Abstract
Gadolinium chelates with octadentate ligands are widely used as contrast agents for magnetic resonance imaging (MRI), with macrocyclic ligands based on DO3A being preferred for the high kinetic inertness of their Gd chelates. A major challenge in the design of new bifunctional MRI probes is the need to control the rotational motion of the chelate, which greatly affects its relaxivity. In this work we explored facile alkylation of a secondary amine in macrocyclic DO3A-like ligands to create a short, achiral linkage to limit the undesired internal motion of chelates within larger molecular constructs. The acetate moiety on the trans nitrogen was also replaced with either a bidentate (ethoxyacetate, L1 or methyl picolinate, L2) or bulky monodentate (methyl phosphonate, L3) donor arm to give octa- or heptadentate ligands, respectively. The resultant Gd(III) complexes were all monohydrated (q = 1) and exhibited water residency times that spanned 2 orders of magnitude (τM = 2190 ± 170, 3500 ± 90 and 12.7 ± 3.8 ns at 37 °C for GdL1, GdL2 and GdL3 respectively). Alkylation of the secondary amine with a non-coordinating biphenyl moiety resulted in coordinatively saturated q = 0 complexes of octadentate ligands L1 and L2. Relaxivities were limited by slow water exchange and/or lack of water co-ligand. All complexes showed decreased inertness compared to [Gd(DO3A)] despite higher ligand denticity, and inertness was further decreased upon N-alkylation. These results demonstrate that high kinetic inertness and in vivo safety of Gd chelates with macrocyclic ligands should not be generalized.
Keywords: Molecular Imaging, Magnetic Resonance Imaging, Kinetic Inertness, Nephrogenic Systemic Fibrosis, Rotational Motion, Water Exchange, Relaxivity, Human Serum Albumin
INTRODUCTION
Molecular magnetic resonance (MR) imaging is a technique that combines the favorable properties of MRI (deep tissue penetration, high resolution, absence of ionizing radiation) with the use of targeted probes. In principle, this technique can provide a safe and quantitative method for mapping of molecular targets in vivo, but so far its full potential has been limited by the low efficiency of MR probes.
For T1 relaxation, Gd(III) is the ion of choice because of its high spin number (S = 7/2) and relatively long electronic relaxation time. Two major aspects need to be considered when designing new gadolinium-based MR probes. The first is relaxivity (the ability to increase the relaxation rate of water), which is a measure of the efficiency of the probe. The second aspect is the in vivo stability of the probe with respect to release of toxic gadolinium ions. It has been long recognized that Gd(III) ion must be bound in a chelate possessing high thermodynamic stability and high kinetic inertness to be safe for in vivo use. This is achieved with octadentate polyaminocarboxylate chelators mainly based on two structural types: macrocyclic 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and acyclic diethylenetriamine pentaacetic acid (DTPA). Under normal circumstances, these nonspecific MR probes are quickly excreted from the human body before significant release of free gadolinium can occur. However, processes that slow the excretion (e.g. impaired kidney function, specific binding of targeted probes) may provide enough time for substantial de-chelation of gadolinium. In renally impaired patients, the toxicity of gadolinium released from MR probes of the acyclic type was linked to occurrence of nephrogenic systemic fibrosis (NSF), a very rare but severe disease.1–4 Such adverse effects were not observed with macrocyclic chelates and studies comparing MR probes in vitro,5–12 in animal models5,13–18 as well as in human subjects19 consistently demonstrated that kinetic inertness of macrocyclic chelates is superior to that of acyclic structures. Based on these results, it is currently believed that octadentate macrocyclic ligands derived from 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (DO3A) provide Gd chelates with the highest kinetic inertness and are therefore most suitable for in vivo applications.
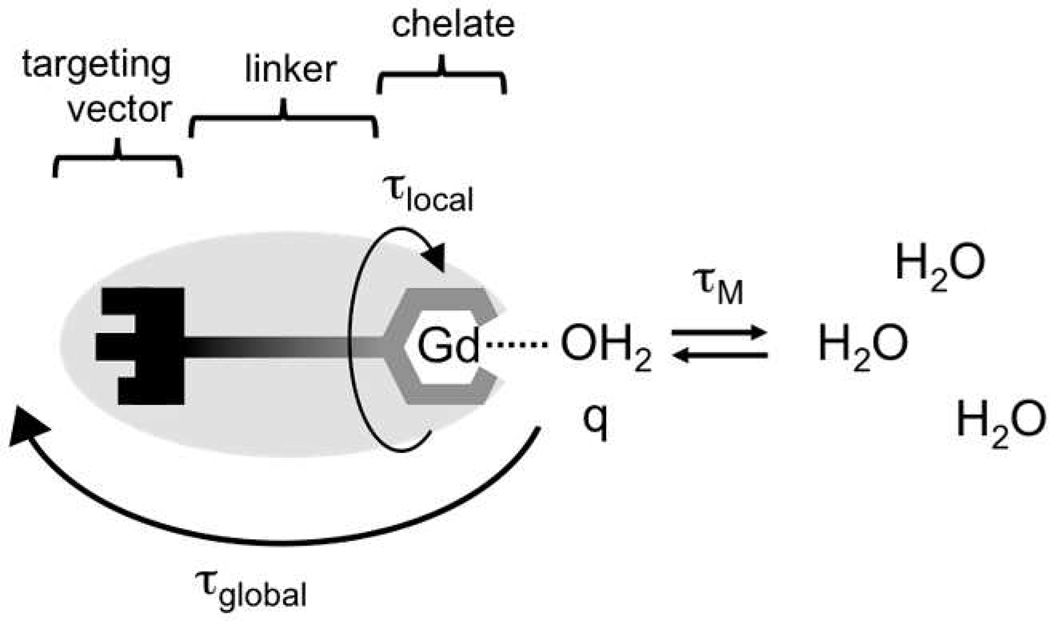
When chelated with octadentate ligands, Gd(III) is 8 or 9 coordinate, with the 9th coordination site occupied by water co-ligand. The coordinated water molecule is quickly relaxed by gadolinium and the relaxation is transmitted to the bulk water through a rapid exchange with surrounding water molecules. The effect on bulk water increases with the number of coordinated water ligands (q). The relaxation of the coordinated water is further modulated by the rotational motion of the molecule, characterized by rotational correlation time τR,. In targeted MR probes, the chelate is covalently linked to a targeting vector that directs the probe to a biological target (Figure 1). Here the chelate "experiences" two different types of motion: local rotation around covalent bonds in the linker group described by a local correlation time (τlocal) and rotation of the whole molecule described by a global correlation time (τglobal). Hence, relaxivity is mostly determined by the number of coordinated water ligands, the water exchange rate (kex = 1/τM, where τM is lifetime of the coordinated water) and the rotational correlation times τlocal and τglobal (Figure 1).
Figure 1.
Schematic representation of a targeted MR probe, showing parameters that influence relaxivity: hydration number q, water residency time τM, rotational correlation times τlocal and τglobal.
The properties of gadolinium chelates are dictated by the number, character and spatial arrangement of the donor atoms, and can be therefore modified by the choice of substituents in the ligand molecule. Unfortunately, the relationship between relaxivity and stability is often inverse, and proper balance must be established. For example, octadentate chelators based on DOTA or DTPA provide sufficient stability. While trading one donor atom from the chelator for an additional water ligand could increase relaxivity, it would also lower the stability of the chelate. Thus, with some notable exceptions,20–22 the hydration number cannot be increased beyond q = 1 without compromising the stability of the chelates. Nevertheless, theoretical calculations predict that relaxivities of the currently clinically used MR probes can be improved multifold by optimizing τM and τR. The water exchange rate can be optimized by modifying the donor groups in the ligand molecule.23–25 Rotational dynamics, on the other hand, are modulated by the overall size of the molecule, and can be tuned by coupling the chelate to molecules of appropriate size. The optimal values of τM and τR strongly depend on the magnetic field of the MR scanner. While the requirements for high relaxivity at low magnetic fields (≤ 1.5 T, most current clinical scanners) are fast water exchange (short τM) and slow rotation (τR > 5 ns), at higher fields (≥ 3 T, preclinical and new generation of clinical scanners) it is short τM and rather intemediate τR (0.5 – 2 ns).26
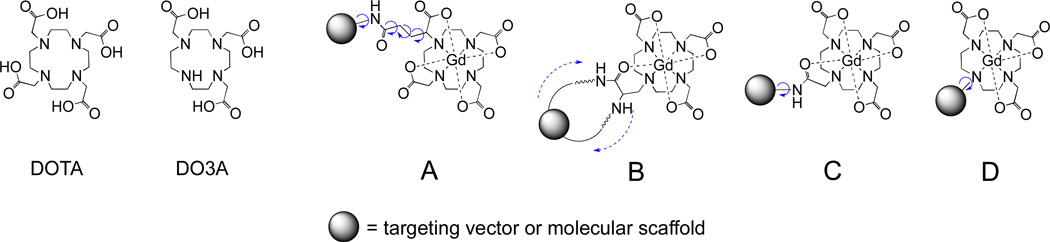
Maintaining precise control over the rotational dynamics is one of the major challenges in the development of high relaxivity probes and it is an actively pursued area of research.27–31 To ensure that the motion of the chelate is dominated by the rotation of the whole molecule, the local rotation of the chelate must be restricted. Figure 2 summarizes approaches for conjugation of DOTA-like chelates. The most common approach is to introduce an additional functional group as a site for conjugation (strategy A). Such a modification can be made on the cyclen backbone, or on the donor arm (as shown here), and the list of available bifunctional chelators is quite extensive.32 However for synthetic reasons, rather lengthy linkers are often used and this results in a relatively flexible attachment and lower relaxivity. One approach to overcoming this flexible linker limitation is to use a "dual anchor" strategy (B) with a specially designed ligand that allows two points of attachment and prevents the chelate from rotating independently of the entire molecule.30 A second approach is to use a very short linker to minimize internal motion. For example, a carboxylic acid group in DOTA can be directly coupled to an amine to form an amide linkage (strategy C). However, coordination of an acetamide oxygen donor to gadolinium results in slower water exchange and consequently low relaxivity.33,34 Another possibility is to use a nitrogen atom from the macrocycle as a point of attachment (strategy D). This offers a synthetically easy and achiral way for short and rigid linkage with potentially broad synthetic applications. This approach was previously used to synthesize multimers of Gd chelates assembled around benzene29,35–37 or pentaerythritol38 frameworks that showed relatively high relaxivities compared to the analogous monomeric Gd chelates. However, by replacing one of the acetate arms in DOTA with a non-coordinating moiety the ligand becomes a derivative of DO3A. Such heptadentate ligands yield coordinatively unsaturated Gd chelates with properties unfavorable for use as MR probes. Firstly, the thermodynamic stability and the kinetic inertness of the chelates are lower compared to chelates of DOTA.6 Secondly, chelates of DO3A derivatives are prone to binding of endogenous anions (e.g bicarbonate, lactate), resulting in displacement of water ligands and low relaxivity.39–44 These aspects were not addressed in previous works.
Figure 2.
Structures of DOTA and DO3A and strategies for conjugation of Gd chelates of DOTA-like ligand (A) and DO3A derivatives (B – D). (A) DOTAGA: an example of substitution on the acetate arm with internal motion possible about multiple single bonds. (B) DOTAla: dual anchor strategy with blocked rotation. (C) DOTA-monoamide: direct amide coupling to an acetate arm; restricted rotation but slow water exchange. (D) DO3A derivative: alkylation of a nitrogen atom in the macrocycle; restricted rotation.
