The similar metabolic and morphological responses to arsenic exposure and sulfur deficiency can be explained by the binding of arsenite to key metabolites that control sulfate uptake and reduction.
Abstract
Treatment of barley (Hordeum vulgare) seedlings with arsenite (AsIII) rapidly induced physiological and transcriptional changes characteristic of sulfur deficiency, even in plants replete with sulfur. AsIII and sulfur deficiency induced 5- to 20-fold increases in the three genes responsible for sulfate reduction. Both treatments also caused up-regulation of a sulfate transporter, but only in the case of sulfur deficiency was there an increase in sulfate influx. Longer-term changes included reduction in transfer of sulfur from roots to shoots and an increase in root growth relative to shoot growth. Genes involved in complexation and compartmentation of arsenic were up-regulated by AsIII, but not by sulfur deficiency. The rate at which arsenic accumulated appeared to be controlled by the rate of thiol synthesis. Over a range of AsIII concentrations and growth periods, the ratio of thiols to arsenic was always close to 3:1, which is consistent with the formation of a stable complex between three glutathione molecules per AsIII. The greater toxicity of arsenic under sulfur-limiting conditions is likely to be due to an intensification of sulfur deficiency as a result of thiol synthesis, rather than to a direct toxicity to metabolism. Because influx of AsIII was nearly 20-fold faster than the rate of synthesis of thiols, it is questionable whether this complexation strategy can be effective in preventing arsenic toxicity, unless arsenic uptake becomes limited by diffusive resistances in the rhizosphere.
The prevailing view is that toxicity of arsenic to plants is principally mediated by the competition between arsenate (AsV) and phosphate in metabolic processes and the binding of arsenite (AsIII) to thiol groups in proteins, thereby disrupting enzymic activity (Léonard and Lauwerys 1980; Meharg and Hartley-Whitaker 2002; Spuches et al., 2005). Metabolic conversion of AsV to AsIII is readily achieved in plants by AsV reductase (Duan et al., 2005; Wang et al., 2008). Xu et al. (2007) showed that in hydroponic culture of rice (Oryza sativa) and tomato (Solanum lycopersicum), virtually all of the AsV supplied appeared in the nutrient solution as AsIII within 3 d and AsIII was the dominant arsenic form in roots and xylem. Many plants respond to high concentrations of AsIII by increasing the synthesis of a range of thiol-containing compounds (Raab et al., 2005; Schulz et al., 2008), including phytochelatins (PCs; Schmöger et al., 2000). PC synthase genes have now been identified in a number of species, including Arabidopsis (Arabidopsis thaliana; Vatamaniuk et al., 1999), wheat (Triticum aestivum; Clemens et al., 1999), Brassica juncea (Heiss et al., 2003), Pteris vittata (Dong et al., 2005), and Lotus japonicus (Ramos et al., 2008). The importance of these genes in arsenic tolerance in Arabidopsis is underlined by the sensitivity of mutants deficient in PC synthase (Ha et al., 1999) and the increased tolerance in plants overexpressing PC synthase (Gasic and Korban 2007). However, Sung et al. (2007) reported Arabidopsis mutants with enhanced tolerance to arsenic in which PC synthesis was not increased. Instead, they found elevated levels of the thiols Cys, γ-glutamyl-Cys, and glutathione. Norton et al. (2008) also showed, using whole genome transcriptional analysis, that a large number of genes were up-regulated following exposure to AsV in rice, including many associated with glutathione metabolism, but not PCs.
The exact role of thiols in arsenic tolerance is still uncertain given the fact that in the arsenic-tolerant Holcus lanatus and in the hyperaccumulating ferns P. vittata and Pteris cretica, the molar ratios of thiols to arsenic are very low, suggesting that thiols play only a minor role in arsenic tolerance in these species (Zhao et al., 2003; Raab et al., 2004). It has been suggested that thiols may act as chaperones in the cytoplasm to prevent AsIII binding to proteins (Zhao et al., 2003), although arsenic is subsequently stored as inorganic species in the vacuole (Raab et al., 2004).
