Abstract
BACKGROUND
A prospective cohort study was undertaken to develop and validate a risk model for neutropenic complications in cancer patients receiving chemotherapy.
METHODS
The study population consisted of 3760 patients with common solid tumors or malignant lymphoma who were beginning a new chemotherapy regimen at 115 practice sites throughout the United States. A regression model for neutropenic complications was developed and then validated by using a random split-sample selection process.
RESULTS
No significant differences in the derivation and validation populations were observed. The risk of neutropenic complications was greatest in cycle 1 with no significant difference in predicted risk between the 2 cohorts in univariate analysis. After adjustment for cancer type and age, major independent risk factors in multivariate analysis included: prior chemotherapy, abnormal hepatic and renal function, low white blood count, chemotherapy and planned delivery ≥85%. At a predicted risk cutpoint of 10%, model test performance included: sensitivity 90%, specificity 59%, and predictive value positive and negative of 34% and 96%, respectively. Further analysis confirmed model discrimination for risk of febrile neutropenia over multiple chemotherapy cycles.
CONCLUSIONS
A risk model for neutropenic complications was developed and validated in a large prospective cohort of patients who were beginning cancer chemotherapy that may guide the effective and cost-effective use of available supportive care.
Keywords: neutropenia, febrile neutropenia, chemotherapy, risk model
Myelosuppression represents a major toxicity of systemic cancer chemotherapy associated with considerable morbidity, mortality, and costs.1 Such complications also result in dose reductions or treatment delays, which may compromise clinical outcomes.2–6 Previous studies have demonstrated that the risk of an initial episode of febrile neutropenia is greatest during the first cycle of treatment when most patients are receiving full dose intensity often without prophylactic measures.7–9 The risk of febrile neutropenia in cancer patients who are receiving systemic chemotherapy is generally based on reported rates in randomized controlled clinical trials (RCTs).10 However, studies have suggested that the risk of chemotherapy-induced neutropenia and its complications are considerably underreported in RCTs.11 Reported rates vary greatly for the same chemotherapy regimen, making it difficult to estimate the actual risk for neutropenic complications for common chemotherapy regimens.11 Variation in reported rates may relate to differences in study populations as well as to the chemotherapy dose intensity delivered, which is also frequently underreported.11 In addition, the selected patient populations eligible for such trials may not be representative of the majority of cancer patients treated in the general cancer population.
Recognized risk factors for chemotherapy-induced neutropenia and its complications can be classified on the basis of patient characteristics, cancer type, or type of treatment.12 Although older age has been identified as a risk factor for neutropenic complications potentially compromising cancer treatment in the elderly, age is less relevant than major in Wiley Online Library (wileyonlinelibrary.com) comorbidities that accompany increasing age.1–3,6,13,14 Low baseline white blood cell and neutrophil counts and hemoglobin levels appear to be predictors for febrile neutropenia.7,8 The intensity of the specific chemotherapy regimen used also represents a major determinant of the risk of chemotherapy-induced neutropenia.4
Risk assessment models for neutropenia developed to date have largely been based on retrospective data with several important methodologic limitations including small sample size, adjustment for different risk factors, and little, if any, validation.12 A prospective cohort study of cancer patients treated at oncology practices throughout the United States was undertaken to further study the incidence of and risk factors for neutropenic events in cancer patients who were receiving systemic chemotherapy. Preliminary observations with this population have confirmed that half to two-thirds of initial febrile neutropenia episodes occur during the first chemotherapy cycle across a broad range of malignancies.9 The primary goal of this investigation was to prospectively develop and validate a clinical risk model for the occurrence of severe or febrile neutropenia. The development of a valid risk model for neutropenic complications in patients receiving cancer chemotherapy should enable the identification of patients at greatest risk, facilitating more targeted application of available supportive care.
MATERIALS AND METHODS
Study Design and Patient Selection
A prospective cohort study was undertaken to collect data on representative cancer patients who were receiving chemotherapy in the community oncology setting. A stratified random-sampling method based on practice chemotherapy volume and geographic location was used to invite sites for participation in this registry from a nationwide inventory of 2382 practices. Patient enrollment was undertaken at 115 institutional review board-approved practice sites throughout the United States. Consecutive eligible patients who were beginning a new chemotherapy regimen were considered for study entry. Data were collected on patients beginning a new chemotherapy regimen between March 2002 and January 2006. The choice of chemotherapy regimen was at the discretion of the treating oncologist who was encouraged to enroll consecutive eligible patients. Data collection and site management were coordinated by an independent clinical research organization, and neither the funding agency nor the investigators had knowledge of the participating sites. Study records and data reside at the study coordinating center at Duke University, and the investigators retain rights to data analysis, interpretation, and the final contents of this publication. Exclusion criteria included a diagnosis of acute leukemia, a history of HIV infection or stem cell transplantation, and the receipt of concomitant myelosuppressive agents for other conditions or participation in blinded clinical trials. Primary tumor types for this study and included in this analysis are cancers of the breast, lung, colorectum or ovary, and malignant lymphoma.
