Abstract
Diabetic retinopathy is the most disabling complication of diabetes, affecting 65% of patients after 10 years of the disease. Current treatment options for diabetic retinopathy are highly invasive and fall short of complete amelioration of the disease. Understanding the pathogenesis of diabetic retinopathy is critical to the development of more effective treatment options. Diabetic hyperglycemia and dyslipidemia are the main metabolic insults that affect retinal degeneration in diabetes. Although the role of hyperglycemia in inducing diabetic retinopathy has been studied in detail, much less attention has been paid to dyslipidemia. Recent clinical studies have demonstrated a strong association between dyslipidemia and development of diabetic retinopathy, highlighting the importance of understanding the exact changes in retinal lipid metabolism in diabetes. This review describes what is known on the role of dyslipidemia in the development of diabetic retinopathy, with a focus on retinal-specific lipid metabolism and its dysregulation in diabetes.
Keywords: acid sphingomyelinase, cholesterol, diabetes, fatty acid, fatty acid elongase, phospholipid, retinopathy, sphingolipid
Stages of diabetic retinopathy
The progression of diabetic retinopathy is divided into a very early nonproliferative stage and a later, proliferative stage. The nonproliferative stage of diabetic retinopathy is characterized by neurodegeneration [1–3], retinal pericyte loss [4–8], vascular cell apoptosis leading to the formation of a cellular capillaries [9–12] and increased permeability [13–16]. Nonproliferative diabetic retinopathy could progress to the advanced proliferative stage with new, uncontrollably growing, retinal blood vessels covering the field of view and leading to vision loss.
The early stages of diabetic retinopathy are believed to involve low-grade chronic inflammatory disease [17–27]. The individual molecular steps leading to inflammation in the retina at early stages and progression to the proliferative stage are not yet well understood, but probably involve hyperglycemia and dyslipidemia associated with diabetes mellitus.
Diabetic dyslipidemia
Diabetic dyslipidemia is the result of an imbalance in the regulation of lipid uptake, metabolism, release by adipocytes and clearance from circulation. Insulin inhibits adipocyte hormone-sensitive lipase and activates lipoprotein lipase [28,29]. In the liver, insulin stimulates conversion of fatty acids to triglycerides, followed by secretion as VLDL, and the induction of fatty-acid desaturases and elongases [29–40]. Thus, insulin resistance in Type 2 diabetes and low-portal insulin levels in Type 1 diabetes are expected to have a profound effect on plasma lipid levels, lipid profiles and fatty-acid composition. Indeed, Type 2 diabetes is characterized by an elevation of blood levels of cholesterol and of esterified and nonesterified fatty acids. In Type 1 diabetes, the overall cholesterol, triglyceride and nonesterified fatty acid levels do not significantly differ from the control values [35,36,41]; however, there is a substantial change in the fatty-acid profile of these pools.
Clinical studies supporting the role of lipids in diabetic retinopathy
Clinical data show that dyslipidemia is a critical factor in the development of diabetic retinopathy in both Type 1 and Type 2 diabetes. Lipid peroxide levels were shown to be significantly elevated in the vitreous of patients with proliferative diabetic retinopathy [42]. A study on the DCCT/EDIC cohort revealed strong associations between the severity of retinopathy in Type 1 diabetes and the size of the particles of three major classes of serum lipoproteins: VLDL, LDL and HDL, as well as LDL concentration [43]. Cross-sectional studies demonstrate positive associations between the severity of retinopathy and total- and LDL-cholesterol levels, and LDL:HDL cholesterol ratio in Type 2 diabetic patients [44]. The Early Treatment Diabetic Retinopathy Study has demonstrated that higher levels of serum lipids are associated with an increased risk of development of hard exudates in the macula and visual loss [45,46]. Several studies demonstrated that lipid-lowering dietary [47] and drug [48] therapy may lead to regression of retinal hard exudates and that a diet high in polyunsaturated fatty acids may protect against retinopathy [49]. The most recent ACCORD Eye study of Type 2 diabetic patients demonstrated that the pairing of fenofibrate with simvastatin for intensive dyslipidemia therapy significantly slowed the progression of diabetic retinopathy over 4 years.
What is known about lipids in the diabetic retina?
The limitations of techniques traditionally used for lipid analysis (e.g., thin-layer chromatography and gas chromatography) have generally precluded comprehensive lipid analysis from the limited amount of material that can typically be obtained from either postmortem human retina samples or the retinas obtained from animal models of the disease (e.g., rats and mice). These limitations were removed with the development of electrospray ionization and matrix-assisted laser desorption/ionization techniques [50–57], coupled with the use of high-resolution mass spectrometry analysis [58–60], or tandem mass spectrometry methods [50–52,54,55,61–65]. Benefiting from the new methodological approaches, a number of recent studies have demonstrated that dyslipidemia plays an important role in the development and progression of diabetic complications. Below, using the comprehensive lipid classification system established by the NIH-funded LIPID MAPS consortium [66], a brief discussion is provided to highlight what is known regarding the importance of specific lipid classes in diabetic retinopathy. The following lipid classes will be discussed:
-
▪
Fatty acyls
-
▪
Oxidized bioactive lipids
-
▪
Glycated phospholipids
-
▪
Sterol lipids
-
▪
Sphingolipids
Fatty acyls
Retinal fatty acid composition in diabetes
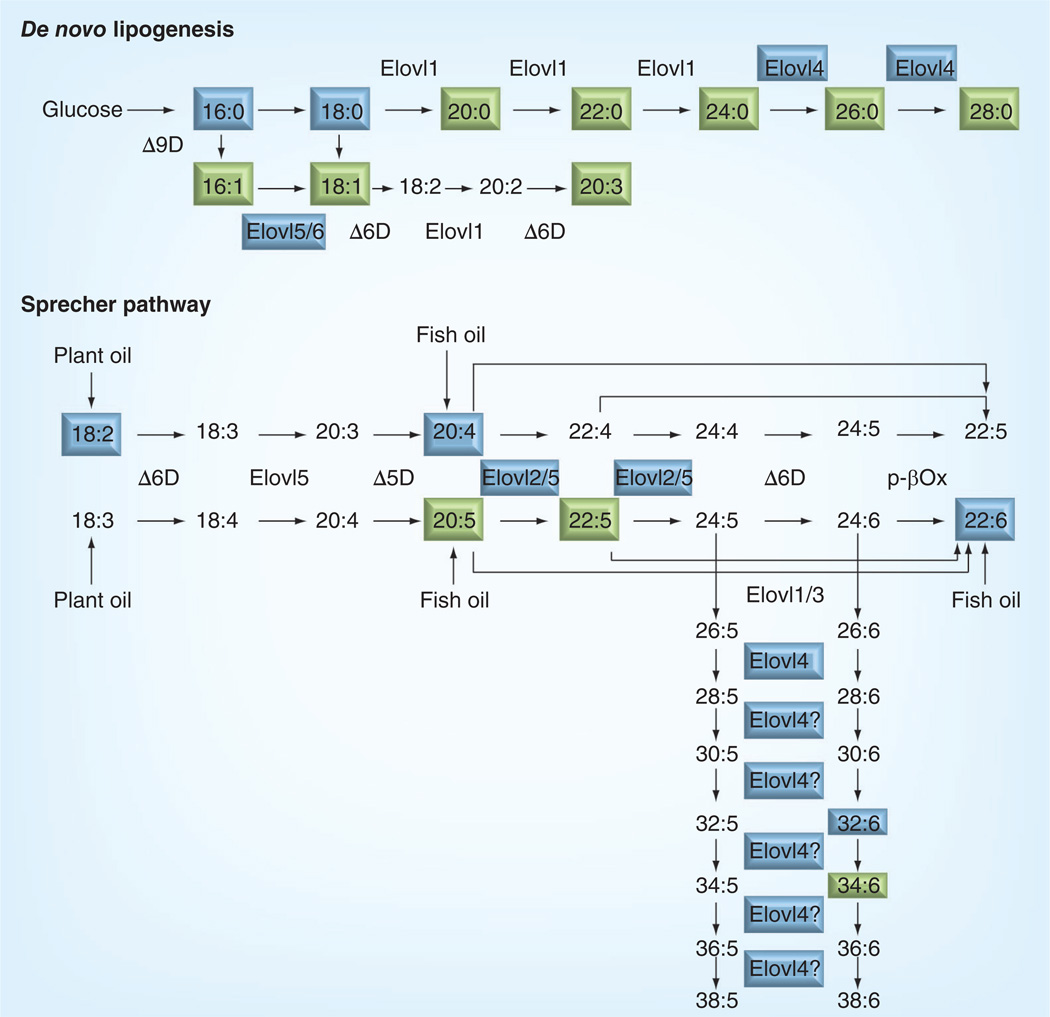
The fatty-acid composition of a particular tissue is determined by fatty-acid uptake from the circulation and local fatty-acid metabolism through de novo lipogenesis and polyunsaturated fatty acid (PUFA) remodeling (Sprecher) pathways [67]. Saturated-, monounsaturated- and polyunsaturated fatty acids are synthesized from dietary precursors (glucose and palmitic, oleic, linoleic, α-linolenic, eicosapentaenoic and docosahexaenoic acid [DHA]) through a series of desaturation (Δ5-, Δ6- and Δ9-desaturases) and elongation (Elovl1–6) reactions. In the recent work by Agbaga et al., the Sprecher pathway was expanded to include very long-chain PUFAs (VLCPUFA) – up to 38 carbon fatty acids in which elongation of shorter-chain fatty acid precursors is performed by Elovl4 (Figure 1) [68]. The retina has a unique fatty-acid profile with the highest levels of n-3 PUFA, especially DHA and VLCPUFA, in the body. This unique profile would suggest high levels of retinal-specific fatty-acid metabolism. Indeed, the retina has a very high expression level of fatty acid elongases. Among retinal elongases, Elovl4 shows the greatest expression level, followed by Elovl2, Elovl1 and Elovl6.