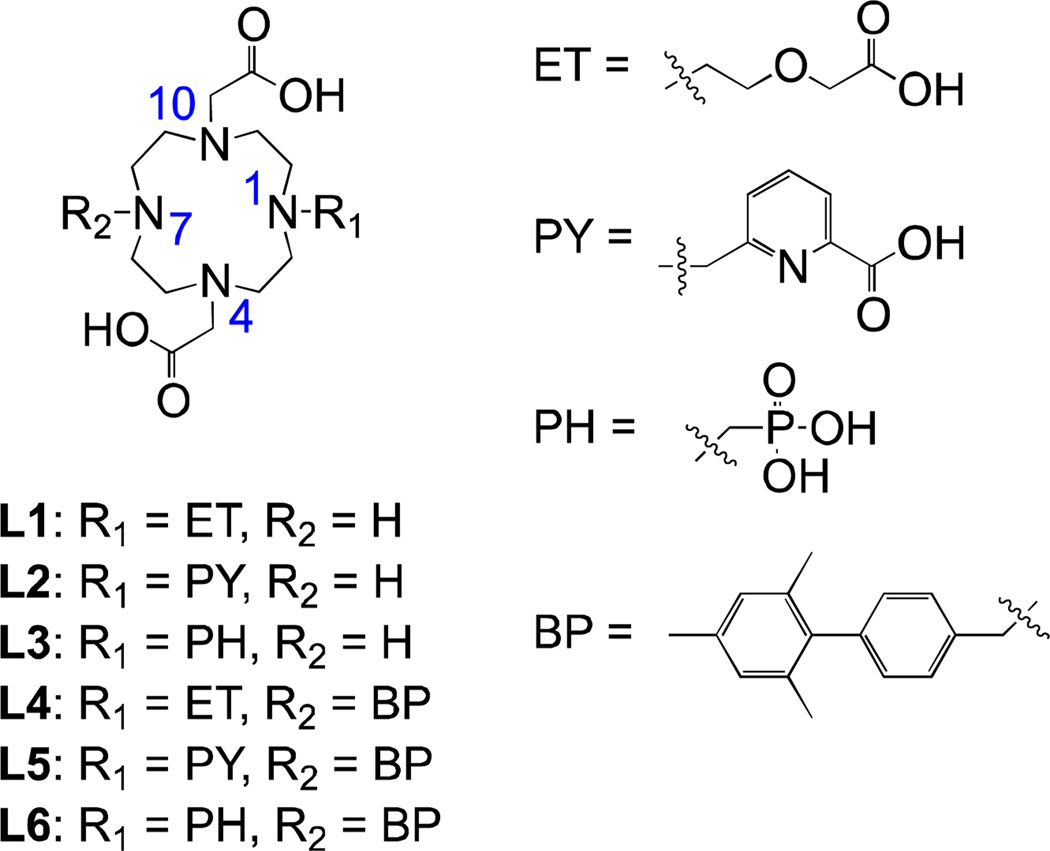
In this work we explore the approach of direct nitrogen conjugation in more detail. We reasoned that the loss of one donor atom at the site of N-conjugation could be compensated by proper modification to the donor arm on the opposite site of the macrocycle. We were interested in the following questions: (i) Can the negative effects of one missing acetate donor arm in the chelate (lower stability, increased kinetic lability, binding of endogenous anions) be offset by employing a bidentate donor arm or a bulky monodentate group? (ii) How does the type and arrangement of donor atoms impact water exchange and relaxivity? (iii) What is the effect of a non-coordinating N-substituent on kinetic inertness and relaxivity of the chelates? To address these questions we synthesized a library of six DO3A-like compounds where we varied the substituents on N1 and N7 atoms of the macrocycle (Figure 3). We studied the effect of these substituents on the hydration, water exchange, relaxivity and kinetic inertness of Gd and Eu chelates.
Figure 3.
New chelators synthesized and studied in this work. The numbering system for N-atoms of the macrocycle used in the text is indicated.
EXPERIMENTAL SECTION
General methods and materials
1H, 13C and 31P NMR spectra were recorded on a Varian 500 MHz NMR system equipped with a 5 mm broadband probe. Concentrations of metal ions were measured with Agilent 7500a Inductively Coupled Plasma Mass Spectrometer (ICP-MS). LC-MS analyses were done on Agilent (Hewlett Packard) 1100 Series LC system with diode array UV absorbance detector and HP 1100 Series Mass Spectrometry Detector (with electrospray ionization). Chemicals were purchased from Aldrich Chemical Co., Inc., and were used without further purification. Solvents (HPLC grade) were purchased from various commercial suppliers and used as received. Bis(tertbutyl) ester of 1,7-DO2A was synthesized according to published procedure.45 The cleavage of tert-butyl groups and complexation of DO2A with gadolinium followed the same procedure as for L1. The precursor for the albumin-binding moiety, 4'-(bromomethyl)-2,4,6-trimethyl-1,1'-biphenyl, was obtained by custom synthesis from SynDesign, LLC. Precursor for introduction of the methylphosphonate arm, tris(t-butyl) phosphite, was synthesized according to a published procedure.46
Measurement of Relaxivity
Longitudinal relaxation times T1, were measured on Bruker Minispecs mq20 (0.47 T) and mq60 (1.41 T) using an inversion recovery method with 10 inversion time values ranging from 0.05 × T1 to 5 × T1. Relaxivity in the absence of HSA was calculated from a linear plot of 3 different concentrations ranging from 0.25 to 1.0 mM versus the corresponding inverse relaxation times. The temperature was controlled at 37 °C or 25 °C. Samples with HSA were prepared in a 4.5% w/v solution of HSA (0.66 mM) in 50 mM HEPES buffer (pH = 7.4) at concentrations 0.02 to 0.06 mM.
Kinetic inertness experiments
Conditions were reproduced from reference9 with minor modifications: Stock solutions were prepared of the phosphate buffer (100 mM, pH = 7.0), Gd chelates (8 – 20 mM) and zinc triflate (78 mM). Exact concentrations of Gd and Zn were determined by ICP-MS. Calculated volumes were pipetted with calibrated pipettes into small glass vials to obtain these concentrations: 30 mM phosphate, 2.5 mM Gd chelate, 2.5 mM zinc. Deionized water was used to complete the volume to 170 µL. The T1 measurements were taken at 37 °C and 1.41 T (60 MHz). Samples were maintained at 37 °C in a heating block for the duration of the experiment except for short periods of time before each T1 measurement when they were stirred by vortexing and centrifugated (equilibration at 37 °C followed before the measurement).
Luminescence
Luminescence lifetime measurements of Eu complexes in H2O and D2O were performed on Hitachi f-4500 fluorescence spectrophotometer. Concentrations of the samples were 40 – 80 mM. For the measurements in D2O, the complexes were first dissolved in D2O (99.98% D), lyophilized and dissolved in D2O again to reduce the amount of residual H2O. Measurements were taken with the following settings: excitation at 396 nm, emission at 616 nm, 80 replicates, 0.04 ms temporal resolution (0 – 20 ms), PMT voltage = 400 V. Lifetimes were obtained from monoexponential fits of the data.
2-(Benzyloxy)ethanol (7)
Schlenk flask with sodium hydride (60% NaH in mineral oil, 820 mg, 20.5 mmol) was evacuated and filled with N2. Hexane (8 mL) was added through a septum, the suspension was stirred for several minutes and left to settle at the bottom. The solvent above the solid was carefully removed through a needle and the residue was dried in vacuum. The flask was then filled with N2 again, cooled in ice bath and anhydrous ethylene glycol (6 mL, 107 mmol) was slowly added. A slow, constant flow of N2 was maintained to remove hydrogen generated. The mixture was stirred for 16 h at RT. Then, a solution of benzyl bromide (3.50 g, 20.5 mmol) in anhydrous THF (15 mL) was added while stirring, resulting in two separated layers of liquid. The flask was placed in an oil bath at 70 °C and the reaction mixture was vigorously stirred to promote mixing of the layers. After 3 d the mixture appeared homogenous, with a precipitate of NaBr. THF was removed on a rotary evaporator, the residue was diluted with distilled water (20 mL) and extracted with ether (3 × 20 mL). Combined organic layers were dried with anhydrous Na2SO4 for 3h, then filtered and evaporated. The residue was purified by flash chromatography on 40g SiO2 column with hexane/ethylacetate mixture and gradient (% ethyl acetate): 20 – 60 in 8 min., flow rate = 40 mL/min., elution time = 5.5 – 8 min. The collected fractions were evaporated and the residue was further purified by vacuum distillation (1.6 Torr, boiling point ca. 90 °C). Product was obtained as colorless liquid (2.50 g, 16.4 mmol, 80% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 2.14 (s, 1H, OH), 3.59 – 3.61 (m, 2H, O-CH2CH2-O), 3.75 – 3.77 (m, 2H, O-CH2CH2-O), 4.56 (s, 2H, phenyl-CH2), 7.26 – 7.36 (m, 5H, C6H5-). 13C NMR (126 MHz, CDCl3, 25 °C) δ 61.9, 71.4, 73.3, 127.8, 127.8, 128.5, 138.0.
tert-Butyl 2-(2-(benzyloxy)ethoxy)acetate (8)
Potassium tert-butoxide (737 mg, 6.57 mmol) was dissolved in anhydrous tert-butanol (8 mL) under N2 atmosphere. Compound 7 (1.00 g, 6.57 mmol) was added through a septum and the mixture was stirred at RT for 30 min. The flask was cooled just enough to keep the tert-butanol in liquid state and tert-butyl bromoacetate (1.281 g, 6.57 mmol) was added through the septum over 5 min., followed by stirring at RT for 16 h. Then, distilled water (30 mL) was added and the mixture was extracted with ether (3 × 20 mL). Combined organic layers were dried with anhydrous Na2SO4 for several hours, filtered and evaporated. The residue was purified by flash chromatography on 40g SiO2 column with hexane/ethylacetate mixture and gradient (% ethyl acetate): 20 – 60 in 12 min., flow rate = 40 mL/min., elution time = 3 min. Collected fractions were evaporated to give product as colorless liquid (0.658 g, 2.47 mmol, 38% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 1.47 (s, 9H, O-C(CH3)3), 3.66 – 3.68 (m, 2H, O-CH2CH2-O), 3.73 – 3.75 (m, 2H, O-CH2CH2-O), 4.04 (s, 2H, O-CH2-CO-), 4.58 (s, 2H, phenyl-CH2), 7.27 – 7.36 (m, 5H, C6H5-). 13C NMR (126 MHz, CDCl3, 25 °C) δ 28.1, 69.1, 69.5, 70.8, 73.3, 81.5, 127.6, 127.8, 128.4, 138.2, 169.7. MS (ESI+): C15H22O4 m/z: calcd. 267.2 [M+H]+; found 267.2 [M+H]+, 289.2 [M+Na]+.
tert-Butyl 2-(2-hydroxyethoxy)acetate (9)
Compound 8 (658 mg, 2.47 mmol) was dissolved in anhydrous MeOH (60 mL) in a hydrogenation vessel and Pd catalyst (10% on charcoal, 150 mg) was added. The mixture was shaken under H2 atmosphere at 2 bar and RT for 3 h. The catalyst was filtered off and the solvent was evaporated. The residual MeOH was removed by repeated addition of a few milliliters of anhydrous MeCN with subsequent evaporation (3-times). The product was obtained as colorless oil (0.392 g, 2.22 mmol, 90% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 1.49 (s, 9H, O-C(CH3)3), 2.97 (bs, 1H, OH), 3.66 – 3.69 (m, 2H, OCH2CH2-O), 3.73 – 3.76 (m, 2H, O-CH2CH2-O), 4.02 (s, 2H, O-CH2-CO-). 13C NMR (126 MHz, CDCl3, 25 °C) δ 28.1, 61.7, 68.8, 73.5, 82.2, 170.5.
Dimethyl pyridine-2,6-dicarboxylate (11)
Thionyl chloride (30 mL) was slowly added to pyridine-2,6-dicarboxylic acid (1.00 g, 5.98 mmol) while stirring and cooling the flask in an ice bath. The ice bath was removed and the reaction mixture was refluxed for 45 min. Thionyl chloride was distilled off to near dryness. Anhydrous MeOH (30 mL) was slowly added at 0 °C followed by reflux for 30 min. The solvent was evaporated, the residue diluted with saturated aqueous solution of NaHCO3 and extracted with DCM (3 × 15 mL). The organic layers were combined and dried with anhydrous Na2SO4. The product was obtained after filtration and evaporation of the solvent as white crystalline powder (1.15 g, 5.87 mmol, 98% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 4.04 (s, 6H, -CH3), 8.04 (t, 1H, aromatic, JHH = 7.8 Hz), 8.33 (d, 2H, aromatic, JHH = 8.0 Hz). 13C NMR (126 MHz, CDCl3, 25 °C) δ 53.2, 128.0, 138.4, 148.2, 165.1. MS (ESI+): C9H9NO4 m/z: calcd. 196.1 [M+H]+; found 196.1 [M+H]+.
Methyl 6-(hydroxymethyl)picolinate (12)
Compound 11 (1.094 g, 5.60 mmol) was dissolved in anhydrous MeOH (35 mL) and stirred at RT under a slow flow of N2. NaBH4 (3.28 mmol) was added in three equal portions at times 0, 60 and 210 min. The mixture was stirred at RT for 14 h following the last addition. The solvent was evaporated and the product isolated by flash chromatography on 40 g SiO2 column with MeOH/DCM (6:94) mixture (isocratic), flow rate = 40 mL/min., elution time = 4 min. Collected fractions were evaporated to give product as white crystalline powder (388 mg, 2.32 mmol, 41% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 3.57 (t, 1H, OH, JHH = 5.5 Hz), 4.00 (s, 3H, -CH3), 4.87 (d, 2H, CH2OH, JHH = 5.4 Hz), 7.54 (d, 1H, aromatic, JHH = 7.5 Hz), 7.85 (t, 1H, aromatic, JHH = 7.75 Hz), 8.04 (d, 1H, aromatic, JHH = 7.0 Hz). 13C NMR (126 MHz, CDCl3, 25 °C) δ 52.9, 64.6, 123.8, 124.0, 137.7, 147.0, 160.1, 165.5. MS (ESI+): C8H9NO3 m/z: calcd. 168.1 [M+H]+; found 168.1 [M+H]+.