Study Variables, Definitions, and Outcomes
Pretreatment and cycle-specific data were collected for up to 4 consecutive cycles of chemotherapy on all patients. Demographics and clinical variables included age, gender, ethnicity, employment and educational status, performance status, body surface area, cancer type, disease stage, history of prior cancer and treatment, concomitant medications, baseline hematology and chemistry results, and planned chemotherapy treatment including drugs, dose, and schedule. Glomerular filtration rate (GFR) was estimated by the method of Cockroft and Gault.15 The planned relative dose intensity (RDI) relative to published standards was estimated for each patient. Standards were based on published phase 3 randomized trials and regimens included in Guidelines from the National Comprehensive Cancer Network. Where more than 1 standard was identified, the least intensive combination of the recognized standards was used. Hematology and chemistry laboratory data were collected along with reported adverse events at the start and midpoint of each treatment cycle. Neutropenic events reported included neutropenia (absolute neutrophil count [ANC] nadir <1.0 × 103/mm3), severe neutropenia (ANC nadir <0.5 × 103/mm3) and febrile neutropenia (fever/infection and ANC nadir <1.0 × 103/mm3). The primary outcome of the study reported here was severe or febrile neutropenia in cycle 1 due to the dominant risk of events in the first cycle and their major impact on subsequent risk and treatment decisions.2,3,9
Statistical Methods and Model Derivation and Validation
Each study measure was assessed individually for completeness, consistency, and quality. Missing data were evaluated for any relation with the primary outcomes or any of the prognostic variables. Summary measures were generated for each variable, including estimates of central tendency and variability. Approximately 1000 evaluable patients would be required in the validation phase of the study to provide a power of 99% to discriminate a 20% risk of cycle 1 severe or febrile neutropenia (high risk) from a 10% risk (low risk) on the basis of the median risk. Therefore, a total of 3750 patients would be required using a 2:1 split-sample approach with a 2-tailed alpha error of .05 and allowing for a 20% rate of inevaluable patients. The 2:1 random split-sample procedure was applied to the final dataset dividing the population into 2500 (67%) patients used for model derivation and 1260 (33%) individuals upon which model validation was applied.16 The association between the primary outcomes and each covariate was studied with both univariate and multivariate analysis. Formal hypothesis testing was limited to associations considered a priori based on the reported literature or clinical experience. Analyses were based on an alpha error of 0.05 and 2-tailed tests of the null hypothesis with no adjustment for multiple testing.17,18
Covariates considered in model derivation were specified a priori based on previous retrospective studies or established biological or clinical rationale. Variables available for model entry in the derivation phase of the study included the specific treatment and possible interactions as well as any other factors of known prognostic significance. A clinical risk model for severe or febrile neutropenia in cycle 1 was developed based on multivariate logistic regression analysis incorporating only variable information available at baseline before or at the time of treatment initiation. A risk score was calculated as a weighted sum of regression coefficients (logarithm of odds ratios) from the model. The model allows calculation of a risk score as a weighted sum of the patient’s specific risk factors where the weights are estimated from the fitted logistic regression model. The covariate weights consist of the model’s beta coefficients corresponding to the natural logarithms of covariate odds ratios. The individual patient risk of cycle 1 severe or febrile neutropenia can then be estimated as the inverse logit of the risk score:
Higher risk score is associated with greater predicted risk of neutropenic events.
Model derivation used a forward stepwise procedure with variable entry based on a score statistic and removal based on a likelihood-ratio statistic using maximal likelihood estimates. Covariate coefficient estimates were based on the method of maximal likelihood along with 95% confidence limits on the estimates. For each model, performance characteristics that were assessed included sensitivity, specificity, positive and negative predictive values, the diagnostic odds ratio, model R2, and the area under the receiver operating characteristic (ROC) curve.19 The R2 estimate was based on the Nagelkerke modification20 of the Cox and Snell R2. First-order interaction terms were assessed, and no significant interactions were found. For each patient, the observed group, predicted probability, predicted group, residual, and standardized residual were estimated. The performance of the derivation model was then assessed in the separate validation population.
RESULTS
Study Population
Of the 4458 patients registered on this study, 3760 (84.3%) were diagnosed with 1 of the primary cancer diagnoses representing the focus of this analysis, ie, cancers of the colorectum (n = 521), lung (n = 907), ovary (n = 312), breast (n = 1473), or lymphoma (n = 547). The average age of included patients was 59.8 years (range, 18–97 years) with 1445 (38.4%) aged 65 and older. Seventy percent (70%) of patients were female with breast cancer representing the most common malignancy (39%) followed by lung cancer (24%), colorectal (14%), lymphoma (15%), and ovary (8%). The majority of patients were Caucasian (85%) with African Americans constituting an additional 10% of the study population. The majority of patients (65%) were stages 1–3, and 34% were stage 4. Of the 1106 (30%) of patients experiencing 1 or more episodes of severe or febrile neutropenia during the period of observation of up to 4 cycles, two-thirds experienced their initial episode in cycle 1. The analysis presented here is based on the 3638 (96.6%) of patients with available data on first cycle neutropenic events. For purposes of model derivation and validation, the study population was randomly divided based on split sampling into a derivation data set (n = 2425, 67%) and a separate validation data set (n = 1213, 33%).
