecting regions of the INs allowed the NTD to participate in the interaction with the IBD engaged to the same or another CCD dimer (Hare et al., 2009a; Hare et al., 2009b. The assemblies observed in the MVV structures could be interpreted as dimers-of-dimers, providing composite LEDGF binding platforms consisting of CCDs from one IN dimer and an NTD from the opposing one (Hare et al., 2009a). Because LEDGF stimulates HIV-1 IN tetramerization (Hare et al., 2009a; McKee et al., 2008), it seems likely that the topology observed in the MVV structures is relevant. The simplicity of the NTD–IBD interface moreover yielded reverse charge IN/LEDGF pairs that partially restored wild-type function in in vitro protein binding and enzymatic assays as well as during HIV-1 infection (Hare et al., 2009b). Importantly, IN multimer arrangements in the MVV NTD-CCD structures were similar to those previously observed in the structure of the isolated HIV-1 IN NTD-CCD construct (Wang et al., 2001) and revealed four independent dimer-dimer interfaces. Despite considerable differences in mutual dimer orientations within the tetramers, they were all formed though NTD–CCD interactions across the dimer-dimer interface (Fig. 4B). Results of mutagenesis experiments moreover proved the functional relevance of these intermolecular interactions in in vitro integration assays and during HIV-1 infection (Hare et al., 2009a). These data unveiled the structural basis for prior observations that the HIV-1 IN NTD functioned in trans to the CCD (Ellison et al., 1995; Engelman et al., 1993) and furthermore extended the relevance of these findings to the context of virus infection. Of note the analogous NTD–CCD interactions in the HIV-2 IN NTD-CCD–IBD crystal structure formed intramolecularly, suggesting that different NTDs might sequentially occupy the same niche during functional intasome assembly (Hare et al., 2009b).
The long-awaited breakthrough: X-ray crystal structure of the functional intasome
Applied to structural biology of retroviral integration, the validity of “shotgun” approaches is limited because full-length IN is required to functionally engage vDNA substrates. Moreover, the urgent need for understanding the mode of INSTI action necessitated work with active IN–vDNA complexes (Hare et al., 2010a). X-ray crystallography of active intasomes could only be approached using biochemical systems that supported efficient integration of relatively short oligonucleotide mimics of vDNA ends. Unfortunately, HIV-1 concerted integration assays primarily relied on vDNA substrates of several hundred bp (Carteau et al., 1999; Goodarzi et al., 1995; Hindmarsh et al., 1999; Li and Craigie, 2005; Sinha and Grandgenett, 2005; Sinha et al., 2002), a mystery that remains unexplained today. In principle, HIV-1 IN reactions containing relatively high concentrations of oligonucleotide vDNA substrate support concerted integration in an LEDGF-dependent manner, yet very high levels of single-end integration persist even under optimal in vitro conditions (Hare et al., 2009b). Moloney murine leukemia virus IN integrated short vDNA substrates in concerted fashion with reasonable efficiency (Craigie et al., 1990; Yang and Roth, 2001), though relatively poor solubility dissuaded extensive structural efforts with this protein. Unlike the situation with transposases (Baus et al., 2005; Davies et al., 2000), there seemed to be no trivial way to select for a hyperactive mutant of a retroviral IN. A search for an IN protein that could be naturally more amenable to in vitro experimentation and crystallography eventually lead to the ortholog from PFV. Recombinant PFV IN is remarkably soluble and could be concentrated to over 10 mg/ml in detergent-free buffers containing just 0.2 M NaCl (Delelis et al., 2008; Valkov et al., 2009). Even more impressively, the enzyme almost exclusively integrated short vDNA substrates in concerted fashion in vitro (Valkov et al., 2009). These results set the stage for the ensuing breakthrough.
Intasomes assembled with full-length wild-type PFV IN, Zn2+, and pre-cleaved 19-mer vDNA substrate retained concerted integration activity during prolonged storage in high salt containing buffers (Hare et al., 2010a). A diffracting crystal form of the complex was identified after over 40,000 crystallization trials, and its structure was initially determined at 3.25 Å resolution. The PFV system has rather quickly yielded 22 additional nucleoprotein complex structures that differ from the basic Zn-IN-vDNA intasome through the presence of biologically or pharmacologically-relevant ligands: Mn2+ or Mg2+ catalytic co-factor, tDNA, or INSTIs (Table 1).
In all PFV intasome crystal structures reported so far, the asymmetric unit harbors an asymmetric IN dimer (Fig. 5A, green and yellow) bound to a single vDNA end, with only one of the monomers (green) contacting the DNA. The trace of this molecule was continuous, lacking electron density for just 9 and 18 N- and C-terminal residues, respectively. By contrast only the CCD of the other IN chain was discernable. The asymmetric nature of the dimer invokes comparison to the HIV-1 reverse transcriptase p66/p51 heterodimer, where two subunits adopt different tertiary structures despite harboring similarly folded sub-domains (Jacobo-Molina et al., 1993; Kohlstaedt et al., 1992). Although N-terminal extension domain (NED), NTD, and CTD electron densities were missing for the yellow PFV IN protomer (Fig. 5A), it seems unlikely this subunit would adopt the same overall fold observed for the DNA-bound monomer. The complete intasome is formed by a pair of symmetry related IN-vDNA assemblies (Fig. 5B) (Hare et al., 2010a). The NTDs, CCDs, and CTDs of the inner IN subunits formed intimate protein and DNA contacts within the highly intertwined nucleoprotein complex. The NED, not strictly essential for PFV IN activity in vitro (Pahl and Flügel, 1995; Valkov et al., 2009) and not present in INs from most retroviral genera, is involved in contacts with the vDNA backbone. As expected from earlier analyses of 2-domain structures (Hare et al., 2009a; Wang et al., 2001), the inner monomers of the PFV IN tetramer (green and cyan) harbored the relevant active sites, the side chains of their catalytic triad residues in close proximity to the reactive vDNA 3′-hydroxyl (Fig. 6A). Concordantly, the NTD of each inner monomer interacted in trans with a CCD from the opposing IN dimer (Fig. 4C and 5B). The extended conformation of the DNA-bound IN molecules was entirely novel, differing significantly from previous IN 2-domain structures (compare Fig. 5A with Fig. 3, 4A, and 4B). The architecture of the PFV intasome was accordingly rather different from previous HIV-1 IN tetramer-vDNA models built using predecessor 2-domain structures as template (Chen et al., 2006; Chen et al., 2008; Gao et al., 2001; Michel et al., 2009; Zhao et al., 2008). The familiar CCD dimer interface was maintained in the structure, but occurred between each outlier (yellow) and DNA-bound CCD, verifying that only one active site per canonical CCD dimer was catalytically competent (Fig. 5).
Fig. 5.
Crystal structure of the active PFV intasome. (A) The crystallographic asymmetric unit housed a dimer of IN bound to a single DNA molecule (PDB code 3L2Q) (Hare et al., 2010a). The non-transferred DNA strand is painted orange whereas the transferred strand terminating in dAOH is magenta. (B) The intasome structure, formed by duplication, C2 symmetry rotation, and merger of the panel A structure, with the second DNA-bound monomer painted cyan. Other labeling as in Figures 1 and 3.
Fig. 6.