Methyl 6-(chloromethyl)picolinate hydrochloride (13)
Compound 12 (388 mg, 2.32 mmol) was dissolved in thionyl chloride (5 mL) and stirred at RT for 5 h. Thionyl chloride was evaporated, the residue dissolved in chloroform (5 mL) and evaporated again (repeated 2-times). The product, as its hydrochloride, was obtained by crystallization from a concentrated solution in toluene as pale yellow crystals (218 mg, 0.89 mmol, 42% yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 4.06 (s, 3H, -CH3), 5.01 (s, 2H, CH2), 6.95 (bs, 1H, pyridine N-H), 7.90 (d, 1H, aromatic, JHH = 7.5 Hz), 8.08 (d, 1H, aromatic, JHH = 7.8 Hz), 8.18 (d, 1H, aromatic, JHH = 8.0 Hz). 13C NMR (126 MHz, CDCl3, 25 °C) δ 44.6, 53.4, 125.1, 127.3, 140.2, 145.8, 156.8, 163.8. MS (ESI+): C8H8ClNO2 m/z: calcd. 186.0 [M+H]+; found 186.0 [M+H]+.
Di-tert-butyl 2,2'-(4-((2',4',6'-trimethyl-[1,1'-biphenyl]-4-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetate (14)
Bis(tert-butyl) ester of 1,7-DO2A (1.51 g, 3.77 mmol) was dissolved in anhydrous MeCN (60 mL) and a solution of 4'-(bromomethyl)-2,4,6-trimethyl-1,1'-biphenyl (281 mg, 972 µmol) in anhydrous MeCN (5 mL) was slowly added with a syringe pump (ca. 3 mL/h) while stirring at RT. The solution was stirred for 30 min. following the complete addition. The solvent was evaporated, the residue dissolved in a 20/80 mixture of H2O/MeCN (10 mL) and the product was isolated by preparative HPLC (method A, elution at 13 min.). The collected fractions were concentrated on rotary evaporator to remove most of the MeCN. The resulting solution was mixed with 5% NaOH (10 mL) and extracted with ether (3 × 20 mL). The organic layers were combined and evaporated. The residual water was removed by co-distillation with anhydrous EtOH (2 × 10 mL) and the residue dried in high vacuum. Product was obtained as a thick colorless oil (443 mg, 727 µmol, 77% yield relative to the alkylating agent). 1H NMR (500 MHz, CDCl3, 25 °C) δ 1.41 (s, 18H, (CH3)3C-), 2.01 (s, 6H, CH3-), 2.33 (s, 3H, CH3-), 2.49 – 2.53 (m, 4H, CH2 macrocycle), 2.62 – 2.67 (m, 4H, CH2 macrocycle), 2.79 – 2.83 (m, 4H, CH2 macrocycle), 2.84 – 2.88 (m, 4H, CH2 macrocycle), 3.08 (s, 4H, N-CH2-CO-), 3.45 – 3.54 (m, 1H, NH), 3.56 (s, 2H, N-CH2-phenyl), 6.94 (s, 2H, aromatic), 7.06 (d, 2H, aromatic, JHH = 8.0 Hz), 7.45 (d, 2H, aromatic, JHH = 8.0 Hz). 13C NMR (126 MHz, CDCl3, 25 °C) δ 20.9, 21.0, 28.2, 47.7, 50.2, 52.2, 53.9, 56.5, 57.6, 80.6, 128.0, 128.96, 128.99, 136.1, 136.4, 138.9, 139.1, 139.3, 171.6. MS (ESI+): C36H56N4O4 m/z: calcd. 609.4 [M+H]+; found 609.3 [M+H]+.
Di-tert-butyl 2,2'-(4-(2-(2-(tert-butoxy)-2-oxoethoxy)ethyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetate (15)
Compound 9 (57.7 mg, 327 µmol) and triethylamine (66.2 mg, 654 µmol) were mixed with CDCl3 (1 mL) in NMR tube. A titration with mesyl chloride was performed in the NMR tube followed by 1H NMR spectra until the conversion to mesylate reached 97%. This required 31.1 µL of mesyl chloride. The solvent was evaporated, the residue dissolved in anhydrous MeCN (4 mL) and insoluble solids were filtered off. Bis(tert-butyl) ester of 1,7-DO2A (216 mg, 540 µmol) and K2CO3 (60 mg, 434 µmol) were mixed together in anhydrous MeCN (8 mL). The mixture was heated to 55 °C and stirred, while solution of the mesylate was very slowly added with a syringe pump over 3 days. The reaction mixture was evaporated and the residue purified by preparative HPLC (method B, elution at 12.4 min.). Combined fractions were evaporated and the residue dried in high vacuum, yielding product as pale yellow oil (0.1655 g, 210 µmol assuming composition 15•2TFA, 64 % yield relative to 9).
For NMR characterization and for synthesis of 16, the product was dissolved in water, made basic (pH ≈ 12) with NaOH and extracted with Et2O (3-times 20 mL). Organic layers were combined, evaporated and the residue was dried in high vacuum. 1H NMR (500 MHz, CDCl3, 25 °C) δ 1.46 (s, 18H, O-C(CH3)3), 1.48 (s, 9H, O-C(CH3)3), 2.53 – 2.57 (m, 4H, CH2 macrocycle), 2.57 – 2.61 (m, 4H, CH2 macrocycle), 2.71 (t, 2H, N-CH2CH2-O, JHH = 6.3 Hz), 2.76 – 2.80 (m, 4H, CH2 macrocycle), 2.81 – 2.85 (m, 4H, CH2 macrocycle), 3.33 (s, 4H, N-CH2-CO-), 3.59 (t, 2H, N-CH2CH2-O, JHH = 6.3 Hz), 4.00 (s, 2H, O-CH2-CO-). 13C NMR (126 MHz, CDCl3, 25 °C) δ 28.1, 28.3, 47.4, 50.9, 52.0, 52.5, 54.1, 56.9, 69.0, 69.5, 80.8, 81.4, 169.9, 171.4. MS (ESI+): C28H54N4O7 m/z: calcd. 559.4 [M+H]+; found 559.4 [M+H]+.
2,2'-(4-(2-(Carboxymethoxy)ethyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L1)
Compound 15•2TFA (0.1655 g, 210 µmol) was treated for 20 h at RT with cleavage cocktail (4 mL; 90% TFA, 5% tris(isopropyl)silane, 5% H2O). The solution was concentrated on rotary evaporator and loaded onto SPE column (C18, 5g). The product was eluted with distilled water. The solution was evaporated and the residue dried in high vacuum to give product as colorless oil that slowly crystallized (104 mg, 168 µmol assuming composition L1•2TFA, 80 % yield). 1H NMR (500 MHz, D2O, 90 °C) δ 2.93 – 3.08 (m, 4H, CH2 macrocycle), 3.09 – 3.19 (m, 4H, CH2 macrocycle), 3.16 – 3.32 (m, 4H, CH2 macrocycle), 3.43 – 3.52 (m, 4H, CH2 macrocycle), 3.54 (bs, 4H, N-CH2-CO-), 3.57 (t, 2H, N-CH2CH2-O, JHH = 5.3 Hz), 3.95 (t, 2H, N-CH2CH2-O, JHH = 5.3 Hz), 4.19 (s, 2H, O-CH2-CO-). 13C NMR (126 MHz, D2O, 90 °C) δ 43.5, 49.2, 50.3, 52.2, 54.4, 54.7, 64.9, 68.2, 173.8, 174.8. MS (ESI+): C16H30N4O7 m/z: calcd. 391.2 [M+H]+; found 391.2 [M+H]+.
2,2'-(4-((6-Carboxypyridin-2-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L2)
Bis(tert-butyl) ester of 1,7-DO2A (400 mg, 1 mmol) was dissolved in anhydrous MeCN (15 mL) and a solution of 13 (84 mg, 378 µmol) in anhydrous MeCN (3 mL) was added with a syringe pump over 15 min. while stirring. The reaction mixture was stirred for 18 h at RT. The solution was concentrated and product was purified by preparative HPLC (method C, elution at 10 min.). Fractions containing product were concentrated, pH was adjusted to pH = 12 with LiOH and the solution was stirred for 4 h at RT. The solvent was evaporated, the residue dried in high vacuum and then dissolved in TFA (6 mL) and stirred for 16 h at RT. TFA was evaporated, the residue dissolved in H2O (6 mL) and evaporated again. After that the residue was dissolved in H2O again and loaded onto SPE column (C18, 5 g), washed with H2O with 0.1% TFA and the product was eluted with 10% MeCN in H2O. The eluate was lyophilized to give product as pale yellow solid foam (0.206 g, 315 µmol assuming composition L2•2TFA, 83% yield relative to 13). 1H NMR (500 MHz, D2O, 80 °C) δ 3.06 – 3.10 (m, 4H, CH2 macrocycle), 3.16 – 3.23 (m, 4H, CH2 macrocycle), 3.24 – 3.27 (m, 4H, CH2 macrocycle), 3.30 (bs, 4H, N-CH2-CO-), 3.52 – 3.57 (m, 4H, CH2 macrocycle), 4.69 (s, 2H, N-CH2-py), 7.78 (d, 1H, aromatic, JHH = 7.5 Hz), 8.14 (t, 1H, aromatic, JHH = 7.5 Hz), 8.22 (d, 1H, aromatic, JHH = 8.0 Hz). 13C NMR (126 MHz, D2O, 80 °C) δ 43.4, 48.6, 49.3, 52.8, 53.7, 58.2, 126.7, 128.8, 140.9, 147.9, 149.9, 167.4, 174.2. MS (ESI+): C19H29N5O6 m/z: calcd. 424.2 [M+H]+; found 424.2 [M+H]+.
2,2'-(4-(Phosphonomethyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L3)
Bis(tert-butyl) ester of 1,7-DO2A (600 mg, 1.5 mmol) was dissolved in anhydrous THF (12 mL). The reaction mixture was stirred under N2 atmosphere at RT and paraformaldehyde (45 mg, 1.5 mmol) and a solution of tris(tert-butyl)phosphite (375 mg, 1.5 mmol) in anhydrous THF (6 mL) were added in 3 equal portions at time 0, 5 and 24 h. The reaction was stopped 24 h after the third addition. The solvent was evaporated and the product isolated by preparative HPLC (method D, elution at 11.2 min.). Fractions containing product were combined and evaporated. The residue was treated with cleavage cocktail (5 mL, 90% TFA, 5% tris(isopropyl)silane, 5% H2O) for 18 h at RT. The mixture was concentrated on rotary evaporator and loaded onto SPE column (C18, 5g). The product was eluted with distilled water. The solution was evaporated and the residue dried in high vacuum to give product as pale yellow oil (0.139 g, 364 µmol assuming composition L3•2TFA, 24% yield). 1H NMR (500 MHz, D2O, 90 °C) δ 3.00 – 3.05 (m, 4H, CH2 macrocycle), 3.11 – 3.17 (m, 4H, CH2 macrocycle), 3.18 – 3.24 (m, 4H, CH2 macrocycle), 3.40 (d, 2H, CH2-P, JPH = 12.5 Hz), 3.52 – 3.59 (m, 4H, CH2 macrocycle), 3.58 (s, 4H, N-CH2-CO-). 13C NMR (126 MHz, D2O, 90 °C) δ 43.5, 49.3, 50.1, 51.5 (d, 1JCP = 135 Hz), 53.3 (d, JCP = 2 Hz), 54.9, 175.1. 31P NMR (202 MHz, D2O, 25 °C) δ 7.42. MS (ESI+): C13H27N4O7P m/z calcd. 383.2 [M+H]+; found 383.2 [M+H]+.