Univariate Analysis
Table 1 displays the frequency of the primary outcome and prognostic covariates in the derivation and validation datasets, respectively. Three variables, ie, a history of chemotherapy (24% vs 22%), anthracycline-based chemotherapy (38% vs 42%), and myeloid growth factor use (20% vs 23%) demonstrated modest imbalance in analysis unadjusted for multiple testing, attesting to the technical adequacy of the splitting process. Table 2 displays the frequency of severe or febrile neutropenia in cycle 1 overall and major prognostic covariates in the derivation and validation datasets, respectively. Cycle 1 severe or febrile neutropenia was reported in 472 (20%) of patients in the derivation dataset compared with 257 (21%) of subjects in the validation population with no significant difference observed for any of the covariates. However, several factors were significantly associated with cycle 1 severe or febrile neutropenia in unadjusted univariate analysis including chemotherapy dose intensity, history of previous chemotherapy, elevated bilirubin, type of cancer, and certain classes of chemotherapeutic agents including anthracyclines, alkylating agents, and topoisomerase II inhibitor, whereas primary prophylaxis with a myeloid growth factor was associated with a significantly reduced risk.
Table 1.
Distribution of Neutropenia Risk Factors by Study Population
| Variables | No. | Derivation N=2500 | Validation N=1260 | P |
|---|---|---|---|---|
| % | % | |||
| Age, y | ||||
| ≥65 | 1445 | 38.4 | 38.6 | .464 |
| Prior chemotherapy | 880 | 24.4 | 21.6 | .029 |
| Baseline labs | ||||
| AST>35 U/L | 479 | 13.5 | 12.5 | .211 |
| AP>120 U/L | 715 | 19.5 | 19.7 | .456 |
| Bilirubin>1 mg/dL | 153 | 4.1 | 4.4 | .353 |
| GFR<60 mL/min | 732 | 19.5 | 19.4 | .508 |
| WBC<5000/mm3 | 499 | 12.8 | 14.3 | .106 |
| Cancer type | ||||
| Colorectum | 521 | 14.0 | 13.7 | |
| Small cell lung | 210 | 5.8 | 5.2 | |
| Nonsmall cell lung | 697 | 19.0 | 17.5 | .714 |
| Ovary | 312 | 8.4 | 8.1 | |
| Breast | 1473 | 38.7 | 40.1 | |
| Lymphoma | 547 | 14.1 | 15.4 | |
| Medications | ||||
| Immunosuppressives | 534 | 14.4 | 13.7 | .296 |
| Planned RDI | ||||
| ≥85% | 2623 | 69.9 | 69.4 | |
| <85% | 880 | 23.2 | 23.9 | .862 |
| Chemotherapy | ||||
| Anthracyclines | 1469 | 37.8 | 41.5 | .016 |
| Platinum(s) | 1200 | 32.5 | 30.8 | .156 |
| Taxanes | 1083 | 29.0 | 28.3 | .340 |
| Alkylating agents | 1615 | 42.1 | 44.6 | .078 |
| Topoisomerase II inhibitors | 232 | 6.2 | 6.0 | .432 |
| Gemcitabine | 257 | 6.9 | 6.7 | .415 |
| Topoisomerase I inhibitors | 41 | 1.1 | 1.01 | .476 |
| Vinorelbine | 120 | 3.4 | 2.8 | .178 |
| Primary CSF prophylaxis | 779 | 19.6 | 22.9 | .012 |
RDI indicates relative dose intensity; GFR, glomerular filtration rate; AST, aspartate aminotransferase; SN, severe neutropenia; FN, febrile neutropenia; WBC, white blood count; AP, alkaline phosphatase; CSF, colony-stimulating factors.
Table 2.
Univariate Analysis for Cycle 1 Severe or Febrile Neutropenia
| Variables | No. | Percentage (%)Cycle 1 SN or FN | P | |
|---|---|---|---|---|
| Derivation N=2425 | Validation N=1213 | |||
| All patients | 3638 | 19.5 | 21.2 | .221 |
| Age, y | ||||
| ≥65 | 1384 | 17.7 | 17.9 | .916 |
| <65 | 2254 | 20.5 | 23.2 | |
| P | .091 | .030 | ||
| Prior chemotherapy | ||||
| Yes | 847 | 15.1 | 14.0 | .662 |
| No | 2787 | 20.8 | 23.2 | |
| P | .002 | .001 | ||
| Baseline Labs | ||||
| AST>35 U/L | ||||
| Yes | 458 | 22.5 | 23.8 | .757 |
| No | 3068 | 18.7 | 21.2 | |
| P | .122 | .454 | ||
| AP>120 U/L | ||||
| Yes | 688 | 19.1 | 20.7 | .615 |
| No | 2853 | 19.3 | 21.7 | |
| P | .947 | .790 | ||
| Bilirubin>1 mg/dL | ||||
| Yes | 147 | 27.7 | 32.1 | .572 |
| No | 3388 | 19.0 | 21.0 | |
| P | .045 | .061 | ||
| GFR<60 mL/min | ||||
| Yes | 703 | 21.9 | 18.8 | .334 |
| No | 2935 | 18.9 | 21.7 | |
| P | .137 | .369 | ||
| WBC<5000/mm3 | ||||
| Yes | 485 | 23.3 | 25.0 | .673 |
| No | 3153 | 18.9 | 20.5 | |
| P | .077 | .195 | ||
| Cancer type | ||||
| Colorectum | 495 | 4.5 | 5.6 | .609 |
| Small cell lung | 198 | 27.7 | 31.1 | .625 |
| Nonsmall cell lung | 665 | 9.3 | 11.1 | .178 |
| Ovary | 303 | 10.8 | 11.1 | .932 |
| Breast | 1443 | 29.0 | 32.0 | .237 |
| Lymphoma | 534 | 23.0 | 25.3 | .555 |
| P | <.001 | <.001 | ||
| Medications | ||||
| Immunosuppressives | ||||
| Yes | 516 | 24.9 | 21.0 | .321 |
| No | 3122 | 18.5 | 21.2 | |