AsIII uptake into plant roots is rapid (Meharg and Jardine 2003) and appears to be mediated by passive permeation through nodulin-26-like integrated protein aquaporins (Bienert et al., 2008; Ma et al., 2008). These aquaporins facilitate bidirectional movement of AsIII and therefore can allow efflux as well as influx, which helps to explain the plant-induced transformation of AsV to AsIII in nutrient solution reported by Xu et al. (2007). Active transport of arsenic-thiol conjugates into plant vacuoles has also been reported (Martinoia et al., 1993). If toxicity is related to the free AsIII concentration in the cytoplasm, then there will be both membrane and metabolic processes that influence this concentration.
It is clear that although plants may differ in the amounts and types of compounds that they synthesize to combat arsenic toxicity, all of these compounds contain thiol groups to which AsIII can be conjugated. The high tissue concentrations of arsenic observed in many plants necessitates a higher uptake of sulfur for thiol synthesis, and it is not clear whether the limit of tolerance is determined by the rate of uptake of sulfur, the rate of uptake of arsenic species, the rate at which thiol compounds can be synthesized, or the rate at which thiol-arsenic complexes can be compartmentalized. Nocito et al. (2006) provided evidence that tolerance to cadmium, which is also complexed by thiols, may be limited by uptake of sulfur, which in turn restricts the capacity of roots to synthesize reduced glutathione (GSH).
In this study, we employed a common plant species not known to have any special tolerance to arsenic toxicity to examine the early adaptive responses to arsenic. We focused on two aspects of arsenic toxicity: the role of sulfur availability and the expression of genes controlling the synthesis and compartmentation of thiol compounds. The results revealed a range of unexpected responses to AsIII, whose origins appear to be related to the disruption of factors regulating sulfur status in plants.
RESULTS
Sulfur-Deficient Plants Are Highly Sensitive to Arsenic
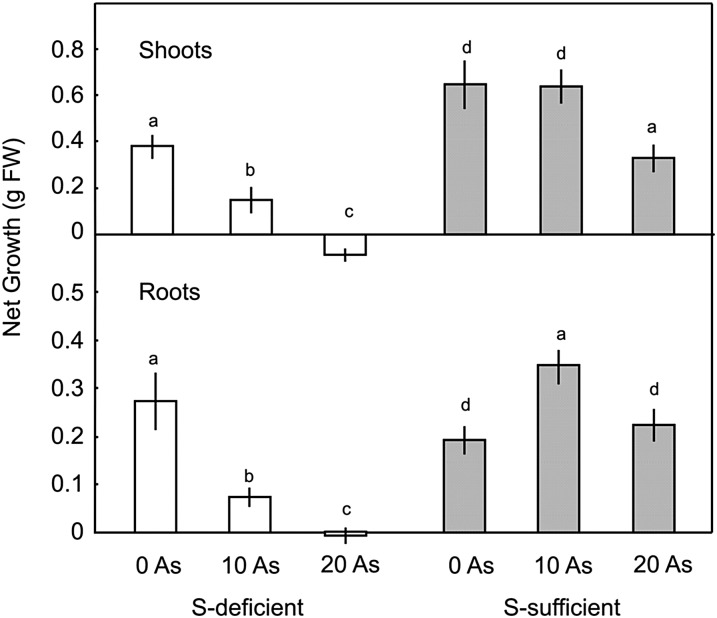
Apart from a mild chlorosis of the shoots, 3 weeks without sulfur did not have a major impact on the overall growth of barley (Hordeum vulgare) seedlings, although there was an obvious shift from shoot growth in favor of root growth (Fig. 1), which is a characteristic response to sulfur deficiency (Clarkson et al., 1989; Elberse et al., 2003). Addition of 10 µm AsIII to sulfur-deficient plants reduced the growth of shoots by 60% and the growth of roots by more than 70%. At 20 µm AsIII, there was no growth of either roots or shoots; in fact, there was a slight loss of biomass over the treatment period (Fig. 1).