Di-tert-butyl 2,2'-(4-(2-(2-(tert-butoxy)-2-oxoethoxy)ethyl)-10-((2',4',6'-trimethyl-[1,1'-biphenyl]-4-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetate (16)
Compound 15 (0.128 g, 229 µmol) and K2CO3 (0.094 g, 682 µmol) were mixed together in anhydrous MeCN (10 mL). Solution of 4'-(bromomethyl)-2,4,6-trimethyl-1,1'-biphenyl (0.066 g, 228 µmol) in MeCN (1 mL) was added in small portions during 3 h while stirring at RT. The solids were filtered off, the filtrate was evaporated and the crude product was purified by preparative HPLC (method B, elution at 11 min.). Combined fractions were concentrated on the rotary evaporator, made basic (pH ≈ 12) with NaOH and extracted with Et2O (3 × 20 mL). Organic layers were combined, evaporated and the residue was dried in high vacuum. Product was obtained as a colorless oil (0.166 g, 216 µmol, 94 % yield). 1H NMR (500 MHz, CDCl3, 25 °C) δ 1.43 (s, 18H, O-C(CH3)3), 1.47 (s, 9H, O-C(CH3)3), 2.00 (s, 6H, Ar-CH3), 2.32 (s, 3H, Ar-CH3), 2.60 – 2.66 (m, 4H, CH2 macrocycle), 2.68 – 2.75 (m, 4H, CH2 macrocycle), 2.72 (t, 2H, N-CH2CH2-O, JHH = 6.5 Hz), 2.83 – 2.90 (m, 8H, CH2 macrocycle), 3.19 (s, 4H, N-CH2-CO-), 3.57 (s, 2H, N-CH2-Ar), 3.64 (t, 2H, N-CH2CH2-O, JHH = 6.5 Hz), 4.00 (s, 2H, O-CH2-CO-), 6.93 (s, 2H, aromatic), 7.05 (d, 2H, aromatic, JHH = 8.5 Hz), 7.40 (d, 2H, aromatic, JHH = 8.5 Hz). 13C NMR (126 MHz, CDCl3, 25 °C) δ 20.8, 21.0, 28.1, 28.2, 52.0, 52.2, 52.4, 53.2, 55.2, 56.4, 60.0, 69.0, 69.9, 80.5, 81.3, 128.0, 128.9, 129.3, 136.0, 136.3, 138.1, 139.0, 139.4, 169.8, 171.1. MS (ESI+): C44H70N4O7 m/z: calcd. 767.5 [M+H]+; found 767.6 [M+H]+.
2,2'-(4-(2-(Carboxymethoxy)ethyl)-10-((2',4',6'-trimethyl-[1,1'-biphenyl]-4-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L4)
Compound 16 (0.166 g, 216 µmol) was dissolved in TFA (4 mL) and stirred for 18 h at RT. TFA was evaporated and the residue was dissolved in distilled water (4 mL) and evaporated again. The residue was loaded onto SPE column (C18, 5g), washed with 5% MeCN in H2O and eluted with 40% MeCN in H2O. The product was obtained as a zwitterion after evaporation of the solvent as white microscopic needles (0.1076 g, 180 µmol, 83% yield). 1H NMR (500 MHz, D2O, 25 °C) δ 1.89 (s, 6H, CH3), 2.23 (s, 3H, CH3), 3.16 – 3.22 (m, 12H, 3xCH2 macrocycle), 3.31 – 3.37 (m, 10H, CH2 macrocycle + 2xN-CH2CO- + N-CH2CH2-O), 3.86 (t, 2H, N-CH2CH2-O, JHH = 4.75), 4.02 (s, 2H, CH2 benzyl), 4.26 (s, 2H, O-CH2-CO-), 6.94 (s, 2H, aromatic), 7.16 (d, 2H, aromatic, JHH = 8 Hz), 7.54 (d, 2H, aromatic, JHH = 8 Hz). 13C NMR (126 MHz, D2O, 90 °C) δ 20.1, 20.4, 50.5, 50.6, 50.8, 51.4, 53.7, 56.9, 58.5, 66.2, 69.7, 128.3, 129.0, 130.7, 131.7, 136.4, 138.0, 138.4, 142.9, 174.8, 176.0. MS (ESI+): C32H46N4O7 m/z: calcd. 599.3 [M+H]+; found 599.4 [M+H]+.
2,2'-(4-((6-Carboxypyridin-2-yl)methyl)-10-((2',4',6'-trimethyl-[1,1'-biphenyl]-4-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L5)
Compounds 14 (176 mg, 290 µmol), 13 (64.5 mg, 290 µmol) and K2CO3 (240 mg, 1.74 mmol) were mixed together in anhydrous MeCN (10 mL) and stirred for 24 h at RT. A solution of LiOH (13.5 mg, 563 µmol) in distilled water (5 mL) was added and the mixture stirred at RT for 90 min. The solution was acidified with TFA (44 µL), concentrated on a rotary evaporator and the product was purified by preparative HPLC (method C, elution at 7 min.). Collected fractions were evaporated, the residue dried in vacuum and then dissolved in TFA (6 mL) and stirred at RT for 24 h. TFA was evaporated, the residue dissolved in distilled water (5 mL) and evaported again. The product was obtained by lyophilization from a small amount of water as white powder (198 mg, 230 µmol assuming composition L5•2TFA, 79% yield). 1H NMR (500 MHz, D2O, 25 °C) δ 1.82 (s, 6H, CH3), 2.17 (s, 3H, CH3), 2.94 – 3.18 (m, 12H, 4xCH2 macrocycle + 2xN-CH2CO-), 3.32 (m, 4H, CH2 macrocycle), 3.45 (m, 4H, CH2 macrocycle), 4.43 (s, 2H, N-CH2- benzyl), 4.53 (s, 2H, N-CH2-py), 6.92 (s, 2H, aromatic), 7.13 (d, 2H, aromatic, JHH = 8 Hz), 7.51 (d, 2H, aromatic, JHH = 8 Hz), 7.74 (d, 1H, aromatic, JHH = 7.5 Hz), 8.03 (t, 1H, aromatic, JHH = 8 Hz), 8.11 (d, 1H, aromatic, JHH = 5 Hz). 13C NMR (126 MHz, D2O, 25 °C) δ 19.7, 19.9, 48.0, 48.3, 50.0, 51.3, 53.2, 57.9, 58.1, 126.0, 127.1, 128.0, 129.1, 130.4, 131.4, 136.1, 137.8, 137.9, 140.4, 142.8, 147.9, 149.4, 167.5, 173.7. MS (ESI+): C29H43N4O7P m/z: calcd. 632.3 [M+H]+; found 632.4 [M+H]+.
2,2'-(4-(Phosphonomethyl)-10-((2',4',6'-trimethyl-[1,1'-biphenyl]-4-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,7-diyl)diacetic acid (L6)
Compound 14 (200 mg, 328 µmol) and paraformaldehyde (25 mg, 833 µmol) were mixed together in anhydrous THF (10 mL). The mixture was maintained under a slow flow of N2 and a solution of tris(tert-butyl)phosphite (82 mg, 328 µmol) in anhydrous THF (1 mL) was added. The reaction mixture was stirred at RT for 16 h. Then, paraformaldehyde (10 mg) and tris(tert-butyl) phosphite (25 mg) were added again, followed by 24 h stirring at RT. The solvent was evaporated and the product purified by preparative HPLC (method E, elution at 9 min.). Combined fractions were evaporated and the residue dried in vacuum. TFA (5 mL) was added and the solution was stirred at RT for 16 h. TFA was evaporated, the residue dissolved in distilled water (5 mL) and evaporated again. The final product was obtained by lyophilization from a small amount of water as a white powder (169 mg, 207 µmol assuming composition L6•2TFA, 63% yield). 1H NMR (500 MHz, D2O, 90 °C) δ 1.92 (s, 6H, CH3), 2.26 (s, 3H, CH3), 3.07 – 3.17 (m, 8H, 4x CH2 macrocycle), 3.37 – 3.45 (m, 10H, 2x CH2 macrocycle + 2x CH2CO + CH2-P), 3.56 – 3.65 (m, 4H, 2x CH2 macrocycle), 4.52 (s, 2H, CH2 benzyl), 6.99 (s, 2H, aromatic), 7.25 (d, 2H, aromatic, JHH = 8 Hz), 7.59 (d, 2H, aromatic, JHH = 8 Hz). 13C NMR (126 MHz, D2O, 90 °C) δ 20.0, 20.4, 49.1, 49.3, 51.3, 51.9 (d, 1JCP = 138 Hz), 52.9, 54.6, 59.1, 127.2, 128.3, 131.1, 131.8, 136.4, 138.1, 138.3, 143.8, 174.5. 31P NMR (202 MHz, D2O, 25 °C) δ 7.46. MS (ESI+): C29H43N4O7P m/z calcd. 591.3 [M+H]+; found 591.3 [M+H]+.
Eu and Gd complexes of ligands L1 – L6
Chelates were prepared at RT by stepwise addition of a solution of the metal salt to a solution of the ligand in distilled water or 20% aqueous MeCN (L4 – L6). After each addition, the pH was adjusted to 7 with a diluted solution of NaOH. When the solution cleared from precipitated lanthanide hydroxide, a sample was taken for LC-MS analysis. The additions were repeated until complete conversion to chelate was observed in UV trace (220 nm, ligands L2, L4, L5, L6) or until disappearance of the free ligand signal in MS trace (ligands L1 and L2). After completion, the chelates were desalted and additionally purified. Chelates of L1 and L3 were purified by preparative HPLC (method F) to remove possible excess of free ligand or free metal. Chelates of L2 and L4 – L6 were desalted on SPE column (C18, 5g) by washing with distilled water followed by elution with 10% MeCN (L2) or 40% MeCN (L4 – L6). Yields were not determined. Tests for the presence of free lanthanide ions were performed with solutions of Xylenol Orange (0.1 M sodium acetate buffer pH = 5.5) and Arsenazo III (10 µM in 0.15 M ammonium acetate buffer pH = 7.0) by mixing 2 µL of the chelate solution with 100 µL of the indicator solution.
MS (ESI+): m/z [Eu(L1)] C16H27EuN4O7 calcd. 541.1 [M+H]+; found 541.2 [M+H]+. [Gd(L1)] C16H27GdN4O7 calcd. 546.1 [M+H]+; found 546.2 [M+H]+. [Eu(L2)] C19H26EuN5O6 calcd. 574.1 [M+H]+; found 574.2 [M+H]+. [Gd(L2)] C19H26GdN5O6 calcd. 579.1 [M+H]+; found: 579.1 [M+H]+. [Eu(L3)] C13H24EuN4O7P calcd. 533.1 [M+H]+; found 533.0 [M+H]+. [Gd(L3)] C13H24GdN4O7P calcd. 538.1 [M+H]+; found 538.0 [M+H]+. [Eu(L4)] C32H43EuN4O7 calcd. 749.2 [M+H]+; found: 749.3 [M+H]+. [Gd(L4)] C32H43GdN4O7 calcd. 754.3 [M+H]+; found 754.3 [M+H]+. [Eu(L5)] C35H42EuN5O6 calcd. 782.2 [M+H]+; found 782.3 [M+H]+. [Gd(L5)] C35H42GdN5O6 calcd. 787.3 [M+H]+; found 787.3 [M+H]+. [Eu(L6)] C29H40EuN4O7P calcd. 741.2 [M+H]+; found 741.2 [M+H]+. [Gd(L6)] C29H40GdN4O7P calcd. 746.2 [M+H]+; found: 746.3 [M+H]+.
STRUCTURAL DESIGN
Octadentate ligands derived from cyclen are excellent chelators for gadolinium. From a synthetic viewpoint, alkylation of the secondary nitrogen atoms in cyclen is an obvious and easy way to introduce other substituents to the molecule. A variety of SN2 active alkylation agents can be used - usually alkyl or aryl bromides/chlorides or activated alcohols (mesylates, tosylates, etc.). This reaction is usually used to introduce coordinating units (most commonly acetate pendants) that participate in binding of gadolinium in the resulting chelates, but in principle can be used to attach any group. In order to use N-alkylation as a general method for linking the chelates to non-coordinating moieties such as targeting vectors, we needed to compensate for the loss of one donor site. Synthetically, the most accessible approach was to modify the donor arm on the opposite site of the macrocycle.
We used three different donor arms that can be expected to result in q = 1 chelates after their coordination to gadolinium. The ethoxy-acetate group (compounds L1 and L4) was selected because it provides two oxygen donor atoms for coordination and represents a relatively flexible chain that can easily accommodate to the coordination environment of the metal ion. This donor arm has not been previously used in cyclen-based chelators, but analogous PEG derivatives showed that the ether oxygen donor could coordinate to lanthanides.47–49 As another bidentate arm we chose the picolinate group (in compounds L2 and L5) that provides one anionic carboxylate O and one neutral pyridyl N atom for coordination. This group has a less flexible and pre-defined structure, and has been shown to coordinate strongly to lanthanides in bidentate fashion.25,50–57 We also used a methylphosphonate donor arm (in compounds L3 and L6). This group provides only one anionic O donor atom, but is significantly bulkier than a carboxylate and will therefore occupy larger space around gadolinium. With the phosphonate, we anticipate an overall 8-coordinate complex with one water co-ligand.