| P | .007 | .999 | ||
| Planned RDI | ||||
| ≥85% | 2546 | 22.3 | 24.2 | .273 |
| <85% | 849 | 12.5 | 11.7 | .737 |
| Nonstandard | 243 | 13.9 | 23.4 | .065 |
| P | <.001 | <.001 | ||
| Chemotherapy | ||||
| Anthracyclines | ||||
| Yes | 1438 | 34.5 | 37.3 | .289 |
| No | 2200 | 10.1 | 9.6 | |
| P | <.001 | <.001 | ||
| Platinums | ||||
| Yes | 1150 | 11.7 | 10.5 | .558 |
| No | 2488 | 23.1 | 25.9 | |
| P | <.001 | <.001 | ||
| Taxanes | ||||
| Yes | 1048 | 13.6 | 15.7 | .356 |
| No | 2590 | 21.9 | 23.3 | |
| P | <.001 | .004 | ||
| Alkylating agents | ||||
| Yes | 1586 | 32.0 | 33.0 | .699 |
| No | 2052 | 10.1 | 11.4 | |
| P | <.001 | <.001 | ||
| Topoisomerase II inhibitors | ||||
| Yes | 223 | 30.7 | 35.6 | .458 |
| No | 3415 | 18.7 | 20.3 | |
| P | .001 | .003 | ||
| Gemcitabine | ||||
| Yes | 241 | 13.8 | 9.9 | .390 |
| No | 3397 | 19.9 | 22.0 | |
| P | .063 | .007 | ||
| Topoisomerase I inhibitors | ||||
| Yes | 39 | 25.0 | 45.5 | .213 |
| No | 3599 | 19.4 | 21.0 | |
| P | .471 | .062 | ||
| Vinorelbine | ||||
| Yes | 111 | 15.4 | 6.1 | .176 |
| No | 3527 | 19.6 | 21.6 | |
| P | .467 | .030 | ||
| Primary CSF prophylaxis | ||||
| Yes | 772 | 6.4 | 9.8 | .081 |
| No | 2866 | 22.8 | 24.7 | |
| P | <.001 | <.001 | ||
RDI indicates relative dose intensity; GFR, glomerular filtration rate; AST, aspartate aminotransferase; SN, severe neutropenia; FN, febrile neutropenia; WBC, white blood count; AP, alkaline phosphatase; CSF, colony-stimulating factors.
Multivariate Analysis and Model Derivation
A multivariate risk model was developed based on the derivation population as outlined in the methods section. All baseline and pretreatment measures were considered candidates for model entry including patient demographics, type and stage of cancer, treatment type, schedule and planned dose intensity, reported comorbidities, abnormal baseline laboratory results, and baseline hematology values. Table 3 presents the resulting multivariate model developed on 2425 (97%) patients with complete data in the derivation data set. Important prognostic factors for cycle 1 severe or febrile neutropenia include a history of previous chemotherapy, patients on other immunosuppressive medications, patients with elevated aspartate aminotransferase, alkaline phosphatase or bilirubin, or reduced white blood count or estimated glomerular filtration rate, patients with small-cell lung cancer, and those with planned RDI ≥85% as well as several specific classes of chemotherapeutic agents including the anthracyclines, taxanes (paclitaxel, docetaxel), certain alkylating agents (cyclophosphamide, ifosphamide), type I and II topoisomerase inhibitors, platinums (cisplatin, carboplatin), gemcitabine, or vinorelbine. Lower risk was apparent in those receiving primary prophylaxis with a myeloid growth factor.
Table 3.
Multivariate Analysis for Cycle 1 Severe or Febrile Neutropenia (N=2425)
| Variables | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Age, y, ≥65 | 1.297 | .961–1.750 | .089 |
| Prior chemotherapy | 1.925 | 1.345–2.755 | <.001 |
| Baseline labs | |||
| AST>35 u/L | 1.422 | .991–2.041 | .056 |
| Alkaline phosphatase>120 u/L | 1.469 | 1.058–2.040 | .022 |
| Bilirubin>1 mg/dL | 2.152 | 1.235–3.747 | .007 |
| GFR mL/min | .993 | .989–.997 | <.001 |
| WBC×103/mm3 | .930 | .892–.969 | .001 |
| Cancer typea | .004 | ||
| Small cell lung | 1.556 | .641–3.781 | .329 |
| Nonsmall cell lung | .594 | .270–1.309 | .196 |
| Ovary | .515 | .214–1.242 | .140 |
| Breast | .842 | .377–1.880 | .675 |
| Lymphoma | .510 | .219–1.188 | .118 |
| Medications | |||
| Immunosuppressives | 1.554 | 1.105–2.187 | .011 |
| Planned RDI≥85% | 2.018 | 1.449–2.819 | <.001 |
| Chemotherapy | |||
| Anthracyclines | 7.353 | 4.577–11.811 | <.001 |
| Platinum(s) | 1.830 | 1.075–3.117 | .026 |
| Taxanes | 2.850 | 1.860–4.368 | <.001 |
| Alkylating agents | 5.853 | 3.215–10.658 | <.001 |
| Topoisomerase II inhibitors | 8.815 | 4.411–17.619 | <.001 |
| Gemcitabine | 3.092 | 1.696–5.638 | <.001 |
| Topoisomerase I inhibitors | 18.579 | 5.366–64.335 | <.001 |
| Vinorelbine | 4.218 | 1.896–9.385 | <.001 |
| Primary CSF prophylaxis | .120 | .079–.180 | <.001 |
Constant Term = −3.423
RDI indicates relative dose intensity; GFR, glomerular filtration rate; AST, aspartate aminotransferase; WBC, white blood count; CSF, colony-stimulating factors.