Figure 1.
Effect of AsIII on growth of barley seedlings over 3 weeks in one-fifth-strength Hoagland solution containing 0 or 0.4 mm sulfate. Each point is the mean ± se of at least five replicates. Different letters indicate significant differences (P < 0.5). S, Sulfur.
By contrast, growth of sulfur-sufficient plants was actually increased by addition of 10 µm AsIII, principally due to an 80% increase in root growth (Fig. 1). At 20 µm AsIII, shoot growth was significantly reduced but root growth was unaffected. This experiment was repeated four times, and stimulated root growth was recorded at either 5 or 10 µm AsIII in three experiments, while in the other experiment, there was neither increase nor decrease in root growth at 10 µm AsIII (data not shown). Liu et al. (2008) also reported stimulatory effects of low arsenic concentrations on growth of rice roots but not shoots.
The impact of AsIII on relative growth of roots and shoots was very similar to that of withdrawal of sulfur. In the absence of arsenic, sulfur-sufficient seedlings had a ratio of root growth to shoot growth of 0.31, which increased to 0.51 at 10 µm AsIII and 0.57 at 20 µm AsIII, which is very similar to the ratio of 0.62 observed under sulfur deficiency (Supplemental Table S1).
ASIII Alters Partitioning of Sulfur
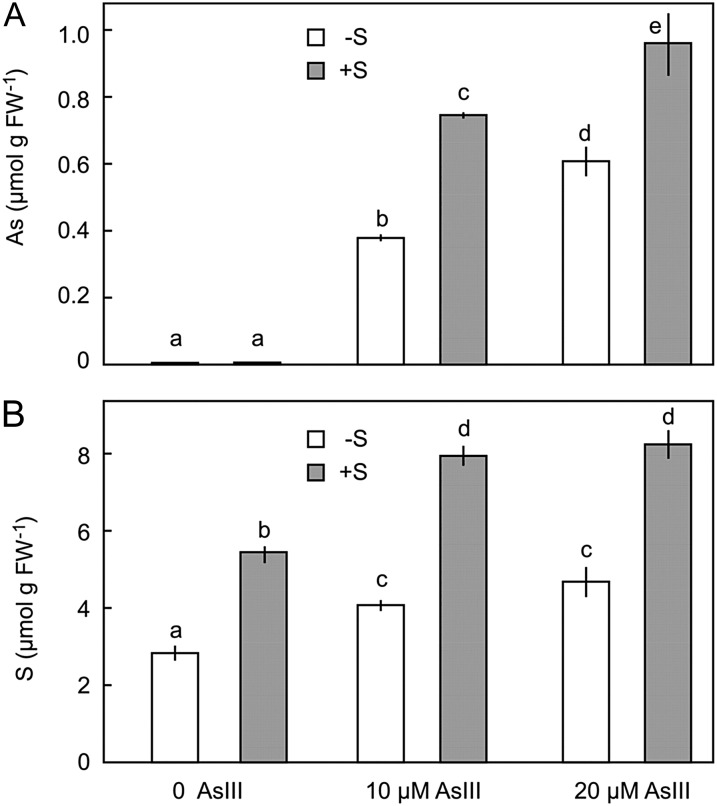
At 10 µm AsIII, the concentration of sulfur in roots increased by 58%, and at 20 µm AsIII, the concentration increased by 51% (Fig. 2). Treatment with AsIII also increased the concentration of sulfur in roots of sulfur-deficient plants, even though there was no sulfur in the nutrient solution. The increase in root sulfur in sulfur-deficient plants was associated with a net reduction in shoot sulfur concentration (Supplemental Table S2) and could therefore be explained by transfer of sulfur from shoot to root in the phloem. In sulfur-sufficient plants, there was also a reduction in shoot sulfur concentration by 16% at 10 µm AsIII and by 23% at 20 µm AsIII. This may have been due either to a remobilization of sulfur from shoots back to the roots, as occurred in sulfur-deficient plants, or to reduced translocation from the root.
