Abstract
Expression of the Evi-1 gene is frequently activated in murine myeloid leukemias by retroviral insertions immediately 5' or 90 kb 5' of the gene. The Evi-1 gene product is a nuclear, DNA-binding zinc finger protein of 145 kDa. On the basis of the properties of the myeloid cell lines in which the Evi-1 gene is activated, it has been hypothesized that its expression blocks normal differentiation. To explore this proposed role, we have constructed a retrovirus vector containing the gene and examined its effects on an interleukin-3-dependent myeloid cell line that differentiates in response to granulocyte colony-stimulating factor (G-CSF). Expression of the Evi-1 gene in these cells did not alter the normal growth factor requirements of the cells. However, expression of the Evi-1 gene blocked the ability of the cells to express myeloperoxidase and to terminally differentiate to granulocytes in response to G-CSF. This effect was not due to altered expression of the G-CSF receptor or to changes in the initial responses of the cells to G-CSF. These results support the hypothesis that the inappropriate expression of the Evi-1 gene in myeloid cells interferes with the ability of the cells to terminally differentiate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Carroll P. M., Lee F. D. Abrogation of IL-3 dependent growth requires a functional v-src gene product: evidence for an autocrine growth cycle. Oncogene. 1990 Mar;5(3):317–325. [PubMed] [Google Scholar]
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C., Morishita K., Askew D., Buchberg A., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral insertions in the CB-1/Fim-3 common site of integration activate expression of the Evi-1 gene. Oncogene. 1989 May;4(5):529–534. [PubMed] [Google Scholar]
- Dudek H., Reddy E. P. Murine myeloid leukemias with aberrant myb loci show heterogeneous expression of novel myb proteins. Oncogene. 1989 Dec;4(12):1489–1495. [PubMed] [Google Scholar]
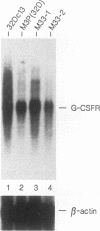
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Keller J. R., Ruscetti S. K., Ruscetti F. W. Introduction of v-abl oncogene induces monocytic differentiation of an IL-3-dependent myeloid progenitor cell line. Oncogene. 1990 Apr;5(4):549–555. [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Reversible dependence on growth factor interleukin-3 in myeloid cells expressing temperature sensitive v-abl oncogene. Oncogene Res. 1988 Feb;2(3):277–284. [PubMed] [Google Scholar]
- Krüger A., Anderson S. M. The v-src oncogene blocks the differentiation of a murine myeloid progenitor cell line and induces a tumorigenic phenotype. Oncogene. 1991 Feb;6(2):245–256. [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville P., Heisterkamp N., Groffen J. Expression of the chronic myelogenous leukemia-associated p210bcr/abl oncoprotein in a murine IL-3 dependent myeloid cell line. Oncogene. 1991 Feb;6(2):275–282. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