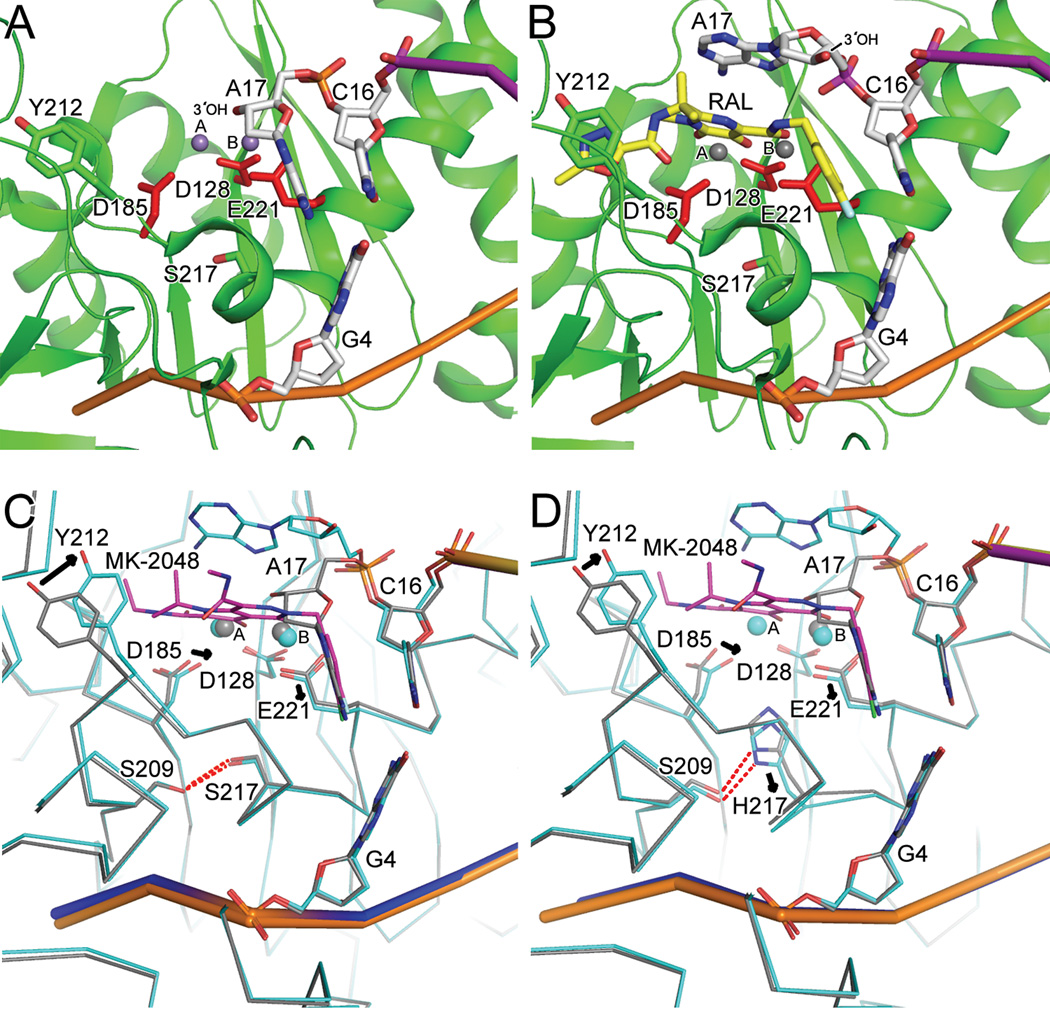
Wild type and S217H mutant intasome structures in committed and drug-bound forms elucidate the mechanism of INSTI action and basis for HIV-1 Q148H/R/K drug resistance. (A) Mn-bound active site (PDB code 3OY9) revealed the coordination of two metal ions by the DDE catalytic triad (see main text for additional details). (B) RAL (yellow scaffold) binding to an induced fit pocket formed through interactions with coordinated metal ion (Mg2+) and the penultimate C16/G4 bp of the vDNA end ejects the terminal adenine nucleotide (A17) and its affiliated 3′-OH nucleophile from the IN active site (PDB code 3OYA). (C) Superposition of wild-type intasome in committed (grey trace; same view as panel A) and MK-2048 (magenta backbone) bound (blue trace; PDB code 3OYB) conformations. (D) Comparison of MK-2048-bound (PDB code 3OYL; blue trace) and unbound S127H mutant (grey trace; PDB code 3OYK) intasome structures revealed significant active site conformational changes (alterations in side chain positions indicated by arrows) elicited by drug binding. Other labeling is same as in Figures 4 and 5.
Soaking PFV intasome crystals in MnCl2 led to visualization of the metal-bound intasomal active site at 2.55 Å resolution (Hare et al., 2010a; Hare et al., 2010b) (Fig. 6A). As predicted from prior work (Nowotny et al., 2005; Steitz and Steitz, 1993), two metal ions were observed at each functional site. Metal ion B, coordinated by the side chains of active site residues Glu221 and Asp128, activates the 3′-OH vDNA nucleophile for DNA strand transfer whereas metal ion A, coordinated by Asp128 and Asp185, would destabilize the scissile tDNA phosphodiester bond (Fig. 6A; see below). Despite lack of detectable sequence homology between Tn5 transposase and PFV IN, their DDE catalytic triads and associated metal ions superimposed remarkably well (Hare et al., 2010a). Mg2+ could also be observed at the PFV intasome active sites after soaking crystals with MgCl2, though only position A was occupied (Hare et al., 2010a; S. Hare and P. Cherepanov, unpublished observations). The presence of scissile phosphodiester bonds during 3′ processing or DNA strand transfer would supply additional ligands to, and predictably increase the affinity of, site B for Mg2+. Concordantly, INSTI-containing structures revealed two coordinated Mg2+ ions per inhibited active site (Hare et al., 2010a; Hare et al., 2010b).
The mechanism of INSTI action based on co-crystal structures with the PFV intasome
Cell infection by PFV as well as PFV IN activity in vitro were importantly sensitive to inhibition by RAL and the related INSTI elvitegravir (EVG) (Valkov et al., 2009), though approximately 10- and greater than 100-fold higher RAL and EVG concentrations, respectively, were required to inhibit 50% of PFV as compared to HIV-1 infection (McColl and Chen, 2010; Vandegraaff and Engelman, 2007). Although INSTI scaffolds are relatively diverse, they share two important chemical features (reviewed in McColl and Chen, 2010). The first is co-planar heteroatoms (predominantly oxygen; otherwise nitrogen) predicted to chelate the crucial divalent metal ion pair in the IN active site (Grobler et al., 2002). The second is halogenated benzyl groups, postulated to bind within a hypothetical hydrophobic pocket that formed upon intasome formation (McColl and Chen, 2010). As predicted, INSTI oxygen atoms interacted intimately with bound metal ions at the IN active site (Hare et al., 2010a; Hare et al., 2010b) (Fig. 6, compare panel B to panel A). Gleaned from the crystal structures, drug halobenzyl groups interacted with the penultimate C/G base pair at the vDNA end, which effectively supplanted the chemical moiety for the base of the vDNA 3′ adenosine and in doing so ejected the nucleotide with its reactive 3′-OH from the active site (Fig. 6B). The ejection of the 3′-OH nucleophile from the active site forms the fundamental basis of INTSI action (Hare et al., 2010a; Hare et al., 2010b).