Figure 2.
Effect of sulfur nutrition on accumulation of AsIII (A) and sulfur (B) in roots of barley seedlings over 7 d. Each result is the mean ± se of five replicates. Treatments with the same letter are not significantly different (P < 0.05). S, Sulfur.
Rates of AsIII Accumulation
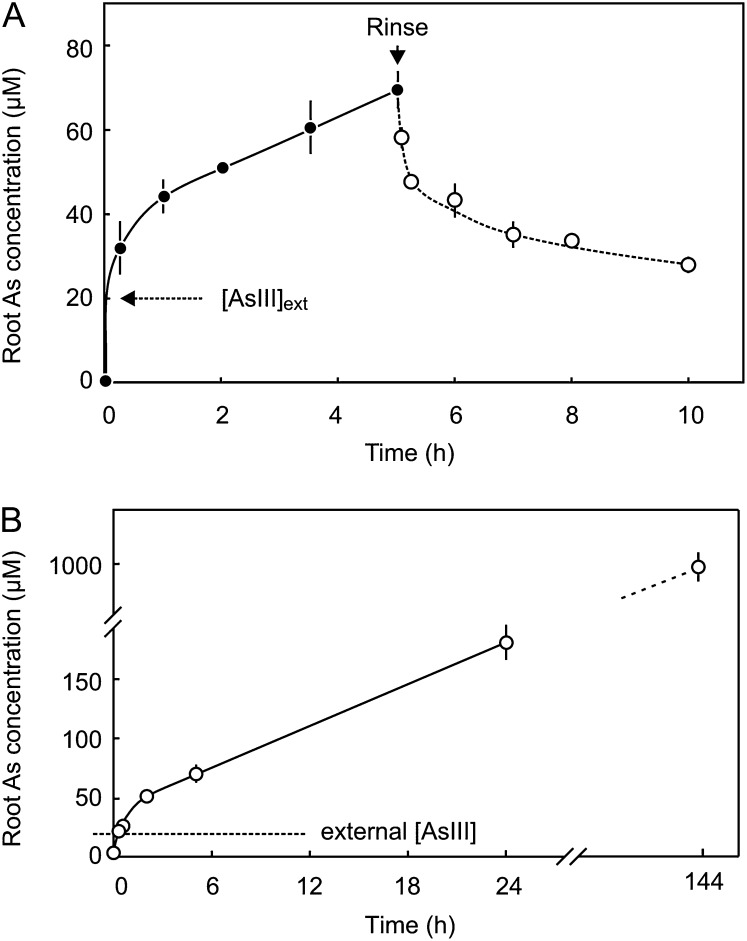
Uptake of AsIII into barley roots was rapid. When placed in a solution of 20 µm AsIII, the concentration in roots exceeded that of the external solution within 15 min, with an average influx of 127 ± 26 nmol g–1 h–1, and thereafter continued to accumulate at a steady rate of 6.7 ± 0.7 nmol g–1 h–1 for at least 6 d, after which the internal arsenic concentration was nearly 1 mm (Fig. 3). Efflux was also initially rapid but slowed after the internal concentration was reduced by around 20 µm. This data suggests that there are two pools of arsenic in the cell, comprised of free AsIII in equilibrium with the external AsIII, and a more slowly exchanging pool of reversibly bound AsIII.
Figure 3.
A, Rapid influx and efflux of AsIII from barley roots. B, Longer time course displaying a linear rate of accumulation of uptake of AsIII from an external solution containing 20 µm AsIII. Each point is the mean ± se of at least five replicates.