Figure 1. De novo lipogenesis and polyunsaturated fatty acid remodeling of the Sprecher pathway.
Fatty acids that accumulated in the retinal tissue are shown in boxes. The enzymes and fatty acids modified in the diabetic retina are highlighted blue.
In the human retina, Elovl4 was shown to be primarily expressed in the inner segment of photoreceptors, extending to photoreceptor cell bodies in the outer nuclear layer [69]. Moderate labeling was also observed in the ganglion cells [69]. Elovl4 has received much attention recently, as an autosomal dominant Stargardt-like macular dystrophy was linked to several dominant negative mutations in Elovl4 [69–72]. The role of VLCPUFA produced by Elovl4 is not known, but because of their localization in the retinal outer segment membranes and their ability to span both leaflets of the lipid bilayer, they are suggested to play a role in stabilizing cellular membranes with high curvature, such as the rims of photoreceptor disks [68]. In addition to VLCPUFA, Elovl4 was shown to be involved in the production of very long-chain saturated fatty acids [73], which are primarily incorporated into sphingolipids in the skin [74]. The role of sphingolipids with very long-chain saturated fatty acids in the retina is not known.
Insulin is the most potent activator of the elongase enzymes [39,40]. Thus, subnormal availability of insulin or insulin resistance in diabetes will result in reduced fatty acid remodeling and, consequently, will lead to accumulation of the substrates and depletion of the products. The overall effect of such metabolic perturbations would lead to a shift in the fatty-acid profile towards a higher saturation index and shorter fatty-acid chains [30–35,38,41]. For example, fatty acids measured from the plasma and erythrocyte phospholipids of diabetic children and young diabetic adults have revealed significantly lower levels of DHA compared with age-matched controls, whereas the level of linoleic acid was significantly higher in the diabetic subjects [41,75]. In the retina, early-stage diabetes induces a marked decrease in the expression levels of Elovl4, Elovl2 and Elovl6.
To determine the effect of this diabetes-induced downregulation of retinal elongases on lipid profiles in the diabetic retina, a comprehensive retinal lipid analysis method utilizing nano-electrospray ionization coupled with tandem mass spectrometry approaches was developed [64,65,76,77]. Using this approach, a significant decrease in total retinal DHA, as well as decreased incorporation of VLCPUFA, particularly 32:6n-3, into retinal phosphatidylcholine, was demonstrated. The retinal lipid profile and diabetes-induced changes observed in the retina were distinct from the liver and erythrocyte changes, supporting the hypothesis that diabetes induces specific changes in retinal lipid metabolism [78].
n-3 & n-6 PUFA in human retinal endothelial cell culture model
The role of fatty-acid profile changes in diabetic retinopathy, especially the most abundant PUFA in the retina, DHA, is beginning to emerge. The effect of n-3 and n-6 PUFA on low-grade inflammation associated with the development of diabetic retinopathy has been a focus of extensive studies in the present authors’ laboratory [79–81]. Using a well-established human retinal endothelial cell (HREC) culture system, the authors demonstrated that n-6 PUFAs, such as linoleic and arachidonic acid, induce adhesion molecule expression and leukocyte adhesion [81], whereas the n-3 PUFA, DHA, inhibits cytokine-induced NF-κB activation and nuclear translocation, as well as adhesion molecule expression [79,80]. They further identified DHA incorporation into phospholipids of caveolar membrane microdomains and displacement of cholesterol from these microdomains as important mechanisms of the anti-inflammatory effects of DHA in HREC [80,82–86].
n-3 PUFA in animal models of diabetic retinopathy
Dysregulation of retinal fatty acid remodeling results in the retinal-specific decrease of the most abundant retinal n-3 PUFA, DHA. However, a previously published study reported acceleration, rather than amelioration, of diabetic retinopathy in animals supplemented with fish oil [87]. In this study, rats were administered 750 mg of fish oil/day by tube feeding. This amount of fish oil per rat would be equivalent to 240 g of fish oil per day for an average-weight man or 60-times higher than the recommended dose of fish oil. Adult rats should receive 10% of caloric intake in the form of lipids, or 587.5 mg of lipids per day per 250 g rat. Thus, the rats in the fish oil treatment group were receiving much higher than recommended lipid content, all in the form of fish oil. Fish oil is deficient in a number of essential fatty acids including linoleic acid (18:2n-6) and the rats maintained on such a diet for 6 months are likely to have serious metabolic problems.
To determine the effect of the recommended dose of fish oil on the development of diabetic retinopathy, a study was performed with the animals maintained on the recommended 10% caloric intake from lipids with 5% coming from the fish oil in the treatment group. Two dietary experimental groups of animals were established in both normal control and diabetic animals: a vegetable oil diet and a fish oil diet group. The diets were custom-made by Dyets Inc. (PA, USA) based on AIN-93M purified rodent diet composition with 10% caloric intake in the form of soybean oil (standard rodent diet ingredient containing 50.8% linoleic acid [18:2n-6]) or 5% in the form of soybean oil and 5% in the form of Menhaden oil (oil from a small plankton-eating ocean fish containing 10.26% of DHA (22:6n-3) and 14.16% of EPA [20:5n-3]). The n-6:n-3 fatty acid ratio in vegetable oil diet was 8:1, reflecting the 15–16.7:1 ratio found in most western diets [88]. The n-6:n-3 ratio in fish oil diet was 1.5:1, reflecting the ratios associated with prevention of cardiovascular disease (4:1 [89]) and suppression of inflammation in patients with rheumatoid arthritis (2–3:1 [90]). This in vivo study revealed that supplementation with a DHA-rich diet at the recommended levels is indeed protective against capillary loss in the diabetic retinopathy animal model. DHA supplementation was also demonstrated to normalize impairment of the b-wave amplitude and latency time in diabetic rats. Total n-3 PUFAs, and especially DHA, were shown to be reduced in the retina of human diabetic eyes [91] and increasing the retinal levels of ω3-PUFAs reduces pathological retinal angiogenesis [92].
Oxidized bioactive lipids
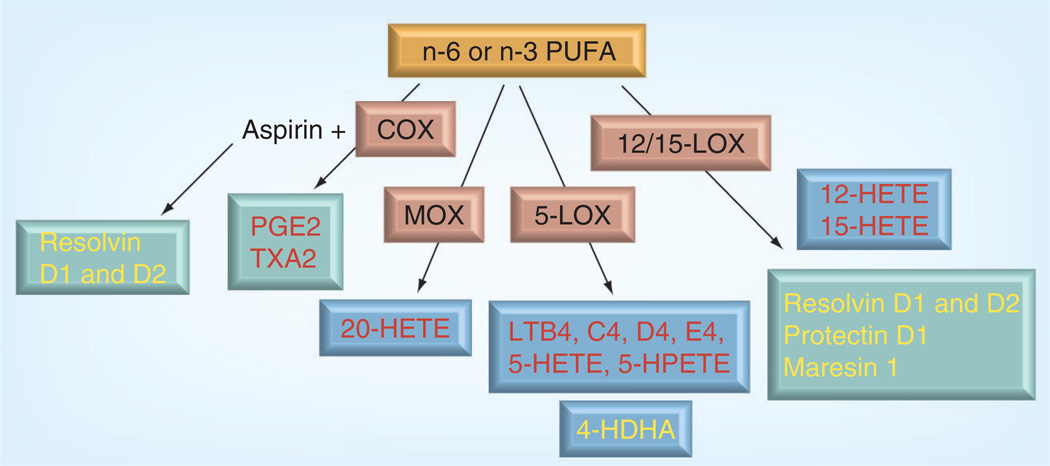
Unsaturated fatty acids are substrates for oxygenases such as COX, lipoxygenases (LOX) and CYP450 monoxygenases (MOX), and for nonenzymatic oxidation. COX-1 and -2, 5-, 12- or 15-LOX, or MOX [93–100] catalyze the conversion of unsaturated fatty acids into a range of biologically active compounds. There is a wealth of literature demonstrating that oxidative products of principal n-6 PUFAs, linoleic18:2n-6 and arachidonic20:4n-6 acids, are precursors of inflammatory mediators, including series 1- and 2-thromboxanes and prostaglandins, series 4 leukotrienes, as well as other bioactive lipid mediators [101], such as hydroxyl and epoxy fatty acids (Figure 2) [93–100]. P450-dependent metabolism of arachidonic acid resulting in the production of 20-hydroxyeicosatetraenoic acids (HETE) was shown to be involved in diabetes-induced vasoconstriction of retinal vessels. Cell culture studies using human retinal endothelial cells demonstrated that LOX, but not COX or P450 MOX pathways, may be essential for the fatty acid-mediated induction of adhesion molecules. LOXs are a diverse family of non-heme ferroproteins that catalyze the hydroperoxidation of polyunsaturated fatty acids. Thus far, six LOXs have been identified in humans: 12-LOX (platelet type), 12(R)-LOX, 15-LOX-1, 15-LOX-2, e-LOX-3 and 5-LOX [102]. LOX products, such as the hydroperoxyeicosatetraenoic acids, HETE, and their metabolites the leukotrienes, play a role in inflammation, especially in modulating cell–cell interactions in human aortic endothelial cells [103]. 12-LOX activity and expression were highly increased in a diabetic pig model [104].