Reference category: colorectum: OR, 1.00.
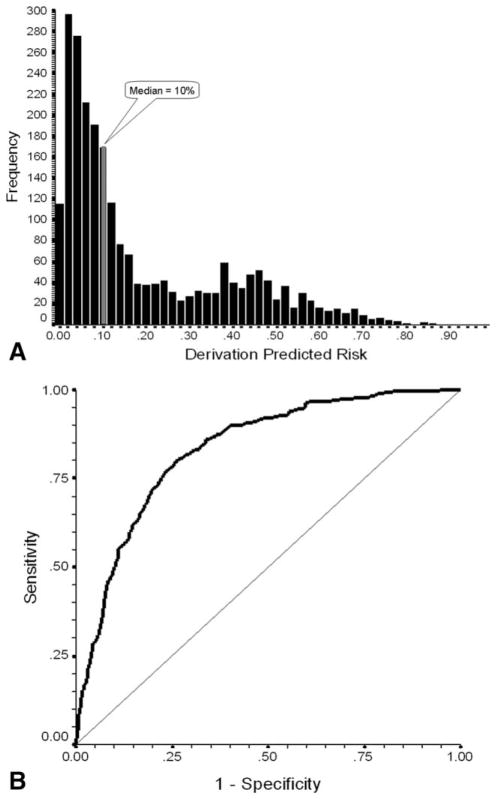
Model Performance
The model estimated the risk of severe or febrile neutropenia in cycle 1 for each patient in the derivation dataset (Fig. 1A). The predicted risk estimates based on the model ranged from 0% to 90% with mean and median values of 19% and 10%, respectively. Model goodness of fit was good (P<.0001). The first-cycle risk model for severe or febrile neutropenia based on the derivation population was associated with good risk discrimination characteristics. Shown in Figure 1B, the area under the ROC curve was 0.83 (95% CI, 0.81–0.85; P<.001). For the purpose of further assessing the predictive performance of the model, the predicted risk of cycle 1 events was stratified as above (high risk) or below (low risk) 10%. The model was associated with an estimated R2 of 0.338 and was capable of separating patients into a high risk half of whom 403 experienced cycle 1 events and a low risk half with observed events in 45 patients. As shown in Table 4, model performance in the derivation dataset was good with 90% of patients who actually experienced an event labeled as high risk by the model (sensitivity) and 59% of those not experiencing an event labeled as low risk (specificity). Neutropenic events occurred in cycle 1 in 34% of patients classified by the model as high risk (positive predictive value) compared with only 4% of those classified as low risk (1 − negative predictive value). As an overall measure of model discrimination, the diagnostic odds ratio (the ratio of the likelihood ratio positive to the likelihood ratio negative) was 12.81 (95% CI, 9.29–17.67).
Figure 1.
(A) Frequency distribution of predicted risk of severe or febrile neutropenia in cycle 1 is shown for patients in the model derivation population. Average risk in low-risk patients below the median risk of 10% is 3.9%, whereas the average risk in those above the median risk is 34.2%. (B) The receiver operating characteristic (ROC) curve plots model sensitivity versus 1-specificity for the risk model developed on the derivation population. The straight line indicates the association between sensitivity and 1-specificity under the null hypothesis of no prognostic discrimination. The area under the ROC curve was 0.833 (95% CI, 0.813–0.852; P<.001).
Table 4.