HIV-1 resistance to RAL arises through one of three clinically-relevant genetic pathways that are named for corresponding HIV-1 IN amino acid substitutions: Y143H/R/C, Q148H/R/K, and N155H (Cooper et al., 2008; reviewed in McColl and Chen, 2010 and Metifiot et al., 2010). Tyr143 in HIV-1 IN is analogous to PFV IN Tyr212 (Valkov et al., 2009). Because the oxadiazole ring in RAL stacks against the phenolic side chain of Tyr212 (Hare et al., 2010a) (Fig. 6B), Y143H/R/C changes likely work by decreasing the affinity of the intasome-RAL interaction through alteration of a direct drug binding contact. PFV IN residues Ser217 and Asn224 correspond to HIV-1 residues Gln148 and Asn155, respectively (Valkov et al., 2009). PFV IN mutant S217Q was viable in vitro and remained sensitive to RAL inhibition whereas S217H IN activity displayed loss of sensitivity to RAL and to a lesser extent to the second-generation INSTI MK-2048 (Hare et al., 2010b). Intasome crystal structures based on wild-type and S217H IN, with and without MK-2048, suggested a mechanism of drug resistance for the predominant RAL resistance Q148H/R/K pathway (Cooper et al., 2008). Ser217 sits at the base of the active site (Fig. 6A–C). The bulky His substituent slightly shifted the position of Asp185, which in turn precluded metal binding to position A (Fig. 6D, grey trace and Mn ion). Binding of MK-2048 to the wild-type intasome induced marginal active site changes, primarily influencing Tyr212 position and to a lesser extent, Asp185 (Fig. 6C, blue trace). By contrast significant changes in S217H IN backbone conformation were observed upon MK-2048 binding: the Cα atom of His217 for example was displaced by as much as 1.1 Å (downward in Fig. 6D), destabilizing the local H-bonding network (Fig. 6D, blue trace). Such a dramatic conformational change is likely to explain lowered drug binding affinity and hence lowered susceptibility of S217H IN to inhibition by MK-2048 and, due to the conserved mode of binding, all other INSTIs (Hare et al., 2010b). The Q148H change in HIV-1 is routinely followed by the secondary G140S mutation, which both restored inherent IN catalytic function and increased RAL resistance (Delelis et al., 2009). The analogous PFV IN residue is naturally serine, and the Ser209 side chain interestingly H-bonded with the mutant His side chain of S217H (Fig. 6D). The G140S change in HIV-1 IN therefore likely increases resistance by constraining the amount of movement allowable for the mutant His148 side chain (Hare et al., 2010b). These observations explain how Gln148 and Gly140 mutations are likely to affect drug resistance despite finding that neither residue directly contacted RAL in a structure-based molecular model of the HIV-1 intasome (Krishnan et al., 2010).
Engagement of tDNA by the intasome and mechanism of DNA strand transfer
Though integration occurs at numerous locations throughout animal cell genomes, it is not entirely random with respect to local DNA sequence at the site of insertion. As examples, HIV-1 preferentially integrates at TDG↓GTWACCHA (Carteau et al., 1998; Holman and Coffin, 2005; Stevens and Griffith, 1996; Wu et al., 2005) whereby PFV favors TD↓VHDBHA (Trobridge et al., 2006; Valkov et al., 2009) (arrows mark scissile phosphodiester bonds; underlines, tDNA duplications – 5 bp for HIV-1 and 4 bp for PFV). Using idealized synthetic tDNA constructs based on the in vitro PFV integration consensus (Valkov et al., 2009), it was possible to co-crystallize the PFV intasome with tDNA (Maertens et al., 2010). Blocking DNA strand transfer by omitting divalent metal cations or by using vDNA lacking the reactive 3′-OH allowed crystallization of the pre-catalytic TCC, while crystals of the post-catalytic STC were obtained in the presence of MgCl2 (Table 1).
The PFV intasome accommodated tDNA in a highly bent conformation, with the major groove widened to 26.3 Å and the minor groove compressed to 9.6 Å at the center of the integration site (Maertens et al., 2010). This deformation allowed the intasomal active sites, separated by as far as 26.5 Å, to access the scissile phosphodiester bonds in the tDNA (Fig. 7A). In the TCC and STC structures, tDNA bending is localized at the central base pair step, with a negative roll of ~60°. Rather impressively, such severe DNA kinking occurs in the absence of direct protein-base stacking interactions. Accounting for the overall aspecific nature of tDNA sequence preference during integration, PFV IN–tDNA base interactions were relatively few. The side chain of CTD residue Arg329 H-bonded with three residues in the expanded major groove whereas CCD residue Ala188 made a van der Waals contact with a minor groove base. Mutations of residues analogous to Ala188 in ASLV (Ser124) and HIV-1 (Ser119) were known to affect phosphodiester bond usage during integration in vitro (Harper et al., 2001; Nowak et al., 2009), and the tDNA signature at the sites of PFV IN mutant A188S integration accordingly differed from the wild-type (Maertens et al., 2010). Target DNA sequence preferences at sites of R329S and R329E mutant IN integration also differed significantly from the wild-type, confirming that the observed side chain–base interactions in the STC crystal structure in large part accounted for the natural sequence preference at sites of PFV integration (Maertens et al., 2010). The crystallographic data also explained the preference for distorted tDNA structures during retroviral integration (Katz et al., 1998; Pruss et al., 1994; Pryciak and Varmus, 1992) and may account for similar preferences among other polynucleotidyl transferase superfamily members (Kuduvalli et al., 2001; Yanagihara and Mizuuchi, 2002).
Fig. 7.

PFV IN TCC and STC crystal structures elucidate the mechanism of DNA strand transfer. (A) The PFV IN TCC structure highlighted the highly bent conformation of bound tDNA (magenta and black strands), with the arrow indicating the spacing between scissile phosphodiester bonds (PDB code 3OS1). Red sticks, DDE side chains. (B) The mechanism of DNA strand transfer elucidated by PFV IN CDC, TCC, and STC overlays (PDB codes 3OY9, 3OS1, and 3OS0, respectively). The dotted line represents the path taken by the adenosine 3′-OH nucleophile during DNA strand transfer; the curved arrow highlights the rotation around the deoxyribose bond that ejects the newly formed vDNA-tDNA phosphodiester bond from the enzyme active site. Color codes are as follows: magenta vDNA, CDC; grey tDNA, TCC; cyan vDNA and black tDNA, STC; green IN protein and DDE side chains, CDC; purple IN ribbon and cyan DDE side chains, STC; grey metal ions, Mn2+ in CDC; black metal ion, position A in STC.
Overlaying metal-bound PFV IN CDC and TCC crystal structures developed static snapshots of the DNA strand transfer reaction mechanism. Metal ion B, coordinated by active site residues Asp128 and Glu221, positioned the vDNA 3′-OH nucleophile for in-line attack of the tDNA scissile phosphodiester bond (Fig. 7B, dotted line). SN2 transesterification reactions like DNA strand transfer are generally reversible, but retroviruses rely on integration for functional gene expression and their inheritance. The mechanistic basis for this apparent paradox was elucidated by visualizing the TCC and STC crystal structures together, as the newly formed vDNA-tDNA phosphodiester bond was displaced from the STC IN active site by 2.3 Å due to an approximate 110° rotation of the corresponding deoxyribose C4-C5 bond (Fig. 7B) (Maertens et al., 2010). The highly distorted nature of bound tDNA likely imparts this dislocation, favoring the forward reaction product after integration.
Conclusions
The approximate 17-year history of retroviral IN structural biology has witnessed piece meal accumulations of structures, starting from individual HIV-1 IN domains and culminating in multiple recent active PFV intasome nucleoprotein complexes. The entire retroviral integration pathway including the initial SSC has now been crystallized (Hare et al., 2010a; Maertens et al., 2010) (S. Hare and P. Cherepanov, unpublished results), revealing unprecedented details of intasome assembly and IN reaction mechanisms. Despite these rather phenomenal advances, several questions in the field of retroviral IN structural biology remain to be answered. For examples, what are the roles of the outer PFV IN domains “missing” in the current structures? We infer these elements are unlikely to interact with vDNA or tDNA, and by extension that they, like the observable outer CCDs, play supportive structural roles in intasome function. A somewhat related question is the physiological relevance of non-DNA bound IN structures. The intasome is comprised of four IN monomers, yet approximately 50 to 100 IN molecules package into each virion (Swanstrom and Wills, 1997). Although the complement of IN molecules in ensuing reverse transcription and preintegration complex replication intermediates is unknown, stoichiometry is likely to exceed the four molecules required for integration. Numerous cellular factors have been shown to interact with IN proteins (Turlure et al., 2004; Van Maele et al., 2006) and some of these, for example karyopherin proteins, could potentially function via interacting with DNA-free IN structures. Also unanswered is the structural role of the IN Cterminal tail (Fig. 1), missing from all structures (Chen, J.C. et al., 2000; Chen, Z. et al., 2000; Hare et al., 2010a; Yang et al., 2000) yet critical for HIV-1 IN function (Dar et al., 2009).