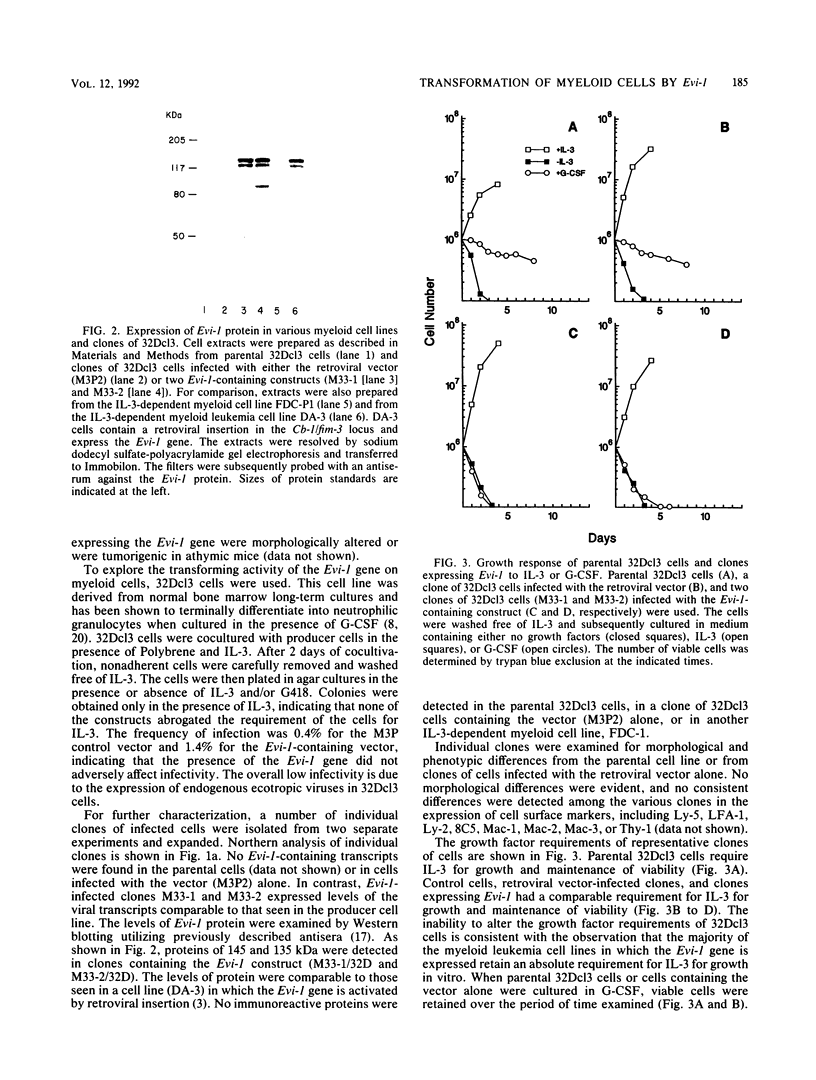
- Matsugi T., Morishita K., Ihle J. N. Identification, nuclear localization, and DNA-binding activity of the zinc finger protein encoded by the Evi-1 myeloid transforming gene. Mol Cell Biol. 1990 Mar;10(3):1259–1264. doi: 10.1128/mcb.10.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F., Kreider B. L., Valtieri M., Naso G., Shirsat N., Venturelli D., Reddy E. P., Rovera G. Alteration of growth and differentiation factors response by Kirsten and Harvey sarcoma viruses in the IL-3-dependent murine hematopoietic cell line 32D C13(G). Oncogene. 1989 Mar;4(3):301–308. [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Kreider B. L., Rovera G., Adamson J. W. Selection of lineage-restricted cell lines immortalized at different stages of hematopoietic differentiation from the murine cell line 32D. J Cell Biol. 1989 Aug;109(2):833–841. doi: 10.1083/jcb.109.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Kubota N., Asano S., Kaziro Y., Nagata S. Molecular cloning and characterization of cDNA for human myeloperoxidase. J Biol Chem. 1987 Mar 15;262(8):3844–3851. [PubMed] [Google Scholar]
- Morishita K., Parganas E., Parham D. M., Matsugi T., Ihle J. N. The Evi-1 zinc finger myeloid transforming gene is normally expressed in the kidney and in developing oocytes. Oncogene. 1990 Sep;5(9):1419–1423. [PubMed] [Google Scholar]
- Morishita K., Parker D. S., Mucenski M. L., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988 Sep 9;54(6):831–840. doi: 10.1016/s0092-8674(88)91175-0. [DOI] [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Copeland N. G., Jenkins N. A. Chromosomal location of Evi-1, a common site of ecotropic viral integration in AKXD murine myeloid tumors. Oncogene Res. 1988 Feb;2(3):219–233. [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Ihle J. N., Hartley J. W., Morse H. C., 3rd, Jenkins N. A., Copeland N. G. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol Cell Biol. 1988 Jan;8(1):301–308. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Perkins A. S., Fishel R., Jenkins N. A., Copeland N. G. Evi-1, a murine zinc finger proto-oncogene, encodes a sequence-specific DNA-binding protein. Mol Cell Biol. 1991 May;11(5):2665–2674. doi: 10.1128/mcb.11.5.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. S., Mercer J. A., Jenkins N. A., Copeland N. G. Patterns of Evi-1 expression in embryonic and adult tissues suggest that Evi-1 plays an important regulatory role in mouse development. Development. 1991 Feb;111(2):479–487. doi: 10.1242/dev.111.2.479. [DOI] [PubMed] [Google Scholar]
- Shirafuji N., Asano S., Matsuda S., Watari K., Takaku F., Nagata S. A new bioassay for human granulocyte colony-stimulating factor (hG-CSF) using murine myeloblastic NFS-60 cells as targets and estimation of its levels in sera from normal healthy persons and patients with infectious and hematological disorders. Exp Hematol. 1989 Feb;17(2):116–119. [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]