Risk Model Performance in the Derivation and Validation Datasets
| Derivation and Validation Models Severe or Febrile Neutropenia Risk Based on Median Predicted Risk | |
|---|---|
| Derivation | Validation |
|
|
Model Validation
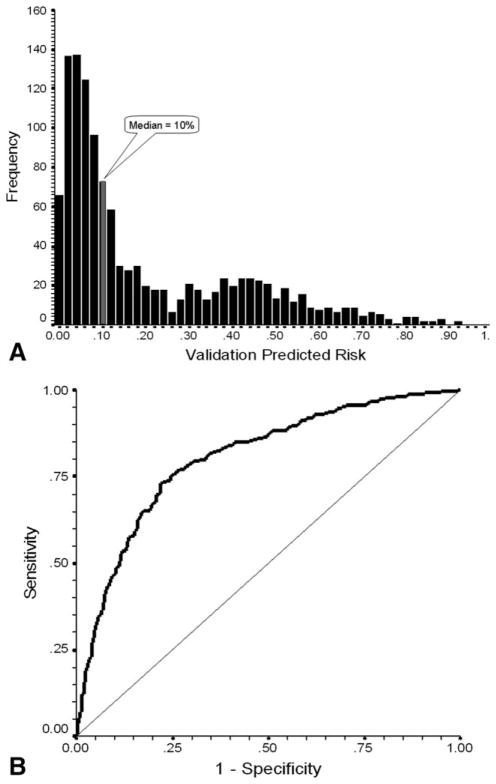
To validate the generated risk model, model parameters were applied to the randomly selected separate population of patients from the same prospective study. Although representing a smaller sample size, this separate population demonstrated very good concordance and discrimination with excellent goodness of fit (P<.0001) and an R2 of 0.349. Figure 2A displays the distribution of individual predicted risks for cycle 1 severe or febrile neutropenia in the validation sample ranging from 0% to 93% with mean and median values of 20% and 10%, respectively. Application of the model to the validation data set was similarly associated with excellent risk discrimination with an area under the ROC curve of 0.81 (95% CI, 0.77–0.84; P<.0001; Fig. 2B). Model test discrimination based on the median of predicted risk performed equally well in the validation patients. Model performance characteristics for validation patients, showing sensitivity and specificity of 85% and 59%, respectively, are also in Table 4. Neutropenic events occurred in 36% and 6% of validation patients classified as high risk and low risk, respectively. The diagnostic odds ratio as a measure of overall model discrimination is 8.03 (95% CI, 5.56–11.62). The close concordance between the predicted risk and the observed risk for cycle 1 severe or febrile neutropenia in the derivation and validation populations is shown in Figure 3.
Figure 2.
(A) Frequency distribution of predicted risk of severe or febrile neutropenia in cycle 1 is shown for patients in the model validation population. Average risk in low-risk patients below the median risk of 10% is 6.6%, whereas the average risk in those above the median risk is 36.1%. (B) The receiver operating characteristic (ROC) curve for the risk model developed on the derivation population was applied to the validation population and plots model sensitivity versus 1-specificity. The straight line indicates the association of sensitivity and 1-specificity under the null hypothesis of no prognostic discrimination. The area under the ROC curve was 0.805 (95% CI, 0.774–0.836; P<.0001]
Figure 3.
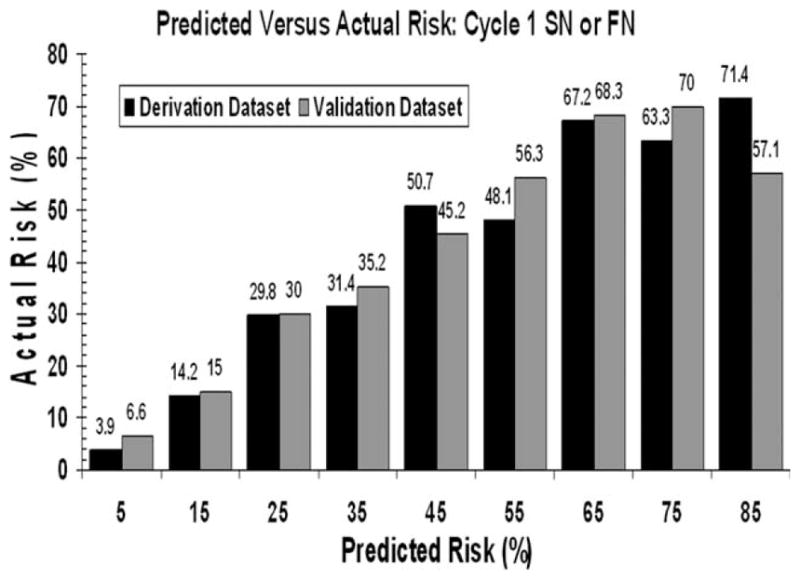
Distribution of the actual risk of severe or febrile neutropenia in cycle 1 for various predicted risks is based on the risk model in both the derivation (black bars) and validation (gray bars) populations.