Another important area of research is obtaining structures for additional retroviral intasomes. Though ‘humanized’ versions of PFV IN like S217Q and S217H behaved similarly in vitro to their HIV-1 counterparts, the ability to substitute PFV IN mutants for important HIV-1 IN drug resistance changes will have its limits (Hare et al., 2010b). Given the relative degrees of amino acid homology among IN proteins (Johnson et al., 1986; Engelman and Craigie, 1992; Hare et al., 2009a; Hare et al., 2010a), we anticipate all retroviral intasomes will assume the basic structure elucidated for PFV, but this has yet to be proven. Considerable variation in interdomain linker lengths among IN proteins (Fig. 1) for example suggests potential constraints on assembled quaternary structures. Recent PFV intasome-based constructions of HIV-1 molecular models (Krishnan et al., 2010; Tang et al., 2010) and subsequent validation of novel IN-vDNA contacts (Krishnan et al., 2010) for the time being is consistent with a retroviral family-wide scaffold. Short of solving 3-dimensional structures of active HIV intasomes, such models are expected to significantly impact INSTI development moving forward.
Acknowledgements
This work was supported by UK Medical Research Council Grant G0900116 (to P.C.) and US National Institutes of Health Grant AI70042 (to A.E.). The content of this paper does not necessarily reflect the views or policies of these funding agencies.
References
- Alexander F, Leis J, Soltis DA, Crowl RM, Danho W, Poonian MS, Pan YC, Skalka AM. Proteolytic processing of avian sarcoma and leukosis viruses pol-endo recombinant proteins reveals another pol gene domain. J. Virol. 1987;61:534–542. doi: 10.1128/jvi.61.2.534-542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alian A, Griner SL, Chiang V, Tsiang M, Jones G, Birkus G, Geleziunas R, Leavitt AD, Stroud RM. Catalytically-active complex of HIV-1 integrase with a viral DNA substrate binds anti-integrase drugs. Proc. Natl. Acad. Sci. USA. 2009;106:8192–8197. doi: 10.1073/pnas.0811919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao KK, Wang H, Miller JK, Erie DA, Skalka AM, Wong I. Functional oligomeric state of avian sarcoma virus integrase. J. Biol. Chem. 2003;278:1323–1327. doi: 10.1074/jbc.C200550200. [DOI] [PubMed] [Google Scholar]
- Baumann H, Knapp S, Lundbäck T, Ladenstein R, Härd T. Solution structure and DNA-binding properties of a thermostable protein from the archaeon Sulfolobus solfataricus. Nat. Struct. Biol. 1994;1:808–819. doi: 10.1038/nsb1194-808. [DOI] [PubMed] [Google Scholar]
- Baus J, Liu L, Heggestad AD, Sanz S, Fletcher BS. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol. Ther. 2005;12:1148–1156. doi: 10.1016/j.ymthe.2005.06.484. [DOI] [PubMed] [Google Scholar]
- Bera S, Pandey KK, Vora AC, Grandgenett DP. Molecular Interactions between HIV-1 integrase and the two viral DNA ends within the synaptic complex that mediates concerted integration. J. Mol. Biol. 2009;389:183–198. doi: 10.1016/j.jmb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoux L, Sebastian S, Muesing MA, Luban J. The role of lysine 186 in HIV-1 integrase multimerization. Virology. 2007;364:227–236. doi: 10.1016/j.virol.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujacz G, Alexandratos J, Qing ZL, Clement-Mella C, Wlodawer A. The catalytic domain of human immunodeficiency virus integrase: ordered active site in the F185H mutant. FEBS Lett. 1996a;398:175–178. doi: 10.1016/s0014-5793(96)01236-7. [DOI] [PubMed] [Google Scholar]
- Bujacz G, Alexandratos J, Wlodawer A, Merkel G, Andrake M, Katz RA, Skalka AM. Binding of different divalent cations to the active site of avian sarcoma virus integrase and their effects on enzymatic activity. J. Biol. Chem. 1997;272:18161–18168. doi: 10.1074/jbc.272.29.18161. [DOI] [PubMed] [Google Scholar]
- Bujacz G, Jaskolski M, Alexandratos J, Wlodawer A, Merkel G, Katz RA, Skalka AM. High-resolution structure of the catalytic domain of avian sarcoma virus integrase. J. Mol. Biol. 1995;253:333–346. doi: 10.1006/jmbi.1995.0556. [DOI] [PubMed] [Google Scholar]
- Bujacz G, Jaskólski M, Alexandratos J, Wlodawer A, Merkel G, Katz RA, Skalka AM. The catalytic domain of avian sarcoma virus integrase: conformation of the active-site residues in the presence of divalent cations. Structure. 1996b;4:89–96. doi: 10.1016/s0969-2126(96)00012-3. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Sanyal G, Bruner MW, Ryan JA, LaFemina RL, Robbins HL, Zeft AS, Middaugh CR, Cordingley MG. Structural implications of spectroscopic characterization of a putative zinc finger peptide from HIV-1 integrase. J. Biol. Chem. 1992;267:9639–9644. [PubMed] [Google Scholar]
- Bushman FD, Craigie R. Activities of human immunodeficiency virus (HIV) integration protein in vitro: Specific cleavage and integration of HIV DNA. Proc. Natl. Acad. Sci. USA. 1991;88:1339–1343. doi: 10.1073/pnas.88.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, Engelman A, Palmer I, Wingfield P, Craigie R. Domains of the integrase protein of human immunodeficiency virus type 1 responsible for polynucleotidyl transfer and zinc binding. Proc. Natl. Acad. Sci. USA. 1993;90:3428–3432. doi: 10.1073/pnas.90.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, Fujiwara T, Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990;249:1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- Busschots K, Vercammen J, Emiliani S, Benarous R, Engelborghs Y, Christ F, Debyser Z. The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding. J. Biol. Chem. 2005;280:17841–17847. doi: 10.1074/jbc.M411681200. [DOI] [PubMed] [Google Scholar]
- Cai M, Huang Y, Caffrey M, Zheng R, Craigie R, Clore GM, Gronenborn AM. Solution structure of the His12 --> Cys mutant of the N-terminal zinc binding domain of HIV-1 integrase complexed to cadmium. Protein Sci. 1998;7:2669–2674. doi: 10.1002/pro.5560071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Zheng R, Caffrey M, Craigie R, Clore GM, Gronenborn AM. Solution structure of the N-terminal zinc binding domain of HIV-1 integrase. Nat. Struct. Biol. 1997;4:567–577. doi: 10.1038/nsb0797-567. [DOI] [PubMed] [Google Scholar]
- Carteau S, Gorelick RJ, Bushman FD. Coupled integration of human immunodeficiency virus type 1 cDNA ends by purified integrase in vitro: Stimulation by the viral nucleocapsid protein. J. Virol. 1999;73:6670–6679. doi: 10.1128/jvi.73.8.6670-6679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteau S, Hoffmann C, Bushman F. Chromosome structure and human immunodeficiency virus type 1 cDNA integration: Centromeric alphoid repeats are a disfavored target. J. Virol. 1998;72:4005–4014. doi: 10.1128/jvi.72.5.4005-4014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G. Studies on a "jumping gene machine": Higher-order nucleoprotein complexes in Mu DNA transposition. Biochem. Cell Biol. 1999;77:487–491. [PubMed] [Google Scholar]
- Chen A, Weber IT, Harrison RW, Leis J. Identification of amino acids in HIV-1 and avian sarcoma virus integrase subsites required for specific recognition of the long terminal repeat ends. J. Biol. Chem. 2006;281:4173–4182. doi: 10.1074/jbc.M510628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wei S-Q, Engelman A. Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J. Biol. Chem. 1999;274:17358–17364. doi: 10.1074/jbc.274.24.17358. [DOI] [PubMed] [Google Scholar]