For a non-coordinating substituent on N1 (see Figure 3) we chose a methylated biphenyl moiety. Similar biphenyl derivatives demonstrated high affinity for binding to human serum albumin (HSA) and have been used to modulate the rotational motion through interaction with this protein.25,50 We synthesized two sets of ligands, with and without this moiety (without = with a secondary amine instead). This allowed us to investigate the effects of coordinating and non-coordinating substituents on the properties of the chelates separately.
RESULTS AND DISCUSSION
Synthesis of precursors
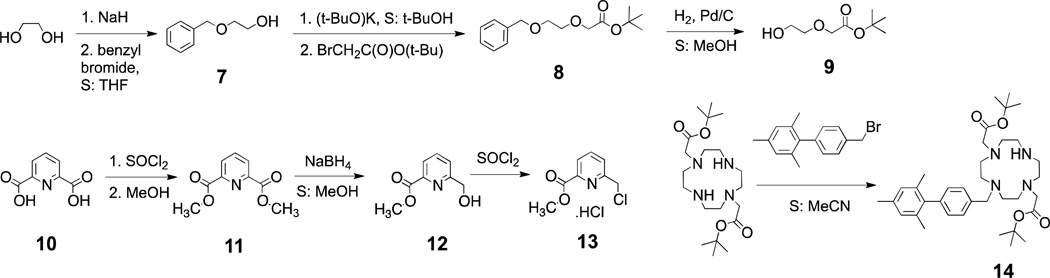
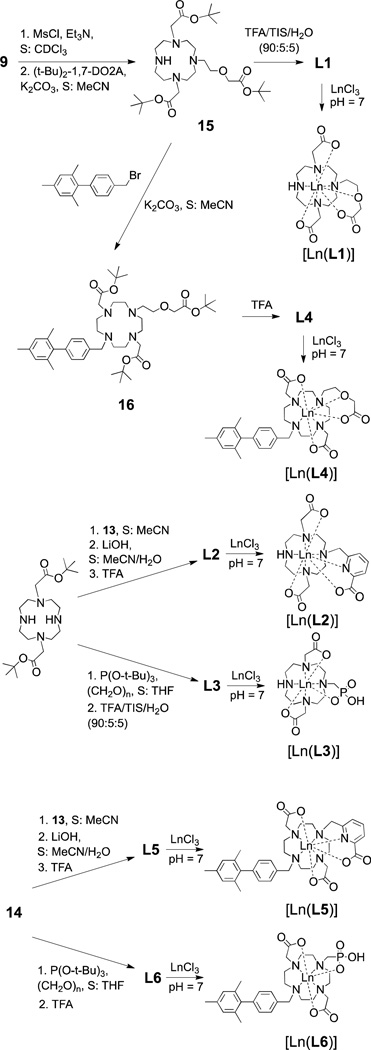
All ligands were synthesized starting from the bis(tert-butyl) ester of 1,7-DO2A by an alkylation reaction with corresponding alkylation agents. Preparation of the precursors is shown in Scheme 1. Alcohol 9, a precursor for the ethoxy-acetate arm, was prepared starting from ethylene glycol by protecting one alcohol group as benzyl ether 7. An alcoholate of the second OH group was reacted with tert-butyl bromoacetate to afford compound 8, which yielded 9 after removal of the benzyl group. Attempts to synthesize 9 directly from ethylene glycol alcoholate by reaction with tert-butyl bromoacetate were unsuccessful.
Scheme 7.
Synthesis of ligand precursors.
The precursor for the pyridine-carboxylate arm (13) was synthesized from pyridine-2,6-dicarboxylic acid that was first transformed into its dimethyl ester 11. Reduction of one ester group with sodium borohydride afforded alcohol 12. The alcohol group was converted to a chloride with thionyl chloride to give the alkylating agent 13.
To facilitate the synthesis of ligands L4, L5 and L6 we first synthesized a common precursor 14, Scheme 1. The alkylation to introduce only one biphenyl group was performed with an excess of bis(tert-butyl) ester of 1,7-DO2A relative to the alkylating agent resulting in statistical mixture of starting material and products. Product 14 was isolated by preparative HPLC.
Synthesis of ligands L1, L2 and L3
Ligands L1 – L3 were prepared from the bis(tert-butyl) ester of 1,7-DO2A. Rather than employing a protection/deprotection strategy to alkylate only one of the secondary amines, we used excess of DO2A and separated the product from the statistical mixture, similar to the synthesis of 14. For the synthesis of L1, the alcohol 9 was first activated as a mesylate. The formation of the mesylate was carried out in an NMR tube and followed by proton NMR. This allowed us to achieve nearly full conversion without using an excess of mesyl chloride that could produce sulfonamide side products. The mesylate was not isolated and was used directly for the next step. The alkylation was slow and required several days for completion (checked by 1H NMR for presence of the mesylate). Adding all the mesylate at once resulted in formation of unidentified byproducts and low yields. It was found that only very slow addition (over days) of mesylate to DO2A provided satisfactory yields of the product.
The alkylation step in the synthesis of L2, on the other hand, was a relatively fast and clean reaction, as the benzylic chloride in alkylating agent 13 is a much better leaving group. Ligand L2 was obtained after base-catalyzed hydrolysis of the methyl ester and acid-catalyzed cleavage of the tert-butyl protecting groups.
The methylphosphonate group in L3 was introduced by a Mannich type reaction with paraformaldehyde and tris(tert-butyl) phosphite. As opposed to other esters of phosphonic acids, the tert-butyl groups can be easily cleaved with TFA, thus affording removal of all the protective groups in the product in one step. Several smaller additions of paraformaldehyde and P(O(t-Bu))3 were found to give better yields than single complete addition.
When neat TFA was used for cleavage of the tert-butyl ester groups, formation of byproducts was observed, probably due to side reaction on the secondary amine. This was avoided by using a cleavage cocktail that contained a scavenging silane and water (TFA/TIPS/H2O, 90:5:5). In any case, pure L1, L2 and L3 could be obtained through cleanup on a disposable C18 column.
Synthesis of ligands L4, L5 and L6
Syntheses of L5 and L6 were conveniently accomplished with the same alkylation reactions as in L2 and L3 by using 14 as the starting material. Because 14 contains only one secondary amine, the alkylations proceeded much more cleanly than in L2 and L3. This approach, however, could not be applied for synthesis of L4. Reactivity of the mesylated 13 was insufficient to overcome the steric hindrance caused by the other three N-substituents. Instead, we used compound 15 (the tert-butyl ester of L1) and alkylated the fourth N-atom with the biphenyl moiety. This reaction proceeded quickly and without complications, yielding 16 that could be deprotected with TFA to provide L4. In all cases, cleavage of the tert-butyl ester groups could be achieved with neat TFA without any side products. All reactions could be easily and quantitatively followed by HPLC due to lipophilicity and high UV absorbance of the biphenyl moiety.
Synthesis of Ln complexes
Complexes were formed under standard conditions by mixing solutions of ligand and metal and adjusting the pH to neutral with NaOH. To increase solubility of amphiphiles L4, L5 and L6 the reaction was done in 20% aqueous acetonitrile. Complexation with ligands containing aromatic chromophores (L2, L4, L5 and L6) could be conveniently followed by reversed-phase HPLC until completion. However, this was not possible for the poorly absorbing ligands L1 and L3. Here, the metal was added in small portions until disappearance of the signal of free ligand in the MS trace on LC-MS. Salts and any possible excess of ligand or metal were removed by preparative HPLC on a C18 column using pure solvents (MeCN and water) with no additives. Use of acidic solvent additives (TFA, formic acid) for HPLC was avoided as these caused decomplexation of the chelates. Surprisingly, tests for the presence of unchelated lanthanide ions based on standard chelating indicators (Xylenol Orange or Arsenazo) could not be used for the chelates of L1, L4 and L5 because they gave positive reaction even with pure chelates. This is probably due to formation of ternary complexes or transchelation of the metal to the indicator dye (vide infra).
Relaxivity
Relaxivities of the Gd chelates were measured at Larmor frequencies of 20 and 60 MHz (0.47 and 1.4T, respectively) at two temperatures, 25 and 37 °C, and are summarized in Table 1. Complexes of the phosphonate derivatives L3 and L6 showed much higher (> 2-fold) relaxivities than complexes of the other ligands. This agrees with the known ability of phosphonate groups to attract strong second-sphere hydration (i.e. water molecules weakly associated with the chelate but not directly bound to gadolinium), thereby providing higher relaxivities.58 The relaxivity of [Gd(L6)] was slightly higher than that of [Gd(L3)], which is expected based on its higher molecular weight and therefore slower rotational motion. Surprisingly, the opposite trend was seen for the other derivatives: the relaxivities of the larger [Gd(L4)] and [Gd(L5)] were actually lower than the relaxivities of their counterparts without the biphenyl moiety. Such unexpected behavior could be explained by a decrease in hydration number. In fact, relaxivities in the range 2 – 3 mM−1s−1 indicate that [Gd(L4)] and [Gd(L5)] lack a water ligand in the coordination environment of gadolinium altogether.25,50
Table 1.
Relaxivities of the Gd chelates.
| relaxivity [mM−1s−1] | ||||
|---|---|---|---|---|
| 20 MHz | 60 MHz | |||
| 25 °C | 37 °C | 25 °C | 37 °C | |
| [Gd(L1)] | 3.6 | 2.9 | 3.5 | 2.8 |
| [Gd(L2)] | 3.6 | 3.5 | 3.4 | 3.0 |
| [Gd(L3)] | 7.5 | 5.5 | 7.2 | 5.1 |
| [Gd(L4)] | 2.4 | 2.2 | 2.2 | 1.9 |
| [Gd(L5)] | 3.0 | 2.5 | 2.8 | 2.6 |
| [Gd(L6)] | 7.8 | 5.9 | 8.0 | 5.8 |
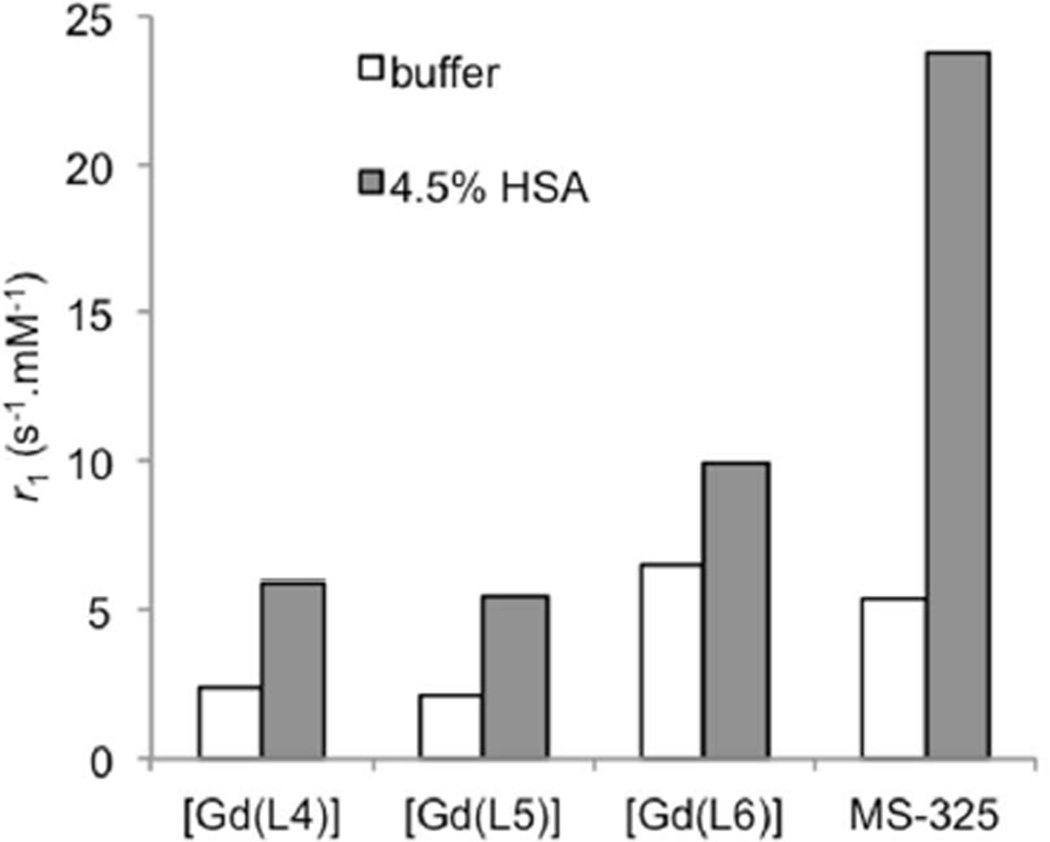
We further investigated how the relaxivity of the chelates changes upon slowing their rotational motion. The biphenyl moiety in ligands L4 – L6 is a targeting vector for binding to human serum albumin. Binding to HSA is based on nonspecific hydrophobic interactions and has proved very efficient in slowing the rotational motion of MR contrast agents. For example, a clinically approved blood pool agent, MS-325 (gadofosveset, Ablavar®) increases its relaxivity from 5.4 to 23.8 mM−1s−1 after binding to HSA (at 60 MHz and 37 °C).59 Similarly we expected increases in the relaxivities of chelates of L4 – L6 after binding to HSA. Relaxivities were determined from 20, 40 and 60 µM solutions in a 4.5% (w/v) solution of HSA (670 µM). Under these conditions, >99% of the complex was bound to HSA. Relaxivities of the unbound (in buffer only) and HSA-bound chelates are compared in Figure 4. However, all three complexes showed only a relatively small increase in relaxivity upon binding to HSA. The low relaxivities of [Gd(L4)] and [Gd(L5)] with HSA were characteristic of q = 0 complexes bound to HSA.25,50,59,60 The relaxivity of [Gd(L6)] was typical of a q = 1 complex when determined in the absence of HSA, and we anticipated a much higher increase in relaxivity upon binding to HSA than was observed. To exclude the possibility that this low relaxivity might be a result of poor affinity to HSA, we determined the unbound fraction of [Gd(L6)] with an independent technique. Protein was separated from solution by ultrafiltration and the concentration of the (unbound) chelate in the filtrate was determined with ICP-MS. We found that the unbound fraction is less than 0.1%. The modest relaxivity of protein-bound [Gd(L6)] suggests that the water ligand was displaced by donor groups from the protein. We did not investigate the HSA adducts further due to the low kinetic inertness of these chelates (vide infra).