Model Discrimination for Cumulative Risk of Febrile Neutropenia
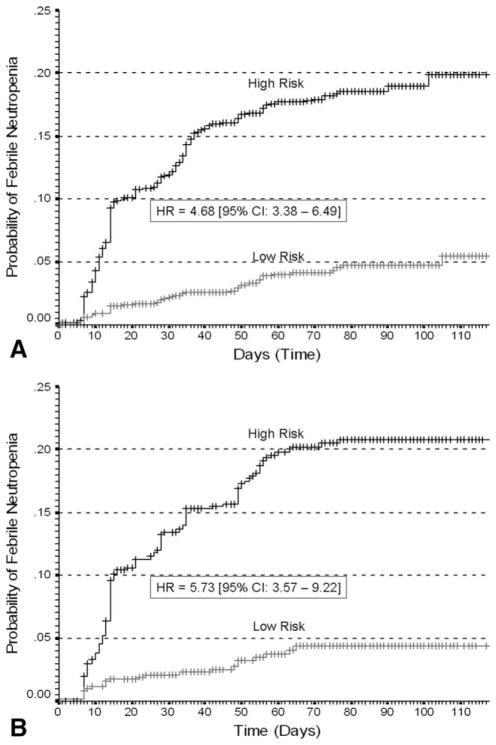
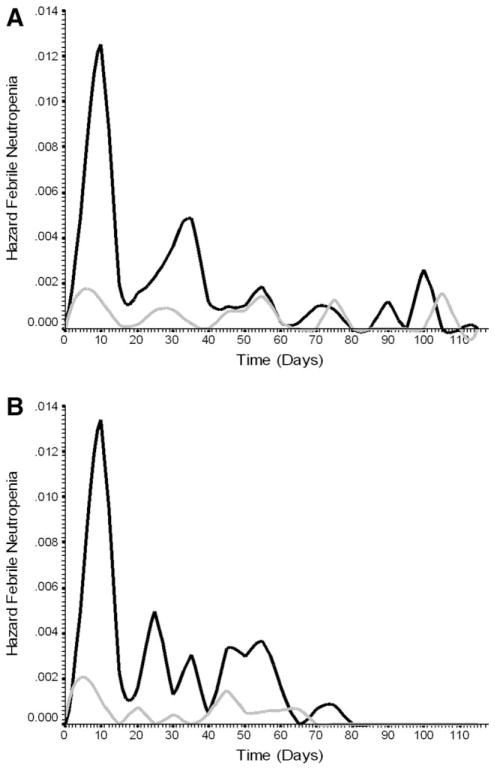
Whereas the model reported here was derived for the composite risk of severe or febrile neutropenia in the first cycle of chemotherapy, model validity for evaluating the risk of febrile neutropenia during the course of chemotherapy was also assessed. The cumulative risk of febrile neutropenia with repeated cycles of chemotherapy for high- and low-risk patients classified by the risk model is shown in Figure 4 for both the derivation (Fig. 4A) and validation populations (Fig. 4B). Kaplan-Meier estimates for the cumulative risk of febrile neutropenia are approximately 20% in high-risk patients compared with 5% in low-risk patients. Figure 5 displays hazard plots for the cumulative risk of febrile neutropenia in both the derivation (Fig. 5A) and validation (Fig. 5B) populations demonstrating that the period of greatest risk and the greatest separation of risk based on the risk model is early in the course of treatment.
Figure 4.
(A) Kaplan-Meier plot displays the cumulative proportion of patients who experienced 1 or more episodes of febrile neutropenia over time in days after chemotherapy initiation for both high-risk and low-risk patients in the derivation population based on the risk model. (B) Kaplan-Meier plot displays the cumulative proportion of patients who experienced 1 or more episodes of febrile neutropenia over time in days after chemotherapy initiation for both high-risk and low-risk patients in the validation population based on the risk model.
Figure 5.
(A) Hazard function plot displays the distribution of hazard rates for febrile neutropenia over time in days after chemotherapy initiation for both high-risk and low-risk patients in the derivation population based on the risk model. (B) Hazard function plot displays the distribution of hazard rates for febrile neutropenia over time in days after chemotherapy initiation for both high risk and low risk patients in the validation population based on the risk model.
DISCUSSION
A prospective cohort study of cancer patients who were beginning systemic chemotherapy for common adult malignancies was conducted between 2002 and 2006 and assessed the risk of treatment-related complications including severe and febrile neutropenia. The clinical risk model presented and validated here was based on a random split-sample methodology in more than 3500 patients with solid tumors and lymphoma who were beginning a new chemotherapy regimen. The results confirm that the risk of neutropenic complications is greatest early in the course of chemotherapy with considerable variation in the overall risk by cancer type and treatment regimen.9 Although retrospective studies have identified several risk factors, formal risk assessment in multivariate analysis has been limited by small patient numbers, missing and incomplete data, variable reporting, use of different outcomes, and differing measure assays and cutpoints applied to continuous measures.12,21 Data from such studies are generally collected for completely different purposes and not suited for rigorous risk modeling that requires complete and carefully collected measures.
Two small prospective studies in patients with hematological malignancies have recently presented preliminary results confirming the major importance of the type and intensity of chemotherapy administered for risk of neutropenic events.22,23 Despite the demonstrated importance of chemotherapy dose intensity to disease-free and overall survival in malignances treated with curative intent, many patients experience substantial reductions in relative dose intensity in clinical practice.2–5,14 Unlike previous studies, the prospective study reported here captured detailed data on chemotherapy dose, schedule, dose reductions, treatment delays, and both planned and received chemotherapy dose intensity. The authors believe that efforts to accurately estimate both efficacy and toxicity in cancer patients who are receiving systemic chemotherapy require a complete understanding of the treatment regimen and the drug delivery as well as patient-specific factors such as those also captured in this study. Other independent risk factors that contributed significantly to the risk of neutropenic complications in this study included a history of previous chemotherapy, specific concomitant medications, and certain medical comorbidities, most notably associated renal or hepatic dysfunction. Likewise, an independent effect of myeloid growth factor support on reducing the risk of severe and febrile neutropenia is consistent with results from RCTs and meta-analyses of such trials.24,25 Clinical practice guidelines from the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the European Organization for Research and Treatment of Cancer (EORTC) recommend consideration of primary myeloid growth factor prophylaxis when the risk of febrile neutropenia reaches or exceeds 20%.26–28 The guidelines also discuss the importance of assessing patient-specific risk factors and the need for prospective data on the risk of febrile neutropenia in the general cancer population.29
Model performance measures demonstrate a fairly high degree of discrimination between patients at high versus low risk for neutropenic events. Although the risk cutpoint based on the median of predicted risk was planned a priori eliminating the temptation to select cut-points based on observed results, the choice of cutpoint will have a direct influence on model sensitivity and specificity. Likewise, it is important to note that the predictive value of the model will depend considerably on the risk of events in the population studied. Nevertheless, application of the risk model to a separate validation population of patients generated similar predicted risk profiles and test performance characteristics, suggesting that the model has general applicability in identifying patients at increased risk for neutropenic complications.