Figure 2. Oxidized products of polyunsaturated fatty acids.
Arachidonic acid products are shown in red, docosahexaenoic products are shown in yellow. The products modified in the diabetic retina are highlighted in blue boxes.
HDHA: 4-hydroxy-docosahexaenoic acid; HETE: 20-hydroxyeicosatetraenoic acid;
HPETE: Hydroperoxyeicosatetraenoic acid; LOX: Lipoxygenase;
MOX: Monoxygenase; PUFA: Polyunsaturated fatty acid.
Recently, a new family of oxidized lipid products of major n-3 fatty acid, DHA (22:6n-3) was discovered in the brain and retina. These products, 17S-hydroxydocosanoids or docosanoids, such as resolvins, protectins and maresins formed either through LOX [92,105,106], COX [106,107] or nonenzymatic pathways [108] are known to possess anti-inflammatory, proresolution and prosurvival properties at nanomolar concentrations, as opposed to the proinflammatory properties of oxidized products of linoleic (18:2n-6) and arachidonic (20:4n-6) acid. Acetylation of COX-2 by aspirin changes to the COX-2 enzymatic scheme that, in the presence of adequate amounts of DHA (22:6n-3), leads to preferential production of 17R series resolvins [107], also has pronounced anti-inflammatory effects.
Lipoxygenases & the development of diabetic retinopathy
Interestingly, the hallmark of early vascular histopathology of diabetic retinopathy, formation of cellular capillaries, was significantly inhibited in 5-LOX-deficient mice, but not in 12/15-LOX-deficient mice [109]. However, the amounts of 12/15-LOX products, as well as 12- and 15-HETE, were significantly higher in the vitreous [110] and epiretinal membranes [111] of diabetic patients with late-stage proliferative diabetic retinopathy and retinal neovascularization was associated with increased 12-LOX expression and 12-, 15- and 5-HETE production. Moreover, retinal neovascularization was markedly reduced in 12-LOX-deficient mice [112]. These studies suggest that both 5-LOX and 12/15-LOX are important players in the development of diabetic microangiopathy with 5-LOX likely to be involved in the initial, vasodegenerative stages and 12/15-LOX at the later proliferative stages of diabetic retinopathy. Moreover, docosanoids were shown to have protective effects against retinal vaso-obliteration and neovascularization in the retinopathy of prematurity model [92]. This antiangiogenic effect was mediated by the product of DHA oxidation through the 5-lipoxygenase pathway, 4-hydroxydocosahexaenoic acid. The antiangiogenic effects of 4-hydroxydocosahexaenoic acid were through the activation of PPARγ, independent of VEGF production and anti-inflammatory effects [113]. Collectively, these results demonstrate the importance of the LOX enzyme in the pathogenesis of diabetic retinopathy, while highlighting that the effects of different LOX isoforms will depend on the stage of retinopathy and the available substrate.
These studies clearly demonstrate the important role of fatty acids in retinal health and disease. However, fatty acids are just the tip of the iceberg and fatty-acid effects are dependent on changes in phospholipids, sterol lipids and sphingolipid metabolism.
Glycated phospholipids
An increase in advanced glycation end products (AGEs) in diabetes is well documented and has been extensively studied over the last decade [114,115]. Moreover, serum levels of AGEs are known to be increased in patients with diabetic complications compared with a complications-free cohort [116,117]. However, AGE inhibitors have been shown to be effective in preventing the development of diabetic complications in animal models [118,119]. Although most prior studies have focused on AGE-modified proteins, the free amino groups of amino-phospholipids, such as GPEtn and GPSer, can also be targeted for nonenzymatic glycation, leading to the formation of glycated phospholipids [120]. Indeed, glycated GPEtn, referred to as Amadori-PE in the literature, has been shown to be increased approximately twofold in the blood plasma of Type 2 diabetic patients compared with controls [121]. The addition of synthetic Amadori-PE to human umbilical vein endothelial cell culture significantly increased angiogenic factors, including MMP2 [122], a pivotal enzyme in the initial step of angiogenesis [16]. A significant increase in the abundance of a modified (glycated) form of GPEtn lipids (i.e., Amadori-PE or GPEtn[Glc]) was observed between the diabetic and control retina in a Type 1 diabetic rat model [76]. For the most abundant Amadori-GPEtn lipid (i.e., Amadori-GPEtn [18:0/22:6]), a 4.8-fold increase (p < 0.05) in the ratio of the Amadori-GPEtn to GPEtn lipid abundance was observed in the diabetic versus control retina lipid extracts. Importantly, an increase in Amadori-GPEtn lipid abundance was also observed between the diabetic and control erythrocyte lipid extracts, where the most abundant Amadori-GPEtn lipid (GPEtn(18:10/20:4)) was observed to undergo a 1.6-fold increase (p < 0.05) [76]. The increase in Amadori-GPEtn levels in diabetic retinas indicates a potential role for this lipid in diabetic retinopathy.
Sterol lipids
A study by the DCCT/EDIC cohort has revealed a strong association between the severity of retinopathy in Type 1 diabetes and the size of the particles of three major classes of serum lipoproteins: VLDL, LDL and HDL, as well as LDL concentration [43]. Cross-sectional studies demonstrate positive associations between the severity of retinopathy and total- and LDL-cholesterol levels, and LDL:HDL cholesterol ratio [44]. High total cholesterol levels and LDL:HDL cholesterol ratio are also known risk factors in diabetic kidney and cardiovascular complications.
The ACCORD EYE study demonstrated that the pairing of fenofibrate with simvastatin for intensive dyslipidemia therapy significantly slowed the progression of diabetic retinopathy over 4 years, whereas glycemia therapy alone to achieve HbA1c levels of <6.0% versus a target of 7.0–7.9% was ineffective [123].
The level of cholesterol in the retina is dependent on the rate of accumulation and elimination. The retina and the brain are barrier tissues that have to rely mostly on the local synthesis of cholesterol as cholesterol cannot freely cross the blood–brain and blood–retinal barriers. The outer blood–retinal barrier is, however, more permeable to LDL-containing cholesterol [124]. The relative contributions of endogenously produced cholesterol versus that obtained from circulating LDL in the retina are not known. Elimination of cholesterol from the tissues involves reverse cholesterol transport and oxidation of cholesterol into more water-soluble oxysterols by CYP450 enzymes CYP46A1, CYP27A1 and CYP11A1. The retina expresses most of the proteins involved in the reverse transport of cholesterol including apoE and A1; cholesterol efflux transporter ABCA1; class B scavenger receptors SR-BI, SR-B-II and CD36; cholesteryl ester transfer protein and lecithin-cholesterol acyltransferase [125–129]. The retina was also shown to have a unique CYP-dependent cholesterol elimination pathway with the main product being 5-cholestenoic acid, rather than 24S-hydroxicholesterol as in the central nervous tissue [130,131]. The effect of diabetes on the rate of cholesterol production and elimination is unknown.
A product of cholesterol auto-oxidation, 7-ketocholesterol (7kCh), is elevated in diabetes [132–134]. Like cholesterol, 7kCh can also form microdomains [135], but the properties 7kCh in microdomains, and the effect of DHA on 7kCh in microdomains could be different from cholesterol. 7kCh is a potent proapoptotic agent shown to activate caspase [136]. Elevated levels of 7kCh were recently shown in photodamaged retina and are suggested to play a role in photoreceptor degeneration after exposure to constant light [137]. The role of 7kCh in the pathogenesis of diabetic retinopathy has not been studied.