- Chen JC, Krucinski J, Miercke LJW, Finer-Moore JS, Tang AH, Leavitt AD, Stroud RM. Crystal structure of the HIV-1 integrase catalytic core and C-terminal domains: A model for viral DNA binding. Proc. Natl. Acad. Sci. USA. 2000;97:8233–8238. doi: 10.1073/pnas.150220297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tsiang M, Yu F, Hung M, Jones GS, Zeynalzadegan A, Qi X, Jin H, Kim CU, Swaminathan S, Chen JM. Modeling, analysis, and validation of a novel HIV integrase structure provide insights into the binding modes of potent integrase inhibitors. J. Mol. Biol. 2008;380:504–519. doi: 10.1016/j.jmb.2008.04.054. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yan Y, Munshi S, Li Y, Zugay-Murphy J, Xu B, Witmer M, Felock P, Wolfe A, Sardana V. X-ray structure of simian immunodeficiency virus integrase containing the core and C-terminal domain (residues 50–293) - an initial glance of the viral DNA binding platform. J. Mol. Biol. 2000;296:521–533. doi: 10.1006/jmbi.1999.3451. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Ambrosio AL, Rahman S, Ellenberger T, Engelman A. Structural basis for the recognition between HIV-1 integrase and transcriptional coactivator p75. Proc. Natl. Acad. Sci. USA. 2005a;102:17308–17313. doi: 10.1073/pnas.0506924102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov P, Devroe E, Silver PA, Engelman A. Identification of an evolutionarily-conserved domain in LEDGF/p75 that binds HIV-1 integrase. J. Biol. Chem. 2004;279:48883–48892. doi: 10.1074/jbc.M406307200. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Sun Z-YJ, Rahman S, Maertens G, Wagner G, Engelman A. Solution structure of the HIV-1 integrase-binding domain in LEDGF/p75. Nat. Struct. Mol. Biol. 2005b;12:526–532. doi: 10.1038/nsmb937. [DOI] [PubMed] [Google Scholar]
- Chiu TK, Davies DR. Structure and function of HIV-1 integrase. Curr. Top. Med. Chem. 2004;4:965–977. doi: 10.2174/1568026043388547. [DOI] [PubMed] [Google Scholar]
- Chow SA, Vincent KA, Ellison V, Brown PO. Reversal of integration and DNA splicing mediated by integrase of human immunodeficiency virus. Science. 1992;255:723–726. doi: 10.1126/science.1738845. [DOI] [PubMed] [Google Scholar]
- Christ F, Voet A, Marchand A, Nicolet S, Desimmie BA, Marchand D, Bardiot D, Van der Veken NJ, Van Remoortel B, Strelkov SV, De Maeyer M, Chaltin P, Debyser Z. Rational design of small-molecule inhibitors of the LEDGF/p75-integrase interaction and HIV replication. Nat. Chem. Biol. 2010;6:442–448. doi: 10.1038/nchembio.370. [DOI] [PubMed] [Google Scholar]
- Cooper DA, Steigbigel RT, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Teppler H, Nguyen B-Y the BENCHMRK Study Teams. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 2008;359:355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- Craigie R, Fujiwara T, Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990;62:829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- Dar MJ, Monel B, Krishnan L, Shun MC, Di Nunzio F, Helland DE, Engelman A. Biochemical and virological analysis of the 18-residue C-terminal tail of HIV-1 integrase. Retrovirology. 2009;6:94. doi: 10.1186/1742-4690-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DR, Goryshin IY, Reznikoff WS, Rayment I. Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. Science. 2000;289:77–85. doi: 10.1126/science.289.5476.77. [DOI] [PubMed] [Google Scholar]
- Delelis O, Carayon K, Guiot E, Leh H, Tauc P, Brochon JC, Mouscadet JF, Deprez E. Insight into the integrase-DNA recognition mechanism. A specific DNA-binding mode revealed by an enzymatically labeled integrase. J. Biol. Chem. 2008;283:27838–27849. doi: 10.1074/jbc.M803257200. [DOI] [PubMed] [Google Scholar]
- Delelis O, Malet I, Na L, Tchertanov L, Calvez V, Marcelin A-G, Subra F, Deprez E, Mouscadet J-F. The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation. Nucleic Acids Res. 2009;37:1193–1201. doi: 10.1093/nar/gkn1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker IB, Samanta HK, Li Z, Hong Y, Tian Y, Banville J, Remillard RR, Walker MA, Langley DR, Krystal M. Changes to the HIV long terminal repeat and to HIV integrase differentially impact HIV integrase assembly, activity, and the binding of strand transfer inhibitors. J. Biol. Chem. 2007;282:31186–31196. doi: 10.1074/jbc.M704935200. [DOI] [PubMed] [Google Scholar]
- Drelich M, Wilhelm R, Mous J. Identification of amino acid residues critical for endonuclease and integration activities of HIV-1 IN protein in vitro. Virology. 1992;188:459–468. doi: 10.1016/0042-6822(92)90499-f. [DOI] [PubMed] [Google Scholar]
- Dyda F, Hickman AB, Jenkins TM, Engelman A, Craigie R, Davies DR. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. Science. 1994;266:1981–1986. doi: 10.1126/science.7801124. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom AP, van den Ent FM, Vos A, Doreleijers JF, Hard K, Tullius TD, Plasterk RH, Kaptein R, Boelens R. The solution structure of the amino-terminal HHCC domain of HIV-2 integrase: a three-helix bundle stabilized by zinc. Curr. Biol. 1997;7:739–746. doi: 10.1016/s0960-9822(06)00332-0. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom AP, van den Ent FM, Wechselberger R, Plasterk RH, Kaptein R, Boelens R. Refined solution structure of the dimeric N-terminal HHCC domain of HIV-2 integrase. J. Biomol. NMR. 2000;18:119–128. doi: 10.1023/a:1008342312269. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom AP, Puras Lutzke RA, Boelens R, Plasterk RH, Kaptein R, Hård K. The DNA-binding domain of HIV-1 integrase has an SH3-like fold. Nat. Struct. Biol. 1995;2:807–810. doi: 10.1038/nsb0995-807. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom AP, Sprangers R, Hård K, Puras Lutzke RA, Plasterk RH, Boelens R, Kaptein R. Refined solution structure of the C-terminal DNA-binding domain of human immunovirus-1 integrase. Proteins. 1999;36:556–564. doi: 10.1002/(sici)1097-0134(19990901)36:4<556::aid-prot18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ellison V, Gerton J, Vincent KA, Brown PO. An essential interaction between distinct domains of HIV-1 integrase mediates assembly of the active multimer. J. Biol. Chem. 1995;270:3320–3326. doi: 10.1074/jbc.270.7.3320. [DOI] [PubMed] [Google Scholar]
- Engelman A. Reverse transcription and integration. In: Kurth R, Bannert N, editors. Retroviruses: Molecular Biology, Genomics and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 129–159. [Google Scholar]