Thiol Accumulation
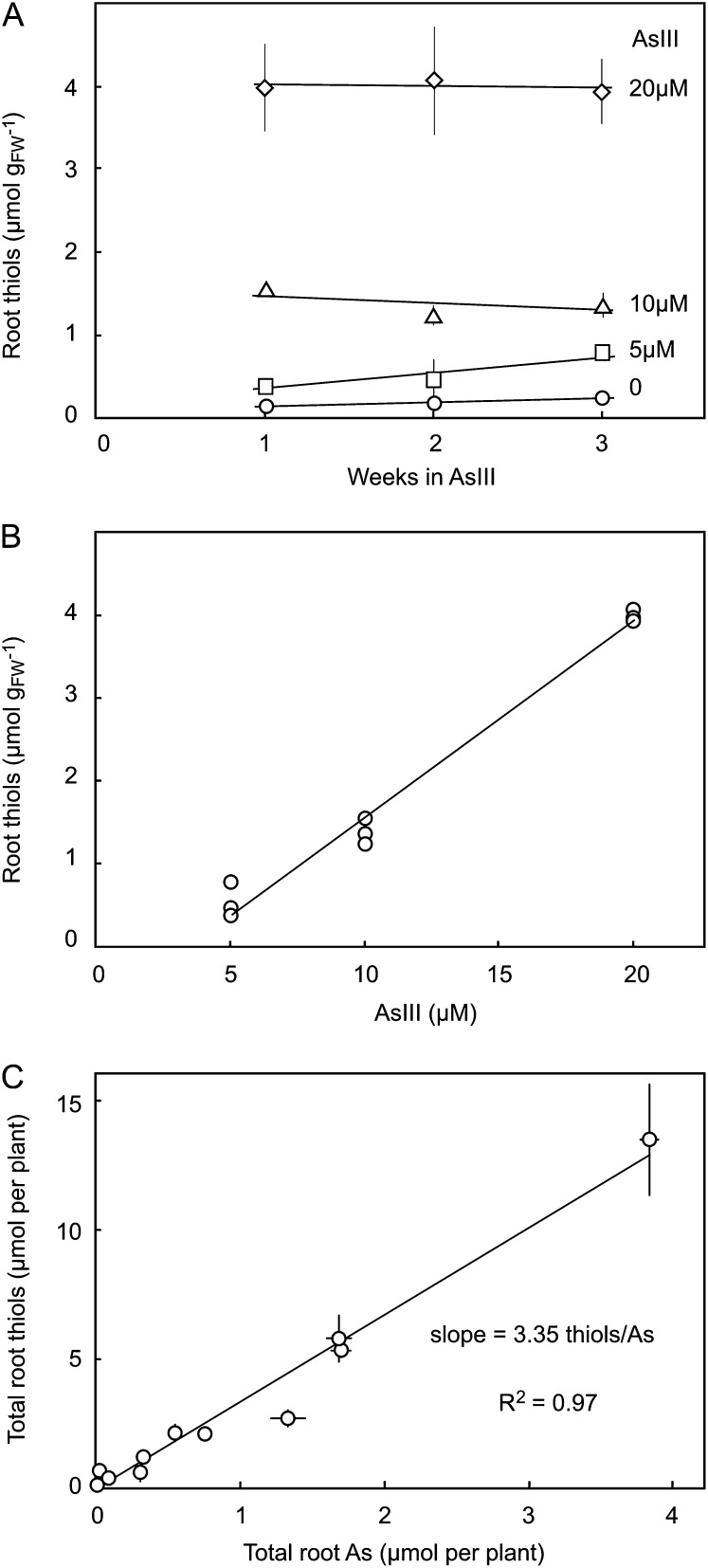
The concentration of total thiols in roots was proportional to the concentration of AsIII in the external solution and did not change over 3 weeks of growth, except at the lowest concentration, where it increased slightly (Fig. 4). The constancy of thiol accumulation was matched by that of arsenic accumulation, such that a ratio of thiol to arsenic close to 3:1 was always obtained (Fig. 4C).
Figure 4.
Key features of arsenic and thiol accumulation in barley roots grown at 5, 10, and 20 µm AsIII for 1, 2, and 3 weeks. A, Thiol concentrations remain constant during root growth. B, Thiol concentrations are linearly related to AsIII treatment concentrations. C, The ratio of thiols to arsenic is approximately 3:1. Each point is the mean of five replicates.