Figure 4.
Relaxivity (60 MHz, 37 °C) of Gd complexes of L3 – L6 in absence (open bars) and presence (filled bars) of 4.5% human serum albumin in 50 mM HEPES buffer, pH = 7.4. Data for MS-325 are shown for comparison (reference59).
Hydration number q
The hydration number is not only an important parameter influencing relaxivity, but also provides an insight into the structure of chelates in solution. The Gd ion is usually 8 or 9 coordinate, and therefore full coordination of the octadentate ligands (L1, L2, L4 and L5) should result in q = 0 or q = 1 complexes, while the heptadentate ligands (L3 and L6) should provide q = 1 or q = 2 species. We determined q in the analogous Eu complexes by measuring the Eu luminescence lifetimes in H2O and D2O solutions following the method of Horrocks61 and calculating q according to the modified Horrocks equation.62 This technique provides an estimate of q with the accuracy ± 0.2. The results are summarized in Table 2.
Table 2.
Luminescence lifetimes and calculated hydration numbers for Eu complexes
| τH2O (ms) | τD2O (ms) | q | |
|---|---|---|---|
| [Eu(L1)] | 0.464 | 0.984 | 0.98 |
| [Eu(L2)] | 0.465 | 0.959 | 0.94 |
| [Eu(L3)] | 0.464 | 1.129 | 1.13 |
| [Eu(L4)] | 0.977 | 1.581 | 0.17 |
| [Eu(L5)] | 0.944 | 1.625 | 0.23 |
| [Eu(L6)] | - | - | 1a |
Data could not be obtained due to low solubility of [Eu(L6)]. q = 1 is assumed based on a similar relaxivity of [Gd(L6)] to [Gd(L3)].
For all six compounds the hydration number was in accordance with coordination of all ligand donor atoms to the central ion. Complexes of ligands L1 – L3 (without the biphenyl moiety) all contained one coordinated water molecule. The value for the derivative L3 was slightly higher than for L1 and L2, and is probably due to the presence of an additional OH oscillator on the phosphonate group. Europium complexes of biphenyl derivatives L4 and L5 contained no water ligand as was expected from the low relaxivities of the gadolinium chelates. Alkylation of the secondary amine with the noncoordinating biphenyl moiety in these compounds shifted the preference of the metal from CN9 to CN8, resulting in displacement of the water ligand. Low solubility precluded the determination of q for [Eu(L6)], but we assume q = 1 based on the relaxivity of [Gd(L6)]. Both L3 and L6 provide seven donor atoms and coordination of one water molecule is needed to complete the 8-coordinate state. Therefore, unlike L4 and L5, the presence of the biphenyl moiety in L6 did not result in displacement of the water ligand. It is known that Gd and Eu complexes of DO3A exist in solution in equilibrium between 8- and 9-coordinate states (q = 1 or 2, respectively).63 Similarly, the N-substituted DO3A derivatives (with non-coordinating N-substituents) have been reported to yield either monohydrated36, dihydrated29,35,38,40,42,44,64,65 or mixed37,66,67 states, with no apparent trends that would help to predict the hydration. Clearly, subtle details can decide the preference of the lanthanide ion for 8- or 9-coordinate state. In our case, all N-alkylated ligands resulted in 8-coordinate chelates.
In view of these results it is interesting that the single water molecule could be displaced from [Gd(L6)] chelate after biding to HSA. Displacement of water ligands by protein residues was previously observed for q = 2 complexes of DO3A derivatives in adducts with HSA40,50 and other proteins.68 However, this is a common problem of q = 2 complexes.25,50,60 In the case of the q = 1 complex [Gd(L6)] the displacement might be accompanied by expansion of the coordination number from 8 to 9 with coordination of two donor groups from HSA. Furthermore, the process might be facilitated by the orientation of the bound chelate: after binding with the biphenyl moiety, the chelate is forced to turn to the protein surface with its water-binding site.
We further tested the ability of common endogenous anions (bicarbonate and lactate) to displace water ligands from complexes [Gd(L1)], [Gd(L2)] and [Gd(L3)] by measuring the relaxivity of 1 mM complexes as a function of added anion (0.1 – 20 mM) at pH = 7.4 (see Supporting Information). In this test, the heptadentate phosphonate L3 demonstrated behavior typical for a DO3A derivative, showing a dramatic decrease of relaxivity with increasing anion concentration. On the other hand, the Gd complexes of octadentate ligands L1 and L2 showed negligible changes in their relaxivities. The bidentate donor arms in L1 and L2 successfully blocked formation of ternary adducts and prevented water displacement.
Water exchange rate
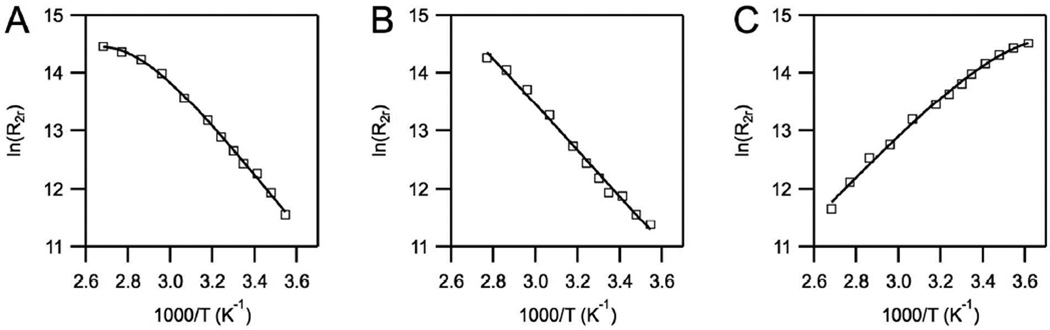
Water exchange plays an important role in the relaxivity of gadolinium chelates. Chelates with an equal number of coordinated water ligands can have very different relaxivities, depending on how fast these water ligands are exchanging. It was therefore interesting to examine the water exchange in q = 1 gadolinium chelates of L1 – L3 that have very different relaxivities. Water exchange can be determined from the temperature dependence of H217O transverse relaxivity of the Gd chelate. We used a 4-parameter model described previously to fit the data.59 Figure 5 shows the temperature dependence of the reduced transverse relaxation rate R2r (R2r is the difference in solvent H217O 1/T2 rates measured in the presence and absence of Gd and normalized to the mol fraction of coordinated water) as a function of reciprocal temperature. Such plots typically show a parabolic behavior with R2r first increasing with increasing temperature (slow exchange regime) and then reaching a maximum and decreasing with further temperature increases (fast exchange regime). It is apparent from Figure 5 that [Gd(L1)(H2O)] and [Gd(L2)(H2O)] exist in the slow exchange regime over the temperature range studied, while [Gd(L3)(H2O)]− shows the opposite behavior. In the slow exchange limit, R2r ≈ kex and for [Gd(L1)(H2O)] and [Gd(L2)(H2O)] we determined the water residency time at 310 K to be τM310 = 2190 ± 170 ns and 3500 ± 90 ns, respectively. These represent very slow exchange rates and are 20- to 30-fold slower than [Gd(DOTA)(H2O)]−.69 These exchange rates can also be compared to complexes with the same donor set but in a different arrangement. For example the Gd complex of DO3A derivative with a pyridyl donor at N1 had a water residency time τM310 = 499 ns,25 while an ether donor at N1 provided τM310 = 91 ns.47
Figure 5.
Temperature dependence of the 17O reduced transverse relaxation rates of solutions of [Gd(L1)(H2O)] (A), [Gd(L2)(H2O)] (B) and [Gd(L3)(H2O)]− (C) at 11.7 T. The solid lines represent fits to the data.
On the other hand, the water exchange rate for the phosphonate derivative [Gd(L3)(H2O)]− was very fast (τM310 = 12.7 ± 3.8 ns), and ideal for relaxivity purposes. The values of τM further explain the differences seen in the relaxivities of these chelates. The relaxivities of [Gd(L1)(H2O)] and [Gd(L2)(H2O)] are limited by both slow water exchange and fast rotational motion and are therefore low. Relaxivity of the phosphonate derivative [Gd(L3)(H2O)]− is almost 2-times higher because it is limited only by rotational motion, and therefore even a small increase in molecular weight from [Gd(L3)] to [Gd(L6)] results in a noticeable increase in relaxivity. It is remarkable that the water exchange rate could be tuned over 2 orders of magnitude by changing just one coordination arm in the structure of the ligand.
Kinetic inertness
The influence of a non-coordinating N-substituent in DO3A-like ligands on the kinetic inertness of gadolinium complexes has not been previously studied. Here we used the transmetallation method described by Laurent and coworkers.7,9,10 In this method, the gadolinium chelate is exposed to an equimolar concentration of Zn(II) in a phosphate buffer. As the zinc complexes are of similar thermodynamic stability to the gadolinium complexes, Gd(III) is gradually replaced in the complex by Zn(II). Released Gd(III) ions then react with phosphate to form a precipitate of insoluble GdPO4 that has negligible relaxivity. This transmetallation process is monitored by measuring the relaxation rate of the solution. For comparison, we performed this test with complexes clinically used as MR contrast agents ([Gd(DTPA)(H2O)]2− and [Gd(HP-DO3A)(H2O)]) and with structurally similar complexes [Gd(DO3A)(H2O)2] and [Gd(DO2A)(H2O)3]+.