- Engelman A, Bushman FD, Craigie R. Identification of discrete functional domains of HIV-1 integrase and their organization within an active multimeric complex. EMBO J. 1993;12:3269–3275. doi: 10.1002/j.1460-2075.1993.tb05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Cherepanov P. The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication. PLoS Pathog. 2008;4:e1000046. doi: 10.1371/journal.ppat.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Craigie R. Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J. Virol. 1992;66:6361–6369. doi: 10.1128/jvi.66.11.6361-6369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Hickman AB, Craigie R. The core and carboxyl-terminal domains of the integrase protein of human immunodeficiency virus type 1 each contribute to nonspecific DNA binding. J. Virol. 1994;68:5911–5917. doi: 10.1128/jvi.68.9.5911-5917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Liu Y, Chen H, Farzan M, Dyda F. Structure-based mutagenesis of the catalytic domain of human immunodeficiency virus type 1 integrase. J. Virol. 1997;71:3507–3514. doi: 10.1128/jvi.71.5.3507-3514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth AS, Felock P, Wolfe A, Witmer M, Grobler J, Anthony N, Egbertson M, Melamed JY, Young S, Hamill T, Cole JL, Hazuda DJ. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc. Natl. Acad. Sci. USA. 2000;97:11244–11249. doi: 10.1073/pnas.200139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Calmels C, Desjobert C, Castroviejo M, Caumont-Sarcos A, Tarrago-Litvak L, Litvak S, Parissi V. HIV-1 integrase crosslinked oligomers are active in vitro. Nucleic Acids Res. 2005;33:977–986. doi: 10.1093/nar/gki241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Butler SL, Bushman F. Human immunodeficiency virus type 1 integrase: arrangement of protein domains in active cDNA complexes. EMBO J. 2001;20:3565–3576. doi: 10.1093/emboj/20.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgur Y, Craigie R, Cohen GH, Fujiwara T, Yoshinaga T, Fujishita T, Sugimoto H, Endo T, Murai H, Davies DR. Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: a platform for antiviral drug design. Proc. Natl. Acad. Sci. USA. 1999;96:13040–13043. doi: 10.1073/pnas.96.23.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgur Y, Dyda F, Hickman AB, Jenkins TM, Craigie R, Davies DR. Three new structures of the core domain of HIV-1 integrase: An active site that binds magnesium. Proc. Natl. Acad. Sci. USA. 1998;95:9150–9154. doi: 10.1073/pnas.95.16.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi G, Im GJ, Brackmann K, Grandgenett D. Concerted integration of retrovirus-like DNA by human immunodeficiency virus type 1 integrase. J. Virol. 1995;69:6090–6097. doi: 10.1128/jvi.69.10.6090-6097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald J, Le V, Butler SL, Bushman FD, Choe S. The mobility of an HIV-1 integrase active site loop is correlated with catalytic activity. Biochemistry. 1999;38:8892–8898. doi: 10.1021/bi9907173. [DOI] [PubMed] [Google Scholar]
- Grobler JA, Stillmock K, Hu B, Witmer M, Felock P, Espeseth AS, Wolfe A, Egbertson M, Bourgeois M, Melamed J, Wai JS, Young S, Vacca J, Hazuda DJ. Diketo acid inhibitor mechanism and HIV-1 integrase: Implications for metal binding in the active site of phosphotransferase enzymes. Proc. Natl. Acad. Sci. USA. 2002;99:6661–6666. doi: 10.1073/pnas.092056199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Diamond T, Hwang Y, Bushman F, Van Duyne GD. Structural properties of HIV integrase. Lens epithelium-derived growth factor oligomers. J. Biol. Chem. 2010;285:20303–20315. doi: 10.1074/jbc.M110.114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Di Nunzio F, Labeja A, Wang J, Engelman A, Cherepanov P. Structural basis for functional tetramerization of lentiviral integrase. PLoS Pathog. 2009a;5:e1000515. doi: 10.1371/journal.ppat.1000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010a;464:232–236. doi: 10.1038/nature08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Shun MC, Gupta SS, Valkov E, Engelman A, Cherepanov P. A novel co-crystal structure affords the design of gain-of-function lentiviral integrase mutants in the presence of modified PSIP1/LEDGF/p75. PLoS Pathog. 2009b;5:e1000259. doi: 10.1371/journal.ppat.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD, Cherepanov P, et al. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc. Natl. Acad. Sci. USA. 2010b;107 doi: 10.1073/pnas.1010246107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AL, Skinner LM, Sudol M, Katzman M. Use of patient-derived human immunodeficiency virus type 1 integrases to identify a protein residue that affects target site selection. J. Virol. 2001;75:7756–7762. doi: 10.1128/JVI.75.16.7756-7762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman AB, Palmer I, Engelman A, Craigie R, Wingfield P. Biophysical and enzymatic properties of the catalytic domain of HIV-1 integrase. J. Biol. Chem. 1994;269:29279–29287. [PubMed] [Google Scholar]
- Hindmarsh P, Ridky T, Reeves R, Andrake M, Skalka AM, Leis J. HMG protein family members stimulate human immunodeficiency virus type 1 and avian sarcoma virus concerted DNA integration in vitro. J. Virol. 1999;73:2994–3003. doi: 10.1128/jvi.73.4.2994-3003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman AG, Coffin JM. Symmetrical base preferences surrounding HIV-1, avian sarcoma/leukosis virus, and murine leukemia virus integration sites. Proc. Natl. Acad. Sci. USA. 2005;102:6103–6107. doi: 10.1073/pnas.0501646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Molina A, Ding J, Nanni RG, Clark AD, Jr, Lu X, Tantillo C, Williams RL, Kamer G, Ferris AL, Clark P. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. USA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskolski M, Alexandratos JN, Bujacz G, Wlodawer A. Piecing together the structure of retroviral integrase, an important target in AIDS therapy. FEBS J. 2009;276:2926–2946. doi: 10.1111/j.1742-4658.2009.07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TM, Hickman AB, Dyda F, Ghirlando R, Davies DR, Craigie R. Catalytic domain of human immunodeficiency virus type 1 integrase: Identification of a soluble mutant by systematic replacement of hydrophobic residues. Proc. Natl. Acad. Sci. USA. 1995;92:6057–6061. doi: 10.1073/pnas.92.13.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TM, Engelman A, Ghirlando R, Craigie R. A soluble active mutant of HIV-1 integrase: involvement of both the core and the C-terminal domains in multimerization. J. Biol. Chem. 1996;271:7712–7718. doi: 10.1074/jbc.271.13.7712. [DOI] [PubMed] [Google Scholar]
- Johnson MS, McClure MA, Feng DF, Gray J, Doolittle RF. Computer analysis of retroviral pol genes: Assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc. Natl. Acad. Sci. USA. 1986;83:7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson C, Donzella G, Gaucan E, Smith C, Roth M. Functional domains of Moloney murine leukemia virus integrase defined by mutation and complementation analysis. J. Virol. 1996;70:4585–4597. doi: 10.1128/jvi.70.7.4585-4597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Li L, Li SS. The SH3 domain--a family of versatile peptide- and protein-recognition module. Front. Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- Katz RA, Gravuer K, Skalka AM. A preferred target DNA structure for retroviral integrase in vitro. J. Biol. Chem. 1998;273:24190–24195. doi: 10.1074/jbc.273.37.24190. [DOI] [PubMed] [Google Scholar]
- Katz RA, Merkel G, Kulkosky J, Leis J, Skalka AM. The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell. 1990;63:87–95. doi: 10.1016/0092-8674(90)90290-u. [DOI] [PubMed] [Google Scholar]