Impact of AsIII on Sulfate Transport
The capacity of roots to take up sulfate was initially assessed in plants without AsIII treatment. For seedlings that were germinated then grown for 7 d on 0.4 mm sulfate, the Km for sulfate uptake was around 31 µm, and saturation of uptake occurred at a rate of 50 nmol g–1 h–1 at around 100 µm sulfate (Supplemental Fig. S1), which corresponds to the concentration required for maximum growth (Supplemental Fig. S2). Seedlings that were grown for 7 d in the absence of sulfur had similar biomass to sulfur-sufficient plants but were beginning to display a mild general chlorosis characteristic of sulfur deficiency. In these plants, the capacity for sulfur uptake was greatly increased, with saturation occurring at 380 nmol g–1 h–1, and the Km was reduced to around 15 µm (Supplemental Fig. S1). AsIII treatment did not significantly affect Km or Vmax in either sulfur-deficient or sulfur-sufficient plants (data not shown).
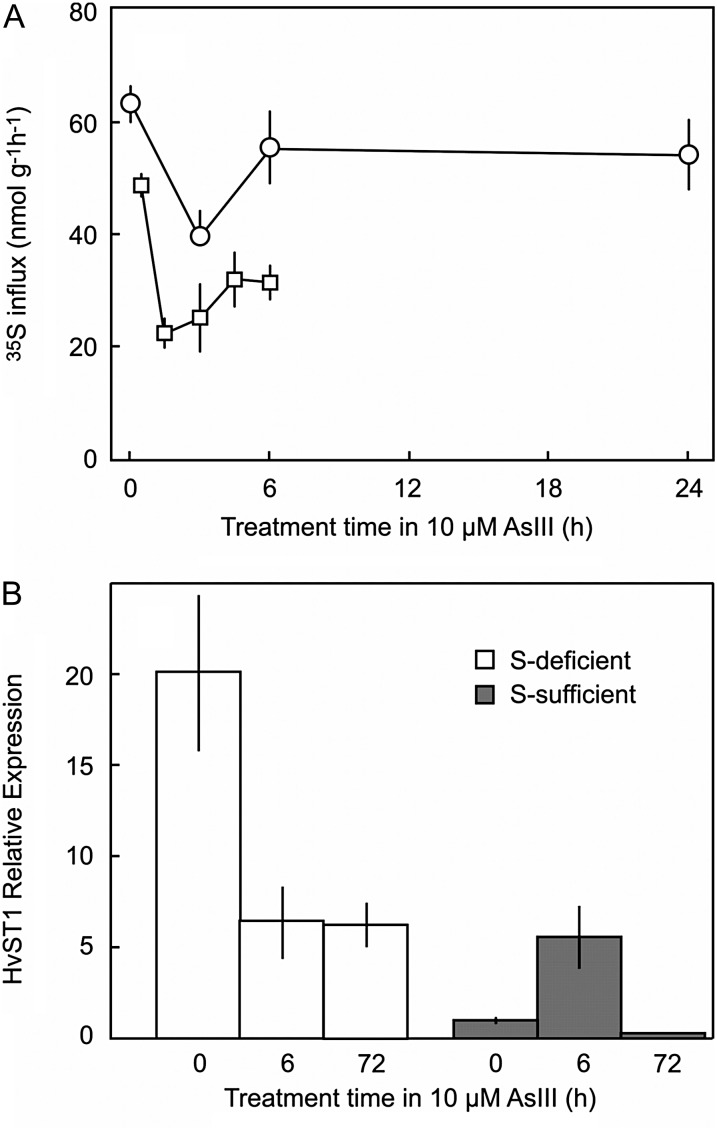
In sulfur-sufficient plants, addition of 10 µm arsenic caused a rapid reduction of sulfate influx for the first 2 h, after which, influx increased but remained lower than that prior to AsIII application (Fig. 5A).
Figure 5.