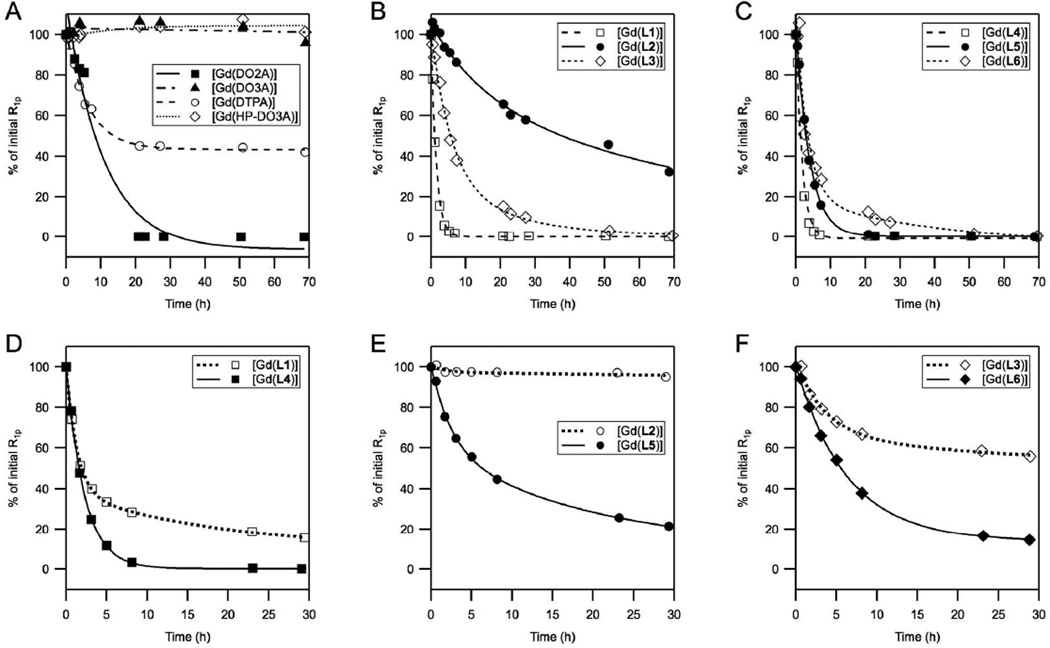
Figure 6A demonstrates the superior inertness of Gd chelates of macrocyclic ligands HP-DO3A and DO3A, both showing very little or no transmetallation over the course of 3 days. The acyclic DTPA ligand provides a substantially less inert Gd(III) chelate, however it qualitatively marks in Figure 6A the region of kinetic inertness that may still be safe for in vivo use. A particularly striking difference was found between the DO3A and DO2A ligands. The further loss of an acetate donor arm results in a very labile Gd complex in the case of hexadentate DO2A. The complex of DO2A showed somewhat different kinetic behavior, with the early decrease of R1 being slower than for DTPA, followed by a rapid decay and complete transmetallation at 20 h. We assume that the reaction in this case proceeds through a soluble intermediate(s) resulting in a seemingly slower transmetallation at early time points. Overall, the results are in accordance with expectations based on literature: for ligands with the same number of donor atoms, the macrocyclic structure (HP-DO3A) is more kinetically inert than acyclic structure (DTPA),5–12 and within the same type (macrocyclic), the kinetic inertness decreases with the decreasing denticity of the ligand.6,70
Figure 6.
Evolution of the paramagnetic contribution to relaxation rates (R1p) of 2.5 mM gadolinium chelates at 37 °C in 30 mM sodium phosphate buffer (pH = 7) with 2.5 mM Zn2+ (A – C). The values are expressed as % of the initial value. Lines represent biexponential fits of the data to guide the eye. (A) Complexes used as standards for comparison ([Gd(HP-DO3A)(H2O)], [Gd(DTPA)(H2O)]2−, [Gd(DO3A)(H2O)2], [Gd(DO2A)(H2O)3]+). (B) Complexes of chelates without biphenyl moiety. (C) Complexes of ligands with biphenyl moiety. (D – F) Comparison of complexes with (filled symbols) and without (empty symbols) biphenyl moiety measured in phosphate buffer only (no Zn2+).
Kinetic profiles of the chelates synthesized here without the biphenyl moiety are shown in Figure 6B. The picolinate derivative [Gd(L2)(H2O)] showed the highest kinetic inertness and was comparable with [Gd(DTPA)(H2O)]2−, however it was significantly more labile than [Gd(HP-DO3A)(H2O)] or [Gd(DO3A)(H2O)2]. The ethoxy-acetate derivative [Gd(L1)(H2O)] on the other hand, was the least stable of the three chelates and comparable to [Gd(DO2A)(H2O)3]+ in terms of lability. It is quite surprising that the ethoxy-acetate arm, although fully coordinated to the Gd ion, has a negligible contribution to the kinetic inertness of the chelate. In other words, the octadentate L1 ligand was comparable to the hexadentate DO2A ligand in terms of inertness. The phosphonate derivative [Gd(L3)(H2O)]− showed somewhat intermediate inertness between that of [Gd(L1)(H2O)] and [Gd(L2)(H2O)]. However, [Gd(L3)(H2O)]− was substantially less inert than structurally similar [Gd(DO3A)(H2O)2]. Again this was somewhat surprising given that the 8-coordinate [Gd(L3)(H2O)]− had only one coordinated water ligand which represented a less open site for initial ternary phosphate coordination compared to a q = 2 complex. On the other hand, the greater lability of [Gd(L3)(H2O)]− is in accordance with a generally lower kinetic inertness of phosphonate derivatives compared to carboxylates.71
When the biphenyl derivatives were tested (Figure 6C), the inertness of the pyridine-carboxylate derivative [Gd(L5)] was drastically reduced compared to [Gd(L2)]. In fact, all three biphenyl derivatives showed fast kinetics of transmetallation that were comparable with [Gd(DO2A)]. To test whether the biphenyl group had a similar effect on inertness in all derivatives, we used milder conditions and followed the decomposition of the chelates in phosphate buffer without the presence of zinc ions (Figure 6, D – F). We confirmed that the presence of the biphenyl moiety lowered kinetic inertness of all chelates, regardless of the choice of coordinating arms. The highest difference, however, was observed between the pyridine-carboxylate derivatives [Gd(L2)] and [Gd(L5)]. It is noteworthy that [Gd(L2)] was the only one of the six chelates that was practically inert towards the reaction with phosphate. The extreme lability of the chelates of L4 and L5 is further remarkable given that these are coordinatively saturated q = 0 complexes with no open site for phosphate to initially coordinate. This increased kinetic lability also explains the observation that chelates of L1, L4 and L5 gave a positive test for the presence of free lanthanide ions with Xylenol Orange and Arsenazo indicators. Both indicators are sufficiently strong chelators to compete for the metal ion, especially at the lower pH used in Xylenol Orange test (pH = 5.5).
From the kinetic experiments we semiquantitatively assessed the inertness of the chelates with two measures. We determined the time needed to reach 80% of the initial R1 value, as well as the percentage of the initial R1 rate remaining at 24 h of reaction (Table 3). This allowed us to rank the ligands according to relative kinetic inertness of their Gd chelates as follows: DO2A ≈ L4 < L1 ≈ L5 ≈ L6 < L3 << DTPA < L2 << DO3A < HP-DO3A.
Table 3.
Time required to reach 80% of R1 (t = 0) and values of R1 (t = 24 h) for Gd complexes
| Compound | Time to 80% R1 [h] |
% R1 (t = 24 h) |
% R1 (t = 24 h) phosphate only |
|---|---|---|---|
| [Gd(DO2A)] | N/Aa | 0 | 0 |
| [Gd(DTPA)] | 2.96 | 45 | inert |
| [Gd(DO3A)] | inert | inert | inert |
| [Gd(HP-DO3A)] | inert | inert | inert |
| [Gd(L1)] | < 0.5 | 0 | 18 |
| [Gd(L2)] | 11.4 | 64 | 96 |
| [Gd(L3)] | 1.81 | 11 | 58 |
| [Gd(L4)] | < 0.5 | 0 | 0 |
| [Gd(L5)] | 1.04 | 0 | 25 |
| [Gd(L6)] | 1.30 | 9 | 16 |
Values calculated from biexponential fits of the experimental data.
Value could not be reliably determined due to nonstandard behavior.
Several important conclusions can be drawn from the data in Figure 6. Figures 6A and 6B teach us that, in DO3A-like ligands, the choice of the third pendant arm (the other two being acetates) has by far the largest influence on the kinetic inertness of the Gd chelates. If the third arm does not coordinate strongly to the metal ion, the resulting chelates might be as kinetically labile as [Gd(DO2A)(H2O)3]+. Moreover, the inertness does not necessarily correlate with the number of donor atoms, e.g. the octadentate L4 formed a Gd complex as labile as with the hexadentate DO2A.
Finally, N-alkylation with a non-coordinating substituent can significantly lower the kinetic inertness of the chelates. We speculate that this may have three causes. (i) The electron withdrawing character of the benzylic biphenyl moiety decreases basicity of the nitrogen donor atom, thus weakening the Gd-N coordination bond. This, however, should result in a similar decrease in kinetic inertness regardless of the other substituents. We have seen that for the pyridine-carboxylate derivatives the effect was much more dramatic than for the other compounds. (ii) The bulky biphenyl may also strongly favor a non-coordinating conformation of the free ligand, thereby providing a thermodynamic driving force for decomplexation. (iii) The steric strain introduced by the bulky biphenyl may also induce a slight deformation to the macrocycle, making the ligand a less perfect fit for gadolinium ion. In such a case, binding of a rigid, pre-oriented donor arm like picolinate could be affected more strongly than binding of a more adaptable arm like ethoxyacetate. 1H NMR spectra of the Eu complexes seem to support this hypothesis. Extremely broad lines were observed for the complexes of biphenyl derivatives, indicating that these possess very flexible and hence unstable structures (see Supporting Information).
There is no doubt that the clinically used MR probes based on a macrocyclic cyclen structure are more kinetically inert compared to probes of the acyclic type, and are therefore safer with respect to the release of toxic Gd(III) ions in vivo. However, this property is often linked in the literature to macrocyclic chelators in general, despite the fact that some modifications to the ligand architecture are known to result in lower kinetic inertness. For example, enlargement of the cyclen ring with additional CH2 groups decreases the inertness of Gd chelates by several orders of magnitude.72 In chelates of cyclen/DOTA derivatives, replacing acetate donors with other donor groups can also result in somewhat lower inertness, however the effect is usually relatively small.52,73 Our findings surprisingly show that even octadentate ligands based on cyclen, while fully coordinated to Gd(III) ion, can yield extremely labile chelates unsuitable for in vivo applications. Our results demonstrate that kinetic inertness cannot be assumed, but should be experimentally determined for new Gd chelates designed for in vivo applications.
CONCLUSIONS
We synthesized several novel macrocyclic chelators of DO3A-like structures with two acetate arms and a variable third donor arm. We investigated the possibility to use alkylation of a secondary amine in the macrocycle as an alternative method to attach gadolinium chelates to targeting vectors. The variable third donor arms were chosen so as to give q = 1 gadolinium chelates and allowed us to control the water exchange rate over two orders of magnitude. Alkylation of the secondary amine in the macrocycle with a bulky non-coordinating substituent (HSA targeting vector) had unexpected and deleterious effects in the chelates. It shifted the preference of the Gd ion from a 9 to 8-coordinate state, thus eliminating the water co-ligand from complexes of octadentate chelators and decreasing the relaxivity. This modification also significantly increased the kinetic lability with respect to decomplexation when compared to the unalkylated versions. The resulting Gd chelates, although coordinatively saturated, showed remarkably poor kinetic inertness that was furthermore independent of the number of donor atoms in the chelator. These results are highly relevant to the problem of NSF and toxicity of MR probes, where the exceptional kinetic inertness of the few clinically used compounds is often wrongly generalized to all macrocyclic Gd chelates.
Supplementary Material
Scheme 2.
Synthesis of ligands L1 – L6 and the corresponding chelates.