- Katzman M, Katz RA, Skalka AM, Leis J. The avian retroviral integration protein cleaves the terminal sequences of linear viral DNA at the in vivo sites of integration. J. Virol. 1989;63:5319–5327. doi: 10.1128/jvi.63.12.5319-5327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Kotova S, Li M, Dimitriadis EK, Craigie R. Nucleoprotein intermediates in HIV-1 DNA integration visualized by atomic force microscopy. J. Mol. Biol. 2010;399:491–500. doi: 10.1016/j.jmb.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L, Li X, Naraharisetty HL, Hare S, Cherepanov P, Engelman A. Structure-based modeling of the functional HIV-1 intasome and its inhibition. Proc. Natl. Acad. Sci. USA. 2010;107:15010–15915. doi: 10.1073/pnas.1002346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuduvalli PN, Rao JE, Craig NL. Target DNA structure plays a critical role in Tn7 transposition. EMBO J. 2001;20:924–932. doi: 10.1093/emboj/20.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J, Jones KS, Katz RA, Mack JP, Skalka AM. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol. Cell. Biol. 1992;12:2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J, Katz RA, Merkel G, Skalka AM. Activities and substrate specificity of the evolutionarily conserved central domain of retroviral integrase. Virology. 1995;206:448–456. doi: 10.1016/s0042-6822(95)80060-3. [DOI] [PubMed] [Google Scholar]
- Langley DR, Samanta HK, Lin Z, Walker MA, Krystal MR, Dicker IB. The terminal (catalytic) adenosine of the HIV LTR controls the kinetics of binding and dissociation of HIV integrase strand transfer inhibitors. Biochemistry. 2008;47:13481–13488. doi: 10.1021/bi801372d. [DOI] [PubMed] [Google Scholar]
- Leavitt AD, Shiue L, Varmus HE. Site-directed mutagenesis of HIV-1 integrase demonstrates differential effects on integrase functions in vitro. J. Biol. Chem. 1993;268:2113–2119. [PubMed] [Google Scholar]
- Lee SP, Han MK. Zinc stimulates Mg2+-dependent 3′-processing activity of human immunodeficiency virus type 1 integrase in vitro. Biochemistry. 1996;35:3837–3844. doi: 10.1021/bi952056p. [DOI] [PubMed] [Google Scholar]
- Lee SP, Xiao J, Knutson JR, Lewis MS, Han MK. Zn2+ promotes the self-association of human immunodeficiency virus type-1 integrase in vitro. Biochemistry. 1997;36:173–180. doi: 10.1021/bi961849o. [DOI] [PubMed] [Google Scholar]
- Li M, Craigie R. Processing of viral DNA ends channels the HIV-1 integration reaction to concerted integration. J. Biol. Chem. 2005;280:29334–29339. doi: 10.1074/jbc.M505367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mizuuchi M, Burke TR, Craigie R. Retroviral DNA integration: reaction pathway and critical intermediates. EMBO J. 2006;25:1295–1304. doi: 10.1038/sj.emboj.7601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi PJ, Ernst JA, Kuszewski J, Hickman AB, Engelman A, Craigie R, Clore GM, Gronenborn AM. Solution structure of the DNA binding domain of HIV-1 integrase. Biochemistry. 1995;34:9826–9833. doi: 10.1021/bi00031a002. [DOI] [PubMed] [Google Scholar]
- Lubkowski J, Dauter Z, Yang F, Alexandratos J, Merkel G, Skalka AM, Wlodawer A. Atomic resolution structures of the core domain of avian sarcoma virus integrase and its D64N mutant. Biochemistry. 1999;38:13512–13522. doi: 10.1021/bi991362q. [DOI] [PubMed] [Google Scholar]
- Lubkowski J, Yang F, Alexandratos J, Merkel G, Katz RA, Gravuer K, Skalka AM, Wlodawer A, et al. Structural basis for inactivating mutations and pH-dependent activity of avian sarcoma virus integrase. J. Biol. Chem. 1998a;273:32685–32689. doi: 10.1074/jbc.273.49.32685. [DOI] [PubMed] [Google Scholar]
- Lubkowski J, Yang F, Alexandratos J, Wlodawer A, Zhao H, Burke TR, Neamati N, Pommier Y, Merkel G, Skalka AM. Structure of the catalytic domain of avian sarcoma virus integrase with a bound HIV-1 integrase-targeted inhibitor. Proc. Natl. Acad. Sci. USA. 1998b;95:4831–4836. doi: 10.1073/pnas.95.9.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens G, Cherepanov P, Pluymers W, Busschots K, De Clercq E, Debyser Z, Engelborghs Y. LEDGF/p75 Is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J. Biol. Chem. 2003;278:33528–33539. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- Maertens GN, Hare S, Cherepanov P. The mechanism of retroviral integration through X-ray structures of its key intermediates. Nature. 2010;468:326–329. doi: 10.1038/nature09517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maignan S, Guilloteau JP, Zhou-Liu Q, Clement-Mella C, Mikol V. Crystal structures of the catalytic domain of HIV-1 integrase free and complexed with its metal cofactor: high level of similarity of the active site with other viral integrases. J. Mol. Biol. 1998;282:359–368. doi: 10.1006/jmbi.1998.2002. [DOI] [PubMed] [Google Scholar]
- McColl DJ, Chen X. Strand transfer inhibitors of HIV-1 integrase: Bringing IN a new era of antiretroviral therapy. Antiviral Res. 2010;85:101–118. doi: 10.1016/j.antiviral.2009.11.004. [DOI] [PubMed] [Google Scholar]
- McKee CJ, Kessl JJ, Shkriabai N, Dar MJ, Engelman A, Kvaratskhelia M. Dynamic modulation of HIV-1 integrase structure and function by cellular lens epithelium-derived growth factor (LEDGF) protein. J. Biol. Chem. 2008;283:31802–31812. doi: 10.1074/jbc.M805843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metifiot M, Marchand C, Maddali K, Pommier Y. Resistance to integrase inhibitors. Viruses. 2010;2:1347–1366. doi: 10.3390/v2071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F, Crucifix C, Granger F, Eiler S, Mouscadet JF, Korolev S, Agapkina J, Ziganshin R, Gottikh M, Nazabal A, Emiliani S, Benarous R, Moras D, Schultz P, Ruff M. Structural basis for HIV-1 DNA integration in the human genome, role of the LEDGF/P75 cofactor. EMBO J. 2009;28:980–91. doi: 10.1038/emboj.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni V, Greenwald J, Rhodes D, Hwang Y, Kwiatkowski W, Bushman FD, Siegel JS, Choe S. Identification of a small-molecule binding site at the dimer interface of the HIV integrase catalytic domain. Acta Crystallogr. D Biol. Crystallogr. 2001;57(Pt 4):536–544. doi: 10.1107/s0907444901001652. [DOI] [PubMed] [Google Scholar]
- Nowak MG, Sudol M, Lee NE, Konsavage WM, Katzman M. Identifying amino acid residues that contribute to the cellular-DNA binding site on retroviral integrase. Virology. 2009;389:141–148. doi: 10.1016/j.virol.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Nowotny M. Retroviral integrase superfamily: the structural perspective. EMBO Rep. 2009;10:144–151. doi: 10.1038/embor.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–1016. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pahl A, Flügel RM. Endonucleolytic cleavages and DNA-joining activities of the integration protein of human foamy virus. J. Virol. 1993;67:5426–5434. doi: 10.1128/jvi.67.9.5426-5434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl A, Flügel RM. Characterization of the human spuma retrovirus integrase by site-directed mutagenesis, by complementation analysis, and by swapping the zinc finger domain of HIV-1. J. Biol. Chem. 1995;270:2957–2966. doi: 10.1074/jbc.270.7.2957. [DOI] [PubMed] [Google Scholar]
- Pandey KK, Bera S, Zahm J, Vora A, Stillmock K, Hazuda D, Grandgenett DP. Inhibition of human immunodeficiency virus type 1 concerted integration by strand transfer inhibitors which recognize a transient structural intermediate. J. Virol. 2007;81:12189–12199. doi: 10.1128/JVI.02863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla EM. Integrase, LEDGF/p75 and HIV replication. Cell. Mol. Life Sci. 2008;65:1403–1424. doi: 10.1007/s00018-008-7540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss D, Bushman F, Wolffe A. Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. Proc. Natl. Acad. Sci. USA. 1994;91:5913–5917. doi: 10.1073/pnas.91.13.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryciak PM, Varmus HE. Nucleosomes, DNA-binding proteins, and DNA sequence modulate retroviral integration target site selection. Cell. 1992;69:769–780. doi: 10.1016/0092-8674(92)90289-o. [DOI] [PubMed] [Google Scholar]