Oxidized and glycated LDL identified in the retina in diabetes were shown to induce retinal pericyte apoptosis. Oxidized LDL immunocomplexes in the retina were implicated in diabetic retinopathy [138,139].
Sphingolipids
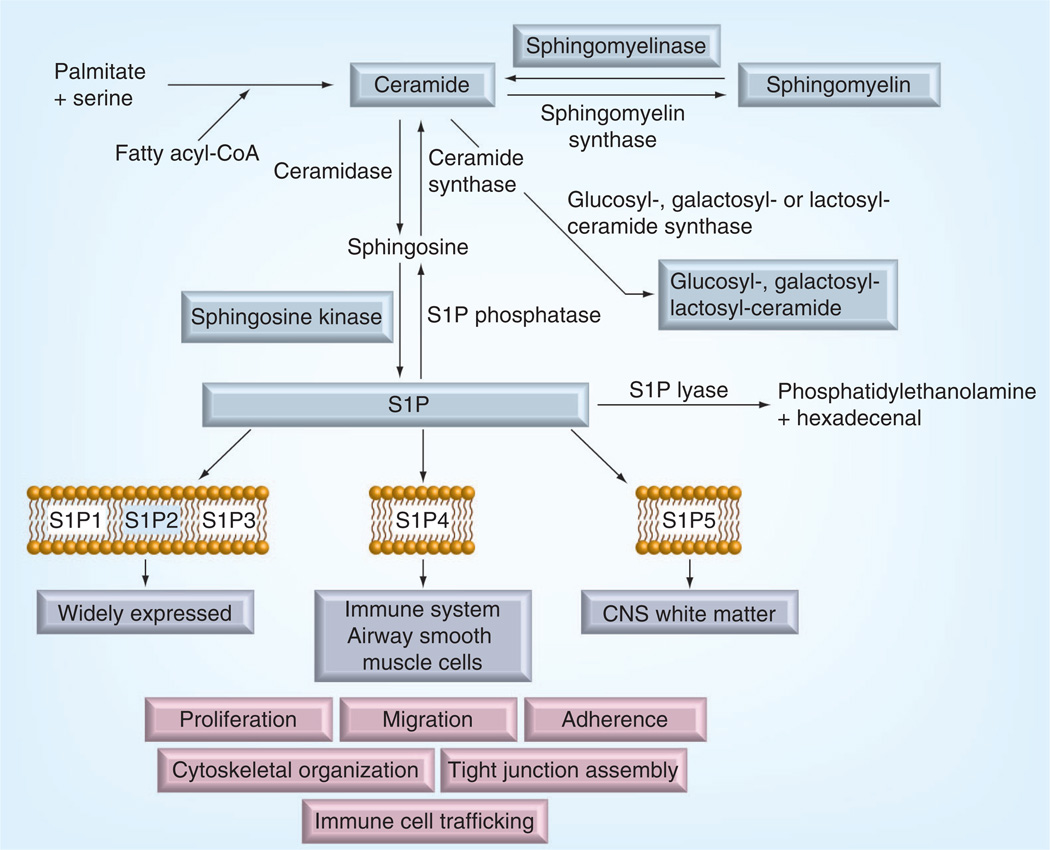
Sphingolipids are a diverse family of lipids derived from the amino alcohol, sphingosine. The synthesis of sphingolipids is initiated by serine palmitoyltransferase as a condensation reaction of palmitate leading to ceramide formation in a sequence of enzymatic reactions. Ceramide can then have different fates (Figure 3). It can be phosphorylated by ceramide kinase, glycosylated by glucosyl or galactosyl ceramide synthases, or converted to sphingomyelin by addition of a phosphocholine head group from phosphatidylcholine through the action of sphingomyelin synthase, which thereby also serves to generate diacylglycerol from phosphatidylcholine. Ceramide may be broken down by several ceramidases leading to the formation of sphingosine. Phosphorylation of sphingosine by one of the two sphingosine kinases leads to generation of sphingosine-1-phosphate (S1P), which can be dephosphorylated by intracellular or extracellular phosphatases to regenerate sphingosine. Alternatively S1P lyase can irreversibly cleave S1P to generate phosphatidylethanolamine and hexadecenal [83].
Figure 3. Sphingolipids synthesis and modification pathway.
The enzymes and products modified in the diabetic retina are highlighted blue.
CoA: Coenzyme A; S1P: Sphingosine-1-phosphate.
The complexity and interconnection of sphingolipid pathways explains the diverse and sometimes opposing effects of sphingolipids in different cell types. In general, ceramides are known to have proinf lammatory and proapoptotic effects [140], whereas S1P has prosurvival, proliferation and migration effects [141]. Thus, sphingolipid metabolism is often called sphingolipid rheostat where cross-conversion between sphingolipid species determines the final outcome. In addition to cross-conversion, it is increasingly recognized that cellular compartmentalization of sphingolipid metabolism determines the signaling pathway activated by sphingolipids. The de novo ceramide synthesis pathway described above occurs in the endoplasmic reticulum and ceramide produced in the de novo pathway is mainly transported to the Golgi where it used for sphingomyelin and glycosphingolipid production [83]. Sphingomyelins and glycosphingolipids are delivered by vesicular transport to the plasma membrane where they are used as important structural elements. This is the predominant direction of the de novo pathway as reflected in the relative amounts of sphingolipid species found in the cells: per each S1P molecule there are 100 sphingosine, 3000 ceramide and 30,000 sphingomyelin molecules [83]. Unless ceramide conversion into sphingomyelin and glucosylceramides is suppressed, de novo pathways would not necessarily lead to ceramide accumulation.
Several sphingolipid classes, including ceramides, glycosphingolipids and S1P have recently been demonstrated to play a role in the development of diabetic complications [142–145]. A discussion of the sphingolipids shown to be involved in the development of diabetic retinopathy is provided below.
Sphingomyelinases & diabetic retinopathy
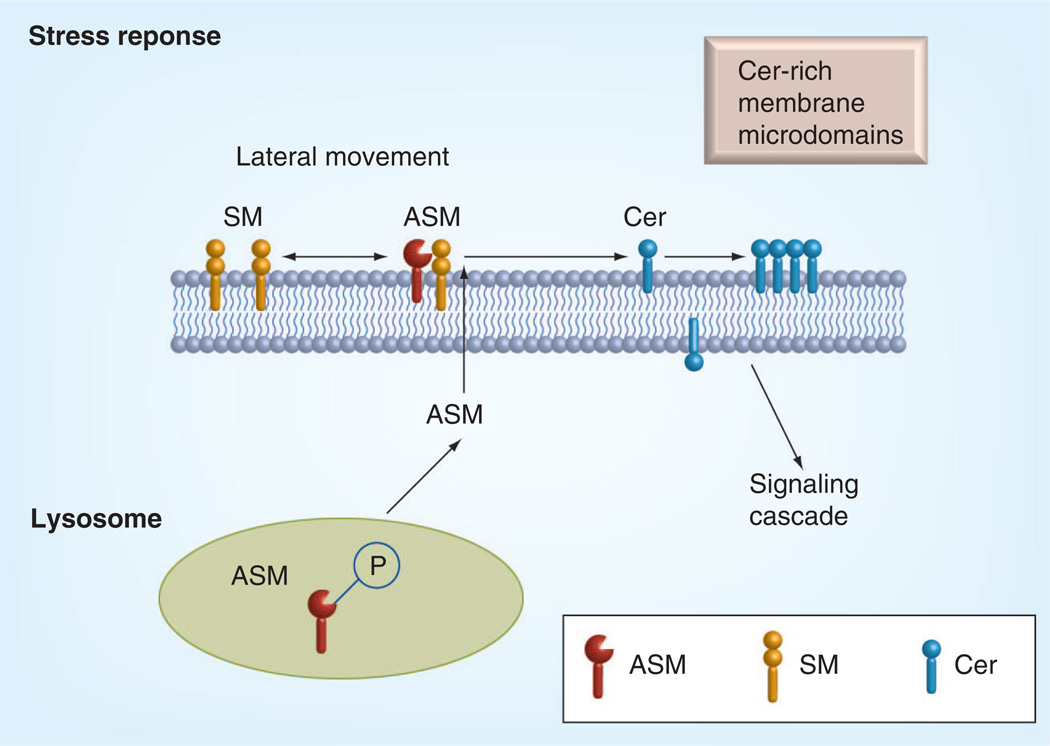
Biologically active ceramide is mainly produced by the action of sphingomyelinases. Based on the pH optimum, sphingomyelinases are classified into three different types: acid, neutral and alkaline [82]. Alkaline sphingomyelinase is only expressed in the gut [146]. Acid and neutral sphingomyelinases are ubiquitously expressed in most tissues. Neutral sphingomyelinase is localized to the plasma membrane inner leaflet [83,147,148]. Acid sphingomyelinase was originally considered as a strictly lysosomal enzyme, however, recent studies demonstrated translocation of acid sphingomyelinase to the plasma membrane outer leaflet induced by various stress stimuli (Figure 4). For example, stimulation of MCF-7 cells with the potent PKC agonist phorbol 12-myristate 13-acetate or UV exposure induces PKCδ-dependent acid sphingomyelinase (ASM) phosphorylation at serine 508 followed by ASM translocation to the plasma membrane and hydrolysis of sphingomyelin into ceramide [85]. This ceramide production initiates membrane reorganization, called membrane capping and facilitates the formation and coalescence of lipid microdomains (Figures 2 & 4) [85,149]. These microdomains are sites of protein–protein interactions that lead to downstream signaling and perturbation of microdomain formation influences the pathophysiology of many diseases. Subsequently, by either changing the physical properties of the membrane or by direct ceramide–protein interactions, these microdomains are thought to enhance the density of proteins, including receptors, which often require dimerization for their activation, as well as facilitate other protein–protein interactions through clustering [150]. Clustering is employed by many receptors for transmembrane signaling. ASM-released ceramide is essential for CD95 clustering. Extracellulary-oriented ceramide released upon CD95-triggered translocation of ASM to the plasma membrane outer surface enabled clustering of CD95 in ceramide-rich membrane rafts and apoptosis induction. ASM deficiency, destruction of rafts or neutralization of surface ceramide prevented CD95 clustering and apoptosis [151].