A, 35S influx following addition of 10 µm AsIII in sulfur-sufficient barley roots. The two experiments are similar except that in the longer time course (circles), the plants were grown for 7 d in sulfur compared with 11 d in the short time course. B, Relative expression of HvST1 in sulfur-deficient and sulfur-sufficient roots of barley after treatment in 10 µm AsIII. Expression is normalized to sulfur-sufficient roots without arsenic (= 1x) and is shown relative to HvGAPDH. Data are presented as means ± se of at least five replicates. S, Sulfur.
In the absence of AsIII, expression in roots of the high-affinity sulfate transporter HvST1 was 20-fold higher in sulfur-deficient plants compared with sulfur-sufficient plants (Fig. 5B). Following addition of 10 µm AsIII, expression of HvST1 decreased in the sulfur-deficient plants, probably due to toxicity. In sulfur-sufficient plants, AsIII treatment caused an increase in expression of HvST1 by more than 5-fold after 6 h, but after 72 h, the expression level had fallen to 30% of that of the control tissue (Fig. 5B).
Effect of AsIII on Expression of Thiol-Related Genes
The synthesis of nonprotein thiols is a complex process involving uptake of sulfate, reduction of sulfate to sulfide, synthesis of Cys, and the incorporation of Cys into glutathione. In addition, there are several downstream enzymes involved in formation of complexes between AsIII and glutathione or the synthesis of PCs. The response to sulfur deficiency and AsIII of genes encoding each of these enzymes was investigated in roots. Because ATP-dependent transfer of AsIII-thiol complexes to the vacuole is likely to be an important step in detoxifying arsenic (Bleeker et al., 2003; Klein et al., 2006), the expression of a gene for the ATP-binding cassette-type transporter HvMRP1 was also examined. The details of all of the genes are provided in Supplemental Table S3.
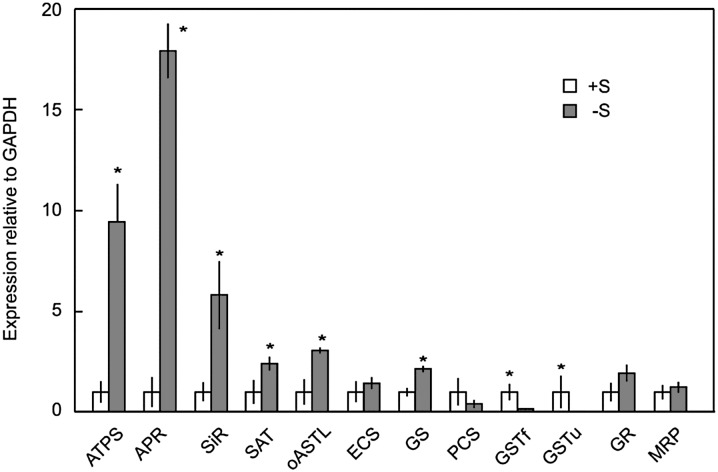
All of the genes associated with sulfate reduction and Cys synthesis were significantly up-regulated under sulfur deficiency (Fig. 6). The strongest expression was recorded for the first three enzymes of the pathway, ATP sulfurylase (9-fold), AP reductase (18-fold), and sulfite reductase (6-fold). Glutathione synthase was also up-regulated, but the genes encoding thiol-complexing enzymes GSTu and GSTf were strongly down-regulated (Fig. 6).
Figure 6.
Relative expression of genes of sulfate reduction, thiol synthesis, and thiol transport in barley roots grown with or without sulfur. Expression is normalized to sulfur-sufficient (+S) plants (relative expression = 1). Each value is the mean ± se of at least four independent extracts. Asterisks indicate significant difference compared with sulfur-sufficient plants (P < 0.05). −S, Sulfur deficient.
