Abstract
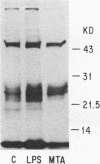
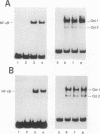
We show that both the lipopolysaccharide (LPS)-induced activation of NF-kappa DNA binding and kappa gene expression are blocked by treating murine pre-B lymphocyte 70Z/3 cells with 5'-methylthioadenosine (MTA), an inhibitor of several S-adenosylmethionine-dependent methylation reactions. We further show that the LPS-induced incorporation of radioactivity from [methyl-3H]methionine into methyl ester-like linkages on a group of membrane polypeptides is also inhibited by MTA treatment, suggesting the involvement of protein methylation reactions in the LPS signal transduction pathway. We also find that NF-kappa B and kappa gene activation in LPS-treated 70Z/3 cells is blocked by mevinolin, an inhibitor that prevents protein isoprenylation. Interestingly, mevinolin-treated cells also exhibited a marked reduction in the methylation of membrane proteins. Neither MTA nor mevinolin significantly inhibited NF-kappa B activation by phorbol myristate acetate, suggesting that these agents act early in signal transduction. These results provide the first evidence that carboxyl methylated and/or isoprenylated proteins play an essential role in the LPS-signaling pathway.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright S. W., Chen T. Y., Flebbe L. M., Lei M. G., Morrison D. C. Generation and characterization of hamster-mouse hybridomas secreting monoclonal antibodies with specificity for lipopolysaccharide receptor. J Immunol. 1990 Jul 1;145(1):1–7. [PubMed] [Google Scholar]
- Briskin M., Kuwabara M. D., Sigman D. S., Wall R. Induction of kappa transcription by interferon-gamma without activation of NF-kappa B. Science. 1988 Nov 18;242(4881):1036–1037. doi: 10.1126/science.3143155. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Sobotka C., O'Neill C. L. Lamin B methylation and assembly into the nuclear envelope. J Biol Chem. 1989 May 5;264(13):7637–7643. [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel-Issakani S., Spiegel A. M., Strulovici B. Lipopolysaccharide response is linked to the GTP binding protein, Gi2, in the promonocytic cell line U937. J Biol Chem. 1989 Dec 5;264(34):20240–20247. [PubMed] [Google Scholar]
- DeFranco A. L., Gold M. R., Jakway J. P. B-lymphocyte signal transduction in response to anti-immunoglobulin and bacterial lipopolysaccharide. Immunol Rev. 1987 Feb;95:161–176. doi: 10.1111/j.1600-065x.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A. J., Vandenbark A. A., Marchitto K. The role of 5'-methylthioadenosine phosphorylase in 5'-methylthioadenosine-mediated inhibition of lymphocyte transformation. Biochim Biophys Acta. 1979 Dec 11;588(3):294–301. doi: 10.1016/0304-4165(79)90337-4. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jondal M., Lanefelt F., Ng J. Effect of 5'-methylthioadenosine, 3-deazaadenosine, and related compounds on human natural killer cell activity. Relation to cyclic AMP and methylation potential. Scand J Immunol. 1984 Dec;20(6):511–518. doi: 10.1111/j.1365-3083.1984.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Galletti P., Oliva A., Manna C., Della Ragione F., Cartenì-Farina M. Effect of 5'-methylthioadenosine on in vivo methyl esterification of human erythrocyte membrane proteins. FEBS Lett. 1981 Apr 20;126(2):236–240. doi: 10.1016/0014-5793(81)80250-5. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Kincade P. W., Mizel S. B. Interleukin 1-mediated induction of kappa-light chain synthesis and surface immunoglobulin expression on pre-B cells. J Immunol. 1984 Jan;132(1):223–228. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Oct;10(10):5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Chin A. F., Abbott J. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2008–2012. doi: 10.1073/pnas.73.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakway J. P., DeFranco A. L. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science. 1986 Nov 7;234(4777):743–746. doi: 10.1126/science.3095921. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law R. E., Sinibaldi R. M., Cummings M. R., Ferro A. J. Inhbition of RNA synthesis in salivary glands of Drosophila melanogaster by 5'-methylthioadenosine. Biochem Biophys Res Commun. 1976 Dec 6;73(3):600–606. doi: 10.1016/0006-291x(76)90852-4. [DOI] [PubMed] [Google Scholar]