Figure 4. Central role of acid sphingomyelinase in the stress response in the retina.
Biologically active Cer is mainly produced by the action of ASMs. Activation of ASM leads to the conversion of SM to Cer in the plasma membrane. Cer-rich membrane microdomains promote activation of signaling cascades.
ASM: Acid sphingomyelinase; Cer: Ceramide; SM: Sphingomyelin.
Apart from its effect on receptor clustering through microdomain formation in the outer leaflet, ceramide produced by acid sphingomyelinase can freely flip-flop to the inner leaflet of the plasma membrane where it was shown to activate protein phosphatase PP2A, JNK and PKCζ [152–155].
In addition, endothelial cells, macrophages and fibroblasts were shown to produce a secretory form of acid sphingomyelinase. In response to cytokine stimulation, endothelial cells produce up to 20-fold more secretory sphingomyelinase than macrophages [156]. Secretory ASM either binds to the outer leaflet of endothelial cells or is secreted into circulation. ASM in circulation is shown to hydrolyze lipoproteins leading to ceramide-dependent LDL aggregation, retention and atherogenesis [157].
ASM was shown to be activated in the diabetic retina and normalizing retinal DHA levels with dietary supplementation lowered ASM expression to basal level. Inhibition of ASM by DHA or genetic manipulations prevented inflammatory cytokine production, adhesion molecule expression, retinal capillary loss and neovascularization in both in vitro and in vivo models [158,159].
Glycosphingolipids & diabetic retinopathy
The diabetic retina was shown to have increased levels of glucosylceramide synthase leading to an increase in glucosylceramide with a concomitant decrease in total ceramide levels. Inhibition of glucosylceramide synthase increased insulin sensitivity and viability of retinal neuronal cells [142].
S1P & diabetic retinopathy
S1P mediates diverse functions in many cell types including proliferation, cytoskeletal organization and migration, adherence and tight junction assembly, and immune cell trafficking and function [141,160–164]. While S1P may function as an intracellular mediator, it is also secreted by and interacts with highly specific cell surface receptors [141].
Five high-affinity S1P receptors, formerly known as the endothelial differentiation gene, or EDG receptors, have been identified [165–167]. S1P1R, S1P2R and S1P3R are widely expressed, whereas S1P4R is expressed at low levels in the immune system and in human airway smooth muscle cells, and S1P5R is expressed in the CNS white matter tracts, particularly in oligodendrocytes. Lymphocyte trafficking from peripheral lymphoid organs to sites of active inflammation is entirely dependent upon S1P1 receptor expression in these cells [168,169]. S1P may provide a concentration gradient along which lymphocytes migrate, or may directly alter the endothelial barrier to migration.
Inhibition of sphingosine kinase has been shown to block VEGF-induced S1P production and markedly attenuate migration and proliferation of human retinal endothelial cells [143]. In addition, sphingosine kinase inhibition reduced retinal vascular leakage in a rat diabetic retinopathy model [143] and a S1P2 receptor knockout mouse was protected from neovascularization in the oxygen-induced retinopathy model [145]. Inhibition of S1P signaling with anti-S1P antibodies prevented angiogenesis in a laser-induced choroidal neovascularization mouse model [170]. A humanized anti-S1P antibody, iSONEP, successfully completed Phase I clinical trials for neovascular age-related macular degeneration [171,172].
Conclusion
With the development of more sensitive techniques, detailed retinal lipid analysis has become a reality. Several previously unknown diabetes-induced changes in lipid metabolism were identified, demonstrating that diabetes induces perturbations of most of the known lipid classes in the retina. Among these are modifications of the fatty-acid chains of retinal phospholipids and oxidized fatty acid products of n-6, as well as n-3 fatty acids, along with changes in cholesterol and oxysterol products, and modifications of the activity of the sphingolipid pathway. Our understanding of how these changes may lead to retinal pathology is just beginning to emerge.
Future perspective
It is clear from the literature review that diabetes affects lipid metabolism of all major lipid classes. However, for the most part, the connections between diabetes-induced lipid changes and the exact pathways leading to the development of retinal pathology are still missing. We are entering into a very exiting era when the amount and quality of data on retinal lipid profiling and metabolism is greatly increasing, and new tools are becoming available for lipid metabolic studies including specific antilipid antibodies, lipid metabolic enzyme inhibitors and mouse genetic models affecting lipid metabolism. These advances are starting to allow for pathway building and connecting the changes between different lipid classes as well as between lipidomic, genomic and proteomic regulation, which will lead to a more complete understanding of the pathogenesis of the disease and allow for better therapeutic options for the prevention and treatment of this disabling diabetic complication.
Executive summary.
Dyslipidemia & diabetic retinopathy
-
▪
An increasing number of basic and clinical studies demonstrate a strong association between dyslipidemia and the development of diabetic retinopathy.
-
▪
Diabetes induces perturbations of most of the known lipid classes in the retina.
Retinal fatty acid composition in diabetes
-
▪
Diabetes induced downregulation of fatty acid elongases 2, 4 and 6 leading to the depletion of long and very long-chain polyunsaturated fatty acids, such as docosahexaenoic acid and 32:6, n-3 polyunsaturated fatty acid.
Retinal oxidized bioactive lipids in diabetes
-
▪
Fatty acid oxidation through the lipoxygenase (LOX), rather than cyclooxygenase or CYP450 monoxygenase, pathway is critically involved in the development of retinopathy.
-
▪
Both 5-LOX and 12/15-LOX are involved in the development of diabetic retinopathy, the effects of different LOX isoforms depend on the stage of retinopathy and the available substrate.
Glycated phospholipids
-
▪
The glycated form of GPEtn lipids is increased in the diabetic retina.
Sterol lipids
-
▪
Data from clinical trials demonstrate the association between total- and LDL-cholesterol levels and LDL:HDL cholesterol ratio, and the development of diabetic retinopathy.
-
▪
Pairing of triglyceride lowering with cholesterol lowering for intensive dyslipidemia therapy significantly slowed the progression of diabetic retinopathy.
-
▪
Oxidized and glycated LDL, and oxidized LDL immunocomplexes in the retina were implicated in diabetic retinopathy.
Sphingolipids
-
▪
Acid sphingomyelinase is upregulated in the diabetic retina and normalization of the acid sphingolmyelinase levels prevents the development of retinopathy.
-
▪
The diabetic retina has increased glucosylceramide levels and a concomitant decrease in total ceramide levels.
-
▪
Inhibition of S1P production or action prevents the increase in retinal vascular permeability and retinal neovascularization.