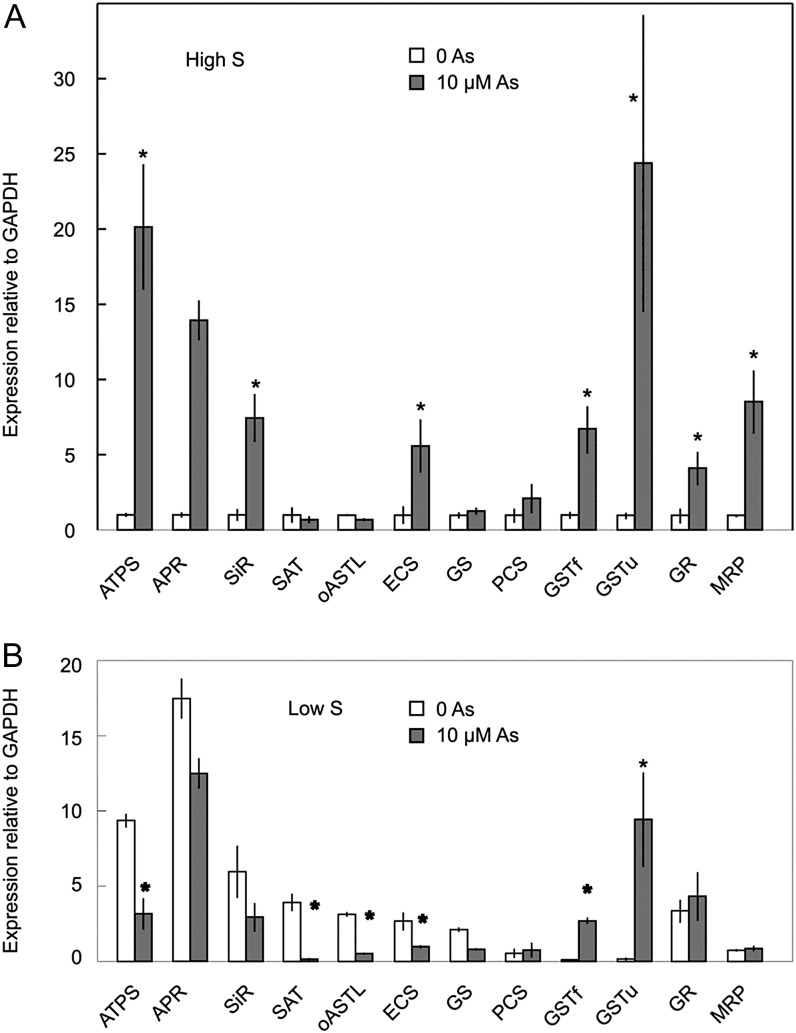
Addition of 10 µm AsIII to sulfur-sufficient roots induced strong up-regulation of ATP sulfurylase, AP reductase, and sulfite reductase (Fig. 7), which follows the same pattern as for sulfur deficiency. However, in contrast to sulfur deficiency, the expressions of GSTu and GSTf were greatly increased, as was the expression of the tonoplast thiol conjugate transporter MRP (Fig. 7). When 10 µm AsIII was added to sulfur-deficient plants, expression of all of the genes was either lower or the same as the sulfur-deficient control, with the exception of GSTu and GSTf, whose expressions increased 10- and 2.5-fold, respectively.
Figure 7.
Effect of treatment with 10 µm AsIII for 6 h on the expression of genes of sulfate reduction, thiol synthesis, and thiol transport in barley roots grown with or without 0.4 mm SO42–. Expression is normalized to sulfur-sufficient plants without AsIII (relative expression = 1). Each value is the mean ± se of at least four independent extracts. Asterisks indicate significant effect of arsenic on expression (P < 0.05). S, Sulfur.
The results shown in Figure 7 reflect the expression levels 6 h after the addition of AsIII. Gene expression responded rapidly, and several genes were already strongly up-regulated 3 h after addition of AsIII. Expression then increased until at least 6 h, but by 24 h, most of the expression levels were close to the preexposure level or were decreasing (Supplemental Fig. S3). Expression of three of the genes, ATP sulfurylase, ECS, and GSTu, was measured again after 72 h. For ATP sulfurylase and ECS, there was no significant difference between control and AsIII-treated roots, but GSTu expression remained slightly elevated (2.24 ± 0.48-fold for independent experiments; data not shown).
The dependence of gene expression on AsIII