This risk model is undergoing extensive additional validation testing in independent institutions and patient populations. With further validation in a range of clinical practice settings, the model will enable more objective identification of patients at increased risk for neutropenic complications that should, in turn, facilitate more effective and cost-effective use of available supportive care measures. Targeted application of primary prophylaxis with a myeloid growth factor starting in cycle 1 in patients identified as high risk for early events based on this model should reduce the risk of these serious and potentially life threatening complications of cancer treatment while allowing for the safe and adequate delivery of effective chemotherapy dose intensity. Alternatively, the identification of patients at low risk for early neutropenic complications may provide reassurance and cost savings in settings where more aggressive supportive care is not warranted. Personalization of supportive care strategies in a fashion similar to the personalization of targeted therapies offers considerable potential for more effective, safe, and cost-effective treatment along with improved survival and quality of life in patients with cancer.
Acknowledgments
This research was supported, in part, by research grant support to Duke University (Dr Lyman, Principal Investigator) from Amgen. The funding agency was not involved in the study design, data collection and analysis or in the preparation and approval of this report. Data collection and site management were coordinated by an independent clinical research organization and neither the funding agency nor the investigators had knowledge of the participating sites. Study records and data reside at the study coordinating center at Duke University and the investigators retain rights to data analysis, interpretation, and the final contents of this publication. No one other than the authors of this manuscript was involved in any phase of its writing.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Gary Lyman is supported by grants from the National Cancer Institute (RC2CA148041-01) and the National Heart, Lung and Blood Institute (1R01HL095109-01). This study was supported, in part, by an unrestricted research grant to Duke University from Amgen. The funding agency was not involved in the study design, data collection and analysis or in the preparation and approval of this report.
References
- 1.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 5.Shayne M, Culakova E, Wolff D, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer. 2009;115:5319–5328. doi: 10.1002/cncr.24560. [DOI] [PubMed] [Google Scholar]
- 6.Shayne M, Culakova E, Poniewierski MS, et al. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer. 2007;110:1611–1620. doi: 10.1002/cncr.22939. [DOI] [PubMed] [Google Scholar]
- 7.Lyman GH, Morrison VA, Dale DC, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44:2069–2076. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003;98:2402–2409. doi: 10.1002/cncr.11827. [DOI] [PubMed] [Google Scholar]
- 9.Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6:109–118. doi: 10.6004/jnccn.2008.0012. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH. Risk assessment in oncology clinical practice. From risk factors to risk models. Oncology (Williston Park) 2003;17:8–13. [PubMed] [Google Scholar]
- 11.Dale D, Crawford J, Lyman GH. Myelotoxicity and Dose Intensity of Chemotherapy: Reporting Practices from Randomized Clinical Trials. J Natl Compr Canc Netw. 2003;1:440–454. doi: 10.6004/jnccn.2003.0038. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 13.Balducci L, Hardy CL, Lyman GH. Hematopoietic growth factors in the older cancer patient. Curr Opin Hematol. 2001;8:170–187. doi: 10.1097/00062752-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shayne M, Crawford J, Dale DC, et al. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100:255–262. doi: 10.1007/s10549-006-9254-4. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Molinaro AM, Simon R, Pfeiffer RM. Prediction error estimation: a comparison of resampling methods. Bioinformatics. 2005;21:3301–3307. doi: 10.1093/bioinformatics/bti499. [DOI] [PubMed] [Google Scholar]
- 17.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 19.Gerds TA, Cai T, Schumacher M. The performance of risk prediction models. Biom J. 2008;50:457–479. doi: 10.1002/bimj.200810443. [DOI] [PubMed] [Google Scholar]
- 20.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 21.Jenkins P, Freeman S. Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol. 2009;20:34–40. doi: 10.1093/annonc/mdn560. [DOI] [PubMed] [Google Scholar]
- 22.Moreau M, Klastersky J, Schwarzbold A, et al. A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol. 2009;20:513–519. doi: 10.1093/annonc/mdn655. [DOI] [PubMed] [Google Scholar]
- 23.Pettengell R, Bosly A, Szucs TD, et al. Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol. 2009;144:677–685. doi: 10.1111/j.1365-2141.2008.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 25.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 27.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Crawford J, Armitage J, Balducci L, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2009;7:64–83. doi: 10.6004/jnccn.2009.0006. [DOI] [PubMed] [Google Scholar]
- 29.Lyman GH, Kleiner JM. Summary and comparison of myeloid growth factor guidelines in patients receiving cancer chemotherapy. J Natl Compr Canc Netw. 2007;5:217–228. doi: 10.6004/jnccn.2007.0021. [DOI] [PubMed] [Google Scholar]