Acknowledgments
This work was supported by grants from NIH (EY-016077 to JV Busik, GM-103508 to GE Reid and JV Busik), MEAS (MICL02163 to JV Busik) and Michigan State University (OVPRGS to JV Busik).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N. Eng. J. Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 3.Gardner TW, Antonetti DA, Barber AJ, Lanoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv. Ophthalmol. 2002;47(Suppl. 2):S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 4.Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab. Rev. 1995;11(2):109–120. doi: 10.1002/dmr.5610110203. [DOI] [PubMed] [Google Scholar]
- 5.Hammes HP, Lin J, Wagner P, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53(4):1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 6.Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53(12):3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 7.Pfister F, Feng Y, vom Hagen F, et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57(9):2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willard AL, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J. Ophthalmol. 2012;2012:209538. doi: 10.1155/2012/209538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engerman RL, Kern TS. Hyperglycemia as a cause of diabetic retinopathy. Metabolism. 1986;35(Suppl. 1)(4):20–23. doi: 10.1016/0026-0495(86)90182-4. [DOI] [PubMed] [Google Scholar]
- 10.Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest. Ophthalmol. Vis. Sci. 2005;46(11):4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 11.Kern TS, Engerman RL. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Arch. Ophthalmol. 1996;114(3):306–310. doi: 10.1001/archopht.1996.01100130302013. [DOI] [PubMed] [Google Scholar]
- 12.Kern TS, Tang J, Mizutani M, et al. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest. Ophthalmol. Vis. Sci. 2000;41(12):3972–3978. [PubMed] [Google Scholar]
- 13.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin. Ophthalmol. 1999;14(4):240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 14.Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr. Eye Res. 2011;36(8):747–753. doi: 10.3109/02713683.2011.585735. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardinger C, Brown LF, Roy S, Mizutani M, Zucker CL, Lorenzi M. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am. J. Pathol. 1998;152(6):1453–1462. [PMC free article] [PubMed] [Google Scholar]
- 16.Navaratna D, Mcguire PG, Menicucci G, Das A. Proteolytic degradation of VE-adherin alters the blood–retinal barrier in diabetes. Diabetes. 2007;56(9):2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 17.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol. 1991;139(1):81–100. [PMC free article] [PubMed] [Google Scholar]
- 18.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 19.Joussen AM, Poulaki V, Qin W, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am. J. Pathol. 2002;160(2):501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol. 2001;158(1):147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mcleod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am. J. Pathol. 1995;147(3):642–653. [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl Acad. Sci. USA. 1999;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest. Ophthalmol. Vis. Sci. 2004;45(11):4161–4166. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- 24.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004;88(10):1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 2002;86(4):363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joussen AM, Huang S, Poulaki V, et al. In vivo retinal gene expression in early diabetes. Invest. Ophthalmol. Vis. Sci. 2001;42(12):3047–3057. [PubMed] [Google Scholar]
- 27.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56(1):224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 28.Coppack SW, Evans RD, Fisher RM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41(3):264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- 29.Weinstock PH, Levak-Frank S, Hudgins LC, et al. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc. Natl Acad. Sci. USA. 1997;94(19):10261–10266. doi: 10.1073/pnas.94.19.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg RB. Lipid disorders in diabetes. Diabetes Care. 1981;4(5):561–572. doi: 10.2337/diacare.4.5.561. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg RB, Capuzzi D. Lipid disorders in Type 1 and Type 2 diabetes. Clin. Lab. Med. 2001;21(1):147–172. vii. [PubMed] [Google Scholar]
- 32.Goldberg IJ. Clinical review 124: diabetic dyslipidemia: causes and consequences. J. Clin. Endocrinol. Metab. 2001;86(3):965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 33.Poisson JP. Essential fatty acid metabolism in diabetes. Nutrition. 1989;5(4):263–266. [PubMed] [Google Scholar]
- 34.Vessby B. Dietary fat and insulin action in humans. Br. J. Nutr. 2000;83(Suppl. 1):S91–S96. doi: 10.1017/s000711450000101x. [DOI] [PubMed] [Google Scholar]
- 35.Brown JE, Lindsay RM, Riemersma RA. Linoleic acid metabolism in the spontaneously diabetic rat: delta6-desaturase activity vs. product/precursor ratios. Lipids. 2000;35(12):1319–1323. doi: 10.1007/s11745-000-0648-1. [DOI] [PubMed] [Google Scholar]
- 36.Brenner RR, Bernasconi AM, Garda HA. Effect of experimental diabetes on the fatty acid composition, molecular species of phosphatidyl-choline and physical properties of hepatic microsomal membranes. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63(3):167–176. doi: 10.1054/plef.2000.0175. [DOI] [PubMed] [Google Scholar]
- 37.Zechner R. The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr. Opin Lipidol. 1997;8(2):77–88. doi: 10.1097/00041433-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot. Essent. Fatty Acids. 2003;68(2):151–162. doi: 10.1016/s0952-3278(02)00265-x. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 2005;46(4):706–715. doi: 10.1194/jlr.M400335-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Botolin D, Xu J, et al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 2006;47(9):2028–2041. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decsi T, Minda H, Hermann R, et al. Polyunsaturated fatty acids in plasma and erythrocyte membrane lipids of diabetic children. Prostaglandins Leukot. Essent. Fatty Acids. 2002;67(4):203–210. doi: 10.1054/plef.2002.0420. [DOI] [PubMed] [Google Scholar]
- 42.Augustin AJ, Breipohl W, Boker T, Lutz J, Spitznas M. Increased lipid peroxide levels and myeloperoxidase activity in the vitreous of patients suffering from proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 1993;231(11):647–650. doi: 10.1007/BF00921959. [DOI] [PubMed] [Google Scholar]
- 43. Lyons TJ, Jenkins AJ, Zheng D, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest. Ophthalmol. Vis. Sci. 2004;45(3):910–918. doi: 10.1167/iovs.02-0648. ▪▪ First study that highlights the association between changes in lipid profile and retinopathy in Type 1 diabetic patient.
- 44.Kissebah AH, Kohner EM, Lewis B, Siddiq YK, Lowy C, Fraser TR. Plasma-lipids and glucose/insulin relationship in non-insulin-requiring diabetics with and without retinopathy. Lancet. 1975;1(7916):1104–1108. doi: 10.1016/s0140-6736(75)92497-6. [DOI] [PubMed] [Google Scholar]
- 45.Ferris FL, 3rd, Chew EY, Hoogwerf BJ. Serum lipids and diabetic retinopathy. Early Treatment Diabetic Retinopathy Study Research Group. Diabetes Care. 1996;19(11):1291–1293. doi: 10.2337/diacare.19.11.1291. [DOI] [PubMed] [Google Scholar]
- 46.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996;114(9):1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 47.Van Eck WF. The effect of a low fat diet on the serum lipids in diabetes and its significance in diabetic retinopathy. Am. J. Med. 1959;27:196–211. doi: 10.1016/0002-9343(59)90340-7. [DOI] [PubMed] [Google Scholar]
- 48.Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes. 1968;17(7):458–467. doi: 10.2337/diab.17.7.458. [DOI] [PubMed] [Google Scholar]
- 49.Houtsmuller AJ, Zahn KJ, Henkes HE. Unsaturated fats and progression of diabetic retinopathy. Doc. Ophthalmol. 1980;48(2):363–371. doi: 10.1007/BF00141465. [DOI] [PubMed] [Google Scholar]
- 50.Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl Acad. Sci. USA. 1997;94(6):2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ejsing CS, Duchoslav E, Sampaio J, et al. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal Chem. 2006;78(17):6202–6214. doi: 10.1021/ac060545x. [DOI] [PubMed] [Google Scholar]
- 52.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005;24(3):367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 53.Jones JJ, Stump MJ, Fleming RC, Lay JO, Jr, Wilkins CL. Strategies and data analysis techniques for lipid and phospholipid chemistry elucidation by intact cell MALDI-FTMS. J. Am. Soc. Mass Spectrom. 2004;15(11):1665–1674. doi: 10.1016/j.jasms.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003;22(5):332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 55.Schwudke D, Oegema J, Burton L, et al. Lipid profiling by multiple precursor and neutral loss scanning driven by the data-dependent acquisition. Anal Chem. 2006;78(2):585–595. doi: 10.1021/ac051605m. [DOI] [PubMed] [Google Scholar]
- 56.Welti R, Shah J, Levine S, Esch SW, Williams TD, Wang X. High throughput lipid profiling to identify and characterize genes involved in lipid metabolism, signaling, and stress response. In: Feng L, Prestwich GD, editors. Functional Lipidomics. NY, USA: Marcel Dekker; 2005. [Google Scholar]
- 57.Welti R, Wang X, Williams TD. Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Anal Biochem. 2003;314(1):149–152. doi: 10.1016/s0003-2697(02)00623-1. [DOI] [PubMed] [Google Scholar]
- 58.Schuhmann K, Almeida R, Baumert M, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J. Mass Spectrom. 2012;47(1):96–104. doi: 10.1002/jms.2031. [DOI] [PubMed] [Google Scholar]
- 59.Schwudke D, Schuhmann K, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on high resolution mass spectrometers. Cold Spring Harb. Perspect. Biol. 2011;3(9) doi: 10.1101/cshperspect.a004614. a004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones JJ, Borgmann S, Wilkins CL, O’Brien RM. Characterizing the phospholipid profiles in mammalian tissues by MALDI FTMS. Anal Chem. 2006;78(9):3062–3071. doi: 10.1021/ac0600858. [DOI] [PubMed] [Google Scholar]
- 61.Ekroos K, Chernushevich IV, Simons K, Shevchenko A. Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Anal Chem. 2002;74(5):941–949. doi: 10.1021/ac015655c. [DOI] [PubMed] [Google Scholar]
- 62.Han X, Gross RW. Shotgun lipidomics: multidimensional MS analysis of cellular lipidomes. Expert Rev. Proteomics. 2005;2(2):253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 63.Kerwin JL, Tuininga AR, Ericsson LH. Identification of molecular species of glycerophospholipids and sphingomyelin using electrospray mass spectrometry. J. Lipid Res. 1994;35(6):1102–1114. [PubMed] [Google Scholar]
- 64.Lydic TA, Busik JV, Esselman WJ, Reid GE. Complementary precursor ion and neutral loss scan mode tandem mass spectrometry for the analysis of glycerophosphatidylethanolamine lipids from whole rat retina. Anal Bioanal Chem. 2009;394(1):267–275. doi: 10.1007/s00216-009-2717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busik JV, Reid GE, Lydic TA. Global analysis of retina lipids by complementary precursor ion and neutral loss mode tandem mass spectrometry. Methods Mol. Biol. 2009;579:33–70. doi: 10.1007/978-1-60761-322-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J. Lipid Res. 2005;46(5):839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Sprecher H, Chen Q. Polyunsaturated fatty acid biosynthesis: a microsomal–peroxisomal process. Prostaglandins Leukot. Essent. Fatty Acids. 1999;60(5–6):317–321. doi: 10.1016/s0952-3278(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 68. Agbaga MP, Brush RS, Mandal MN, Henry K, Elliott MH, Anderson RE. Role of stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl Acad. Sci. USA. 2008;105(35):12843–12848. doi: 10.1073/pnas.0802607105. ▪ Important study demonstrating the role of Elovl4 in the elongation of very long-chain fatty acids with a chain length of 26–28 carbons.