- Réty S, Reaeábková L, Dubanchet B, Silhán J, Legrand P, Lewit-Bentley A. Structural studies of the catalytic core of the primate foamy virus (PFV-1) integrase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010;66:881–886. doi: 10.1107/S1744309110022852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman PA, Fyfe JA. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proc. Natl. Acad. Sci. USA. 1990;87:5119–5123. doi: 10.1073/pnas.87.13.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Grandgenett DP. Recombinant human immunodeficiency virus type 1 integrase exhibits a capacity for full-site integration in vitro that is comparable to that of purified preintegration complexes from virus-infected cells. J. Virol. 2005;79:8208–8216. doi: 10.1128/JVI.79.13.8208-8216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Pursley MH, Grandgenett DP. Efficient concerted integration by recombinant human immunodeficiency virus type 1 integrase without cellular or viral cofactors. J. Virol. 2002;76:3105–3113. doi: 10.1128/JVI.76.7.3105-3113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. USA. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SW, Griffith JD. Sequence analysis of the human DNA flanking sites of human immunodeficiency virus type 1 integration. J. Virol. 1996;70:6459–6462. doi: 10.1128/jvi.70.9.6459-6462.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa V, Petrocchi A, Bonelli F, Crescenzi B, Donghi M, Ferrara M, Fiore F, Gardelli C, Gonzalez Paz O, Hazuda DJ, Jones P, Kinzel O, Laufer R, Monteagudo E, Muraglia E, Nizi E, Orvieto F, Pace P, Pescatore G, Scarpelli R, Stillmock K, Witmer MV, Rowley M. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008;51:5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- Swanstrom R, Wills JW. Synthesis, assembly, and processing of viral proteins. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview, New York: Cold Spring Harbor Laboratory Press; 1997. pp. 263–334. [PubMed] [Google Scholar]
- Tang J, Maddali K, Pommier Y, Sham YY, Wang Z. Scaffold rearrangement of dihydroxypyrimidine inhibitors of HIV integrase: Docking model revisited. Bioorg. Med. Chem. Lett. 2010;20:3275–3279. doi: 10.1016/j.bmcl.2010.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Miller DG, Jacobs MA, Allen JM, Kiem H-P, Kaul R, Russell DW. Foamy virus vector integration sites in normal human cells. Proc. Natl. Acad. Sci. USA. 2006;103:1498–1503. doi: 10.1073/pnas.0510046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlure F, Devroe E, Silver PA, Engelman A. Human cell proteins and human immunodeficiency virus DNA integration. Front. Biosci. 2004;9:3187–3208. doi: 10.2741/1472. [DOI] [PubMed] [Google Scholar]
- Valkov E, Gupta SS, Hare S, Helander A, Roversi P, McClure M, Cherepanov P. Functional and structural characterization of the integrase from the prototype foamy virus. Nucleic Acids Res. 2009;37:243–255. doi: 10.1093/nar/gkn938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent DC, Groeneger AA, Plasterk RH. Mutational analysis of the integrase protein of human immunodeficiency virus type 2. Proc. Natl. Acad. Sci. USA. 1992;89:9598–9602. doi: 10.1073/pnas.89.20.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent DC, Vink C, Groeneger AA, Plasterk RH. Complementation between HIV integrase proteins mutated in different domains. EMBO J. 1993;12:3261–3267. doi: 10.1002/j.1460-2075.1993.tb05995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele B, Busschots K, Vandekerckhove L, Christ F, Debyser Z. Cellular cofactors of HIV-1 integration. Trends Biochem. Sci. 2006;31:98–105. doi: 10.1016/j.tibs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Vandegraaff N, Engelman A. Molecular mechanism of HIV integration and therapeutic intervention. Expert Rev. Mol. Med. 2007;9:1–19. doi: 10.1017/S1462399407000257. [DOI] [PubMed] [Google Scholar]
- Vanegas M, Llano M, Delgado S, Thompson D, Peretz M, Poeschla E. Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering. J. Cell Sci. 2005;118:1733–1743. doi: 10.1242/jcs.02299. [DOI] [PubMed] [Google Scholar]
- Vink C, Oude Groeneger AM, Plasterk RH. Identification of the catalytic and DNA-binding region of the human immunodeficiency virus type I integrase protein. Nucleic Acids Res. 1993;21:1419–1425. doi: 10.1093/nar/21.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-Y, Ling H, Yang W, Craigie R. Structure of a two-domain fragment of HIV-1 integrase: implications for domain organization in the intact protein. EMBO J. 2001;20:7333–7343. doi: 10.1093/emboj/20.24.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-Q, Mizuuchi K, Craigie R. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. EMBO J. 1997;16:7511–7520. doi: 10.1093/emboj/16.24.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielens J, Headey SJ, Jeevarajah D, Rhodes DI, Deadman J, Chalmers DK, Scanlon MJ, Parker MW. Crystal structure of the HIV-1 integrase core domain in complex with sucrose reveals details of an allosteric inhibitory binding site. FEBS Lett. 2010;584:1455–1462. doi: 10.1016/j.febslet.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Woerner AM, Klutch M, Levin JG, Marcus-Sekura CJ. Localization of DNA binding activity of HIV-1 integrase to the C-terminal half of the protein. AIDS Res. Hum. Retroviruses. 1992;8:297–304. doi: 10.1089/aid.1992.8.297. [DOI] [PubMed] [Google Scholar]
- Woerner AM, Marcus-Sekura CJ. Characterization of a DNA binding domain in the Cterminus of HIV-1 integrase by deletion mutagenesis. Nucleic Acids Res. 1993;21:3507–3511. doi: 10.1093/nar/21.15.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B, Burgess SM, Munroe DJ. Weak palindromic consensus sequences are a common feature found at the integration target sites of many retroviruses. J. Virol. 2005;79:5211–5214. doi: 10.1128/JVI.79.8.5211-5214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K, Mizuuchi K. Mismatch-targeted transposition of Mu: a new strategy to map genetic polymorphism. Proc. Natl. Acad. Sci. USA. 2002;99:11317–11321. doi: 10.1073/pnas.132403399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Roth MJ. Assembly and catalysis of concerted two-end integration events by Moloney murine leukemia virus integrase. J. Virol. 2001;75:9561–9670. doi: 10.1128/JVI.75.20.9561-9570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z-N, Mueser TC, Bushman FD, Hyde CC. Crystal structure of an active two-domain derivative of rous sarcoma virus integrase. J. Mol. Biol. 2000;296:535–548. doi: 10.1006/jmbi.1999.3463. [DOI] [PubMed] [Google Scholar]
- Yao X, Fang S, Qiao W, Geng Y, Shen Y. Crystal structures of catalytic core domain of BIV integrase: implications for the interaction between integrase and target DNA. Protein Cell. 2010;1:363–370. doi: 10.1007/s13238-010-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, McKee CJ, Kessl JJ, Santos WL, Daigle JE, Engelman A, Verdine G, Kvaratskhelia M. Subunit-specific protein footprinting reveals significant structural rearrangements and a role for N-terminal Lys-14 of HIV-1 integrase during viral DNA binding. J. Biol. Chem. 2008;283:5632–5641. doi: 10.1074/jbc.M705241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Jenkins TM, Craigie R. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc. Natl. Acad. Sci. USA. 1996;93:13659–13664. doi: 10.1073/pnas.93.24.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]