ACKNOWLEDGMENT
This work was supported in part by award R01EB009062 from the National Institute of Biomedical Imaging and Bioengineering.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Fitted parameters from the variable-temperature 17O T2 experiments, LC-MS traces of all prepared ligands and chelates, time-dependent luminescence data of Eu chelates, relaxivities as a function of added bicarbonate and lactate, 1H NMR spectra of Eu chelates, list of HPLC methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Grobner T. Nephrol. Dial. Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 2.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Am. J. Roentgenol. 2007;188:586–592. doi: 10.2214/ajr.06.1094. [DOI] [PubMed] [Google Scholar]
- 3.Rofsky NM, Sherry AD, Lenkinski RE. Radiology. 2008;247:608–612. doi: 10.1148/radiol.2473071975. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen HS. Radiol. Clin. N. Am. 2009;47:827–831. doi: 10.1016/j.rcl.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Tweedle MF. Invest. Radiol. 1992;27:S2–S6. [PubMed] [Google Scholar]
- 6.Kumar K, Chang CA, Tweedle MF. Inorg. Chem. 1993;32:587–593. [Google Scholar]
- 7.Laurent S, Elst LV, Copoix F, Muller RN. Invest. Radiol. 2001;36:115–122. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Botteman F, Vander Elst L, Muller RN. Helv. Chim. Acta. 2004;87:1077–1089. [Google Scholar]
- 9.Laurent S, Elst LV, Muller RN. Contrast Media Mol. Imaging. 2006;1:128–137. doi: 10.1002/cmmi.100. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Vander Elst L, Henoumont C, Muller RN. Contrast Media Mol. Imaging. 2010;5:305–308. doi: 10.1002/cmmi.388. [DOI] [PubMed] [Google Scholar]
- 11.Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Invest. Radiol. 2008;43:817–828. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 12.Telgmann L, Wehe CA, Kunnemeyer J, Bulter AC, Sperling M, Karst U. Anal. Bioanal. Chem. 2012;404:2133–2141. doi: 10.1007/s00216-012-6404-x. [DOI] [PubMed] [Google Scholar]
- 13.Tweedle MF, Wedeking P, Kumar K. Invest. Radiol. 1995;30:372–380. doi: 10.1097/00004424-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, Walter J, Weinmann HJ, Pietsch H. Eur. Radiol. 2008;18:2164–2173. doi: 10.1007/s00330-008-0977-y. [DOI] [PubMed] [Google Scholar]
- 15.Sieber MA, Lengsfeld P, Walter J, Schirmer H, Frenzel T, Siegmund F, Weinmann HJ, Pietsch H. J. Magn. Reson. Imaging. 2008;27:955–962. doi: 10.1002/jmri.21368. [DOI] [PubMed] [Google Scholar]
- 16.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. Invest. Radiol. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 17.Pietsch H, Lengseld P, Steger-Hartmann T, Lowe A, Frenzel T, Huetter J, Sieber MA. Invest. Radiol. 2009;44:226–233. doi: 10.1097/RLI.0b013e3181998eb7. [DOI] [PubMed] [Google Scholar]
- 18.Fretellier N, Idee JM, Dencausse A, Karroum O, Guerret S, Poveda N, Jestin G, Factor C, Raynal I, Zamia P, Port M, Corot C. Invest. Radiol. 2011;46:292–300. doi: 10.1097/RLI.0b013e3182056ccf. [DOI] [PubMed] [Google Scholar]
- 19.White GW, Gibby WA, Tweedle MF. Invest. Radiol. 2006;41:272–278. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 20.Kim WD, Kiefer GE, Maton F, McMillan K, Muller RN, Sherry AD. Inorg. Chem. 1995;34:2233–2243. [Google Scholar]
- 21.Aime S, Calabi L, Cavallotti C, Gianolio E, Giovenzana GB, Losi P, Maiocchi A, Palmisano G, Sisti M. Inorg. Chem. 2004;43:7588–7590. doi: 10.1021/ic0489692. [DOI] [PubMed] [Google Scholar]
- 22.Werner EJ, Datta A, Jocher CJ, Raymond KN. Angew. Chem.-Int. Edit. 2008;47:8568–8580. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 23.Ruloff R, Toth E, Scopelliti R, Tripier R, Handel H, Merbach AE. Chem. Commun. 2002:2630–2631. doi: 10.1039/b207713b. [DOI] [PubMed] [Google Scholar]
- 24.Helm L, Merbach AE. Chem. Rev. 2005;105:1923–1959. doi: 10.1021/cr030726o. [DOI] [PubMed] [Google Scholar]
- 25.Dumas S, Jacques V, Sun W-C, Troughton JS, Welch JT, Chasse JM, Schmitt-Willich H, Caravan P. Invest. Radiol. 2010;45:600–612. doi: 10.1097/RLI.0b013e3181ee5a9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caravan P, Farrar CT, Frullano L, Uppal R. Contrast Media Mol. Imaging. 2009;4:89–100. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henig J, Toth E, Engelmann J, Gottschalk S, Mayer HA. Inorg. Chem. 2010;49:6124–6138. doi: 10.1021/ic1007395. [DOI] [PubMed] [Google Scholar]
- 28.Mastarone DJ, Harrison VSR, Eckermann AL, Parigi G, Luchinat C, Meade TJ. J. Am. Chem. Soc. 2011;133:5329–5337. doi: 10.1021/ja1099616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mieville P, Jaccard H, Reviriego F, Tripier R, Helm L. Dalton Trans. 2011;40:4260–4267. doi: 10.1039/c0dt01597k. [DOI] [PubMed] [Google Scholar]
- 30.Boros E, Polasek M, Zhang Z, Caravan P. J. Am. Chem. Soc. 2012;134:19858–19868. doi: 10.1021/ja309187m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courant T, Roullin VG, Cadiou C, Callewaert M, Andry MC, Portefaix C, Hoeffel C, de Goltstein MC, Port M, Laurent S, Vander Elst L, Muller R, Molinari M, Chuburu F. Angew. Chem.-Int. Edit. 2012;51:9119–9122. doi: 10.1002/anie.201203190. [DOI] [PubMed] [Google Scholar]
- 32.Frullano L, Caravan P. Curr. Org. Synth. 2011;8:535–565. doi: 10.2174/157017911796117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth E, Burai L, Brucher E, Merbach AE. J. Chem. Soc.-Dalton Trans. 1997:1587–1594. [Google Scholar]
- 34.Dunand FA, Aime S, Merbach AE. J. Am. Chem. Soc. 2000;122:1506–1512. [Google Scholar]
- 35.Pope SJA, Kenwright AM, Boote VA, Faulkner S. Dalton Trans. 2003:3780–3784. [Google Scholar]
- 36.Costa J, Balogh E, Turcry V, Tripier R, Le Baccon M, Chuburu F, Handel H, Helm L, Toth E, Merbach AE. Chem.-Eur. J. 2006;12:6841–6851. doi: 10.1002/chem.200501335. [DOI] [PubMed] [Google Scholar]
- 37.Placidi MP, Natrajan LS, Sykes D, Kenwright AM, Faulkner S. Helv. Chim. Acta. 2009;92:2427–2438. [Google Scholar]
- 38.Jebasingh B, Alexander V. Inorg. Chem. 2005;44:9434–9443. doi: 10.1021/ic050743r. [DOI] [PubMed] [Google Scholar]
- 39.Bruce JI, Dickins RS, Govenlock LJ, Gunnlaugsson T, Lopinski S, Lowe MP, Parker D, Peacock RD, Perry JJB, Aime S, Botta M. J. Am. Chem. Soc. 2000;122:9674–9684. [Google Scholar]
- 40.Aime S, Gianolio E, Terreno E, Giovenzana GB, Pagliarin R, Sisti M, Palmisano G, Botta M, Lowe MP, Parker D. J. Biol. Inorg. Chem. 2000;5:488–497. doi: 10.1007/pl00021449. [DOI] [PubMed] [Google Scholar]
- 41.Dickins RS, Aime S, Batsanov AS, Beeby A, Botta M, Bruce J, Howard JAK, Love CS, Parker D, Peacock RD, Puschmann H. J. Am. Chem. Soc. 2002;124:12697–12705. doi: 10.1021/ja020836x. [DOI] [PubMed] [Google Scholar]
- 42.Botta M, Aime S, Barge A, Bobba G, Dickins RS, Parker D, Terreno E. Chem.-Eur. J. 2003;9:2102–2109. doi: 10.1002/chem.200204475. [DOI] [PubMed] [Google Scholar]
- 43.Terreno E, Botta M, Fedeli F, Mondino B, Milone L, Aime S. Inorg. Chem. 2003;42:4891–4897. doi: 10.1021/ic034321y. [DOI] [PubMed] [Google Scholar]
- 44.Terreno E, Botta M, Boniforte P, Bracco C, Milone L, Mondino B, Uggeri F, Aime S. Chem.-Eur. J. 2005;11:5531–5537. doi: 10.1002/chem.200500129. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs Z, Sherry AD. Synthesis. 1997:759–763. [Google Scholar]
- 46.Mark V, Vanwazer JR. J. Org. Chem. 1964;29:1006–1008. [Google Scholar]
- 47.Botta M, Quici S, Pozzi G, Marzanni G, Pagliarin R, Barra S, Crich SG. Org. Biomol. Chem. 2004;2:570–577. doi: 10.1039/b313677a. [DOI] [PubMed] [Google Scholar]
- 48.Raghunand N, Guntle GP, Gokhale V, Nichol GS, Mash EA, Jagadish B. J. Med. Chem. 2010;53:6747–6757. doi: 10.1021/jm100592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mamedov I, Logothetis NK, Angelovski G. Org. Biomol. Chem. 2011;9:5816–5824. doi: 10.1039/c1ob05463e. [DOI] [PubMed] [Google Scholar]
- 50.Zech SG, Sun WC, Jacques V, Caravan P, Astashkin AV, Raitsimring AM. ChemPhysChem. 2005;6:2570–2577. doi: 10.1002/cphc.200500250. [DOI] [PubMed] [Google Scholar]
- 51.D'Aleo A, Allali M, Picot A, Baldeck PL, Toupet L, Andraud C, Maury O. C. R. Chimie. 2010;13:681–690. [Google Scholar]
- 52.Regueiro-Figueroa M, Bensenane B, Ruscsak E, Esteban-Gomez D, Charbonniere LJ, Tircso G, Toth I, de Blas A, Rodriguez-Blas T, Platas-Iglesias C. Inorg. Chem. 2011;50:4125–4141. doi: 10.1021/ic2001915. [DOI] [PubMed] [Google Scholar]
- 53.Roca-Sabio A, Bonnet CS, Mato-Iglesias M, Esteban-Gomez D, Toth E, de Blas A, Rodriguez-Blas T, Platas-Iglesias C. Inorg. Chem. 2012;51:10893–10903. doi: 10.1021/ic301369z. [DOI] [PubMed] [Google Scholar]
- 54.Nonat A, Gateau C, Fries PH, Mazzanti M. Chem.- Eur. J. 2006;12:7133–7150. doi: 10.1002/chem.200501390. [DOI] [PubMed] [Google Scholar]
- 55.Nonat A, Giraud M, Gateau C, Fries PH, Helm L, Mazzanti M. Dalton Trans. 2009:8033–8046. doi: 10.1039/b907738c. [DOI] [PubMed] [Google Scholar]
- 56.Nonat AM, Gateau C, Fries PH, Helm L, Mazzanti M. Eur. J. Inorg. Chem. 2012:2049–2061. [Google Scholar]
- 57.Nocton G, Nonat A, Gateau C, Mazzanti M. Helv. Chim. Acta. 2009;92:2257–2273. [Google Scholar]
- 58.Kotkova Z, Pereira GA, Djanashvili K, Kotek J, Rudovsky J, Hermann P, Elst LV, Muller RN, Geraldes C, Lukes I, Peters JA. Eur. J. Inorg. Chem. 2009:119–136. [Google Scholar]
- 59.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Inorg. Chem. 2007;46:6632–6639. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 60.Caravan P. Accounts Chem. Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 61.Horrocks WD, Sudnick DR. J. Am. Chem. Soc. 1979;101:334–340. [Google Scholar]
- 62.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods M. J. Chem. Soc.-Perkin Trans. 2. 1999:493–503. [Google Scholar]
- 63.Toth E, Ni Dhubhghaill OM, Besson G, Helm L, Merbach AE. Magn. Reson. Chem. 1999;37:701–708. [Google Scholar]
- 64.Quici S, Cavazzini M, Raffo MC, Botta M, Giovenzana GB, Ventura B, Accorsi G, Barigelletti F. Inorg. Chim. Acta. 2007;360:2549–2557. [Google Scholar]
- 65.Tu C, Nagao R, Louie AY. Angew. Chem.-Int. Edit. 2009;48:6547–6551. doi: 10.1002/anie.200900984. S6547/1-S6547/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koullourou T, Natrajan LS, Bhavsar H, Pope SJA, Feng JH, Narvainen J, Shaw R, Scales E, Kauppinen R, Kenwright AM, Faulkner S. J. Am. Chem. Soc. 2008;130:2178–2179. doi: 10.1021/ja710859p. [DOI] [PubMed] [Google Scholar]
- 67.Regueiro-Figueroa M, Esteban-Gomez D, de Blas A, Rodriguez-Blas T, Platas-Iglesias C. Eur. J. Inorg. Chem. 2010:3586–3595. doi: 10.1021/ic902461g. [DOI] [PubMed] [Google Scholar]
- 68.Strauch RC, Mastarone DJ, Sukerkar PA, Song Y, Ipsaro JJ, Meade TJ. J. Am. Chem. Soc. 2011;133:16346–16349. doi: 10.1021/ja206134b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell DH, NiDhubhghaill OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J. Am. Chem. Soc. 1996;118:9333–9346. [Google Scholar]
- 70.Lin CC, Chen CL, Liu KY, Chang CA. Dalton Trans. 2011;40:6268–6277. doi: 10.1039/c0dt01440k. [DOI] [PubMed] [Google Scholar]
- 71.Kotek J, Kalman FK, Hermann P, Brucher E, Binnemans K, Lukes I. Eur. J. Inorg. Chem. 2006:1976–1986. [Google Scholar]
- 72.Balogh E, Tripier R, Ruloff R, Toth E. Dalton Trans. 2005:1058–1065. doi: 10.1039/b418991d. [DOI] [PubMed] [Google Scholar]
- 73.Balogh E, Tripier R, Fouskova P, Reviriego F, Handel H, Toth E. Dalton Trans. 2007:3572–3581. doi: 10.1039/b706353a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.