- 69.Grayson C, Molday RS. Dominant negative mechanism underlies autosomal dominant stargardt-like macular dystrophy linked to mutations in ELOVL4. J. Biol Chem. 2005;280(37):32521–32530. doi: 10.1074/jbc.M503411200. [DOI] [PubMed] [Google Scholar]
- 70.Karan G, Lillo C, Yang Z, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl Acad. Sci. USA. 2005;102(11):4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasireddy V, Vijayasarathy C, Huang J, et al. Stargardt-like macular dystrophy protein ELOVL4 exerts a dominant negative effect by recruiting wild-type protein into aggresomes. Mol. Vis. 2005;11:665–676. [PubMed] [Google Scholar]
- 72.Zhang K, Kniazeva M, Han M, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet. 2001;27(1):89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 73. Ohno Y, Suto S, Yamanaka M, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl Acad. Sci. USA. 2010;107(43):18439–18444. doi: 10.1073/pnas.1005572107. ▪ Describes the role of Elovl1 and Elovl4 in very long-chain (C24–28) sphingolipid synthesis
- 74.Li W, Sandhoff R, Kono M, et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol Sci. 2007;3(2):120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Decsi T, Szabo E, Burus I, et al. Low contribution of n-3 polyunsaturated fatty acids to plasma and erythrocyte membrane lipids in diabetic young adults. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76(3):159–164. doi: 10.1016/j.plefa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Lydic TA, Renis R, Busik JV, Reid GE. Analysis of retina and erythrocyte glycerophospholipid alterations in a rat model of Type 1 diabetes. JALA. 2009;14(6):383–399. doi: 10.1016/j.jala.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kielczewski JL, Jarajapu YP, Mcfarland EL, et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ. Res. 2009;105(9):897–905. doi: 10.1161/CIRCRESAHA.109.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tikhonenko M, Lydic TA, Wang Y, et al. Remodeling of retinal fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2009;59(1):219–227. doi: 10.2337/db09-0728. ▪ First study demonstrating the effect of diabetes on retinal fatty-acid elongases and very long-chain polyunsaturated fatty acids in the retina.
- 79.Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46(11):4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 2007;48(1):18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W, Jump DB, Grant MB, Esselman WJ, Busik JV. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44(11):5016–5022. doi: 10.1167/iovs.03-0418. [DOI] [PubMed] [Google Scholar]
- 82.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531(1):38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 83.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 84.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Invest. 2002;110(1):3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J. Biol Chem. 2007;282(15):11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 86.Zeidan YH, Wu BX, Jenkins RW, Obeid LM, Hannun YA. A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 2008;22(1):183–193. doi: 10.1096/fj.07-8967com. [DOI] [PubMed] [Google Scholar]
- 87. Hammes HP, Weiss A, Fuhrer D, Kramer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996;39(3):251–255. doi: 10.1007/BF00418338. ▪ Demonstrated detrimental effects of very high-dose fish oil in an animal model of diabetic retinopathy.
- 88.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev. Nutr. Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 89.de Lorgeril M, Salen P. Dietary prevention of coronary heart disease: focus on omega-6/omega-3 essential fatty acid balance. World Rev. Nutr. Diet. 2003;92:57–73. doi: 10.1159/000073792. [DOI] [PubMed] [Google Scholar]
- 90.Cleland LG, James MJ, Proudman SM. Omega-6/omega-3 fatty acids and arthritis. World Rev. Nutr. Diet. 2003;92:152–168. doi: 10.1159/000073798. [DOI] [PubMed] [Google Scholar]
- 91.Futterman S, Kupfer C. The fatty acid composition of the retinal vasculature of normal and diabetic human eyes. Invest. Ophthalmol. 1968;7(1):105–108. [PubMed] [Google Scholar]
- 92. Connor KM, Sangiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13(7):868–873. doi: 10.1038/nm1591. ▪ The first to demonstrate protective effects of docosahexenoic acid against retinal pathological neovascularization.
- 93.Hwang D. Fatty acids and immune responses – a new perspective in searching for clues to mechanism. Annu. Rev. Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- 94.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 95.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- 96.Dubois RN. Leukotriene A4 signaling, inflammation, and cancer. J. Natl Cancer Inst. 2003;95(14):1028–1029. doi: 10.1093/jnci/95.14.1028. [DOI] [PubMed] [Google Scholar]
- 97.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 98.Sellmayer A, Koletzko B. Long-chain polyunsaturated fatty acids and eicosanoids in infants – physiological and pathophysiological aspects and open questions. Lipids. 1999;34(2):199–205. doi: 10.1007/s11745-999-0354-z. [DOI] [PubMed] [Google Scholar]
- 99.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J. Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 100.Smith WL, Garavito RM, Dewitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and-2. J. Biol Chem. 1996;271(52):33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 101.Delerive P, Gervois P, Fruchart J-C, Staels B. Induction of Ikappa Balpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J. Biol. Chem. 2000;275(47):36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 102.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol Chem. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 103.Patricia MK, Kim JA, Harper CM, et al. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1999;19(11):2615–2622. doi: 10.1161/01.atv.19.11.2615. [DOI] [PubMed] [Google Scholar]
- 104.Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia. 2002;45(1):125–133. doi: 10.1007/s125-002-8253-x. [DOI] [PubMed] [Google Scholar]
- 105.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 106.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musiek ES, Brooks JD, Joo M, et al. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J. Biol Chem. 2008;283(29):19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57(5):1387–1393. doi: 10.2337/db07-1217. ▪ First study demonstrating the importance of 5-lipoxygenase in the animal model of the vasodegenerative stage of diabetic retinopathy.
- 110. Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest. Ophthalmol. Vis. Sci. 2008;49(7):3239–3244. doi: 10.1167/iovs.08-1755. ▪ First study demonstrating the importance of 12-lipoxygenase in the animal model of the proliferative stage of diabetic retinopathy and the increased activity of 12-lipoxigenase in the patients with proliferative diabetic retinopathy.
- 111.Augustin AJ, Grus FH, Koch F, Spitznas M. Detection of eicosanoids in epiretinal membranes of patients suffering from proliferative vitreoretinal diseases. Br. J. Ophthalmol. 1997;81(1):58–60. doi: 10.1136/bjo.81.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Shabrawey M, Mussell R, Kahook K, et al. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011;60(2):614–624. doi: 10.2337/db10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sapieha P, Stahl A, Chen J, et al. 5-lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci. Transl Med. 2011;3(69) doi: 10.1126/scitranslmed.3001571. 69RA12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed N. Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 115.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 116.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000;28(12):1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 117.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53(2):131–142. [PubMed] [Google Scholar]
- 118.Gardiner TA, Anderson HR, Stitt AW. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J. Pathol. 2003;201(2):328–333. doi: 10.1002/path.1429. [DOI] [PubMed] [Google Scholar]
- 119.Kern TS, Engerman RL. Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes. 2001;50(7):1636–1642. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- 120.Ravandi A, Kuksis A, Marai L, et al. Isolation and identification of glycated aminophospholipids from red cells and plasma of diabetic blood. FEBS Lett. 1996;381(1–2):77–81. doi: 10.1016/0014-5793(96)00064-6. [DOI] [PubMed] [Google Scholar]
- 121.Nakagawa K, Oak JH, Miyazawa T. Angiogenic potency of amadori-glycated phosphatidylethanolamine. Ann. NY Acad. Sci. 2005;1043:413–416. doi: 10.1196/annals.1333.048. [DOI] [PubMed] [Google Scholar]
- 122.Oak JH, Nakagawa K, Oikawa S, Miyazawa T. Amadori-glycated phosphatidylethanolamine induces angiogenic differentiations in cultured human umbilical vein endothelial cells. FEBS Lett. 2003;555(2):419–423. doi: 10.1016/s0014-5793(03)01237-7. [DOI] [PubMed] [Google Scholar]
- 123. Chew EY, Ambrosius WT. Update of the ACCORD Eye Study. N. Eng. J. Med. 2011;364(2):188–189. doi: 10.1056/NEJMc1011499. ▪▪ Data from the ACCORD Eye Study in Type 2 diabetic patients demonstrating that intensive dyslipidemia therapy significantly slows the progression of retinopathy.
- 124.Tserentsoodol N, Sztein J, Campos M, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- 125.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 126.Li CM, Chung BH, Presley JB, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest. Ophthalmol. Vis. Sci. 2005;46(7):2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 127.Hayes KC, Lindsey S, Stephan ZF, Brecker D. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest. Ophthalmol. Vis. Sci. 1989;30(2):225–232. [PubMed] [Google Scholar]
- 128.Duncan KG, Bailey KR, Kane JP, Schwartz DM. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun. 2002;292(4):1017–1022. doi: 10.1006/bbrc.2002.6756. [DOI] [PubMed] [Google Scholar]
- 129.Amaratunga A, Abraham CR, Edwards RB, Sandell JH, Schreiber BM, Fine RE. Apolipoprotein E is synthesized in the retina by Muller glial cells, secreted into the vitreous, and rapidly transported into the optic nerve by retinal ganglion cells. J. Biol Chem. 1996;271(10):5628–5632. doi: 10.1074/jbc.271.10.5628. [DOI] [PubMed] [Google Scholar]
- 130.Mast N, Reem R, Bederman I, et al. Cholestenoic acid is an important elimination product of cholesterol in the retina: comparison of retinal cholesterol metabolism with that in the brain. Invest. Ophthalmol. Vis. Sci. 2011;52(1):594–603. doi: 10.1167/iovs.10-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liao WL, Heo GY, Dodder NG, et al. Quantification of cholesterol-metabolizing P450s CYP27A1 and CYP46A1 in neural tissues reveals a lack of enzyme-product correlations in human retina but not human brain. J. Proteome Res. 2011;10(1):241–248. doi: 10.1021/pr1008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferderbar S, Pereira EC, Apolinario E, et al. Cholesterol oxides as biomarkers of oxidative stress in Type 1 and Type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2007;23(1):35–42. doi: 10.1002/dmrr.645. [DOI] [PubMed] [Google Scholar]
- 133.Matsui H, Okumura K, Mukawa H, Hibino M, Toki Y, Ito T. Increased oxysterol contents in diabetic rat hearts: their involvement in diabetic cardiomyopathy. Can. J. Cardiol. 1997;13(4):373–379. [PubMed] [Google Scholar]
- 134.Yoshioka N, Adachi J, Ueno Y, Yoshida K. Oxysterols increase in diabetic rats. Free Radic. Res. 2005;39(3):299–304. doi: 10.1080/10715760400023002. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Megha, London E. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function. Biochemistry. 2004;43(4):1010–1018. doi: 10.1021/bi035696y. [DOI] [PubMed] [Google Scholar]
- 136.Luthra S, Dong J, Gramajo AL, et al. 7-ketocholesterol activates caspases-3/7, -8, and-12 in human microvascular endothelial cells in vitro . Microvasc. Res. 2008;75(3):343–350. doi: 10.1016/j.mvr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Rodriguez IR, Fliesler SJ. Photodamage generates 7-keto- and 7-hydroxycholesterol in the rat retina via a free radical-mediated mechanism. Photochem. Photobiol. 2009;85(5):1116–1125. doi: 10.1111/j.1751-1097.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu M, Chen Y, Abdel-samie SA, et al. Ox-LDL immunocomplexes are implicated in diabetic retinopathy. Presented at: The American Federation for Medical Research 2009 Southern Regional Meeting; 12–14 February 2009; New Orelans, LA, USA. [Google Scholar]
- 139.Wu M, Chen Y, Wilson K, et al. Highly oxidized and glycated LDL induced human retinal capillary pericyte loss: oxidative stress, proteasome inhibition and mitochondrial-mediated apoptosis. Presented at: The American Federation for Medical Research 2009 Southern Regional Meeting; 12–14 February 2009; New Orelans, LA, USA. [Google Scholar]
- 140.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 141.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 2004;15(5):513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 142.Fox TE, Han X, Kelly S, et al. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55(12):3573–3580. doi: 10.2337/db06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maines LW, French KJ, Wolpert EB, Antonetti DA, Smith CD. Pharmacologic manipulation of sphingosine kinase in retinal endothelial cells: implications for angiogenic ocular diseases. Invest. Ophthalmol. Vis. Sci. 2006;47(11):5022–5031. doi: 10.1167/iovs.05-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Masson E, Troncy L, Ruggiero D, Wiernsperger N, Lagarde M, El Bawab S. a-Series gangliosides mediate the effects of advanced glycation end products on pericyte and mesangial cell proliferation: a common mediator for retinal and renal microangiopathy? Diabetes. 2005;54(1):220–227. doi: 10.2337/diabetes.54.1.220. [DOI] [PubMed] [Google Scholar]
- 145.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Invest. 2007;117(9):2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nilsson A, Duan RD. Alkaline sphingomyelinases and ceramidases of the gastrointestinal tract. Chem. Phys Lipids. 1999;102(1–2):97–105. doi: 10.1016/s0009-3084(99)00078-x. [DOI] [PubMed] [Google Scholar]
- 147.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. J. Biol Chem. 2007;282(13):10047–10056. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- 148.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 2007;19(2):229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 149.Dumitru CA, Zhang Y, Li X, Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid. Redox Signal. 2007;9(9):1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- 150.Montes LR, Ruiz-Arguello MB, Goni FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol Chem. 2002;277(14):11788–11794. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- 151.Grassme H, Jekle A, Riehle A, et al. CD95 signaling via ceramide-rich membrane rafts. J. Biol Chem. 2001;276(23):20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 152.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol Chem. 1998;273(26):16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 153.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol Chem. 2002;277(5):3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 154.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14(9):1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J. Biol Chem. 2005;280(28):26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 156.Marathe S, Schissel SL, Yellin MJ, et al. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J. Biol Chem. 1998;273(7):4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 157.Schissel SL, Jiang X, Tweedie-Hardman J, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol Chem. 1998;273(5):2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 158.Opreanu M, Lydic TA, Reid GE, Mcsorley KM, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 2010;51(6):3253–3263. doi: 10.1167/iovs.09-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Opreanu M, Tikhonenko M, Bozack S, et al. The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes. 2011;60(9):2370–2378. doi: 10.2337/db10-0550. ▪▪ Demonstrated a critical role for acid sphingomyelinase in the development of diabetic retinopathy.
- 160.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 161.Spiegel S, English D, Milstien S. Sphingosine 1-phosphate signaling: providing cells with a sense of direction. Trends Cell Biol. 2002;12(5):236–242. doi: 10.1016/s0962-8924(02)02277-8. [DOI] [PubMed] [Google Scholar]
- 162.Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16(9):1596–1602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- 163.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol Chem. 2002;277(29):25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 164.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 165.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 166.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 167.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J. Biol Chem. 1999;274(39):27351– 27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 168.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 169.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 170. Caballero S, Swaney J, Moreno K, et al. Antisphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp. Eye Res. 2009;88(3):367–377. doi: 10.1016/j.exer.2008.07.012. ▪ Demonstrated the role of sphingosine-1-phosphate in pathological retinal neovascularization.
- 171.Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br. J. Pharmacol. 2011;162(6):1225–1238. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stoller GL, Lapierre-Holme F, Peterkin J, Garland W, Sabbadini R. iSONEP, an antisphingosine-1-phosphate (anti-S1P) monoclonal antibody for investigation in exudative AMD: results from a Phase 1 prospective open-label dose-escalating multi-center study. Invest. Ophthalmol. Vis. Sci. 2010;51:1253. [Google Scholar]