- Law R. E., Sinibaldi R. M., Ferro A. J., Cummings M. R. Effect of 5'-methylthioadenosine on gene action during heat shock in Drosophila melanogaster. FEBS Lett. 1979 Mar 15;99(2):247–250. doi: 10.1016/0014-5793(79)80965-5. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenylated proteins in cultured cells: subcellular distribution and changes related to altered morphology and growth arrest induced by mevalonate deprivation. J Cell Physiol. 1987 Dec;133(3):471–481. doi: 10.1002/jcp.1041330307. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Inducible binding of a factor to the c-fos enhancer. Cell. 1986 Dec 5;47(5):777–784. doi: 10.1016/0092-8674(86)90520-9. [DOI] [PubMed] [Google Scholar]
- Pérez-Sala D., Tan E. W., Cañada F. J., Rando R. R. Methylation and demethylation reactions of guanine nucleotide-binding proteins of retinal rod outer segments. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3043–3046. doi: 10.1073/pnas.88.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repko E. M., Maltese W. A. Post-translational isoprenylation of cellular proteins is altered in response to mevalonate availability. J Biol Chem. 1989 Jun 15;264(17):9945–9952. [PubMed] [Google Scholar]
- Rosoff P. M., Stein L. F., Cantley L. C. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984 Jun 10;259(11):7056–7060. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley P. J., Rukenstein A., Connolly J. L., Greene L. A. Differential inhibition of nerve growth factor and epidermal growth factor effects on the PC12 pheochromocytoma line. J Cell Biol. 1984 Feb;98(2):417–426. doi: 10.1083/jcb.98.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Azrolan N., Coleman P. S. Cellular distribution of cholesterogenesis-linked, phosphoisoprenylated proteins in proliferating cells. FEBS Lett. 1989 Mar 13;245(1-2):110–116. doi: 10.1016/0014-5793(89)80202-9. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M., Beck L. A., Leonard S., Evans R. Differential inhibitory effects of lovastatin on protein isoprenylation and sterol synthesis. J Biol Chem. 1990 Nov 15;265(32):19937–19941. [PubMed] [Google Scholar]
- Sinensky M., Logel J. Defective macromolecule biosynthesis and cell-cycle progression in a mammalian cell starved for mevalonate. Proc Natl Acad Sci U S A. 1985 May;82(10):3257–3261. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Smith D. S., King C. S., Pearson E., Gittinger C. K., Landreth G. E. Selective inhibition of nerve growth factor-stimulated protein kinases by K-252a and 5'-S-methyladenosine in PC12 cells. J Neurochem. 1989 Sep;53(3):800–806. doi: 10.1111/j.1471-4159.1989.tb11776.x. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990 Sep 25;265(27):16248–16254. [PubMed] [Google Scholar]
- Stimmel J. B., Deschenes R. J., Volker C., Stock J., Clarke S. Evidence for an S-farnesylcysteine methyl ester at the carboxyl terminus of the Saccharomyces cerevisiae RAS2 protein. Biochemistry. 1990 Oct 16;29(41):9651–9659. doi: 10.1021/bi00493a021. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Clarke S., Koshland D. E., Jr The protein carboxylmethyltransferase involved in Escherichia coli and Salmonella typhimurium chemotaxis. Methods Enzymol. 1984;106:310–321. doi: 10.1016/0076-6879(84)06031-6. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Ferro A. J., Barney C. L. Inhibition of lymphocyte transformation by a naturally occurring metabolite: 5'-methylthioadenosine. Cell Immunol. 1980 Jan;49(1):26–33. doi: 10.1016/0008-8749(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Wall R., Briskin M., Carter C., Govan H., Taylor A., Kincade P. A labile inhibitor blocks immunoglobulin kappa-light-chain-gene transcription in a pre-B leukemic cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(2):295–298. doi: 10.1073/pnas.83.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Seidenfeld J., Galletti P. Trends in the biochemical pharmacology of 5'-deoxy-5'-methylthioadenosine. Biochem Pharmacol. 1982 Feb 1;31(3):277–288. doi: 10.1016/0006-2952(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Wolberg G., Zimmerman T. P., Schmitges C. J., Duncan G. S., Deeprose R. D. Inhibition of lymphocyte cyclic AMP phosphodiesterase and lymphocyte function by 5'-methylthioadenosine. Biochem Pharmacol. 1982 Jun 15;31(12):2201–2203. doi: 10.1016/0006-2952(82)90518-4. [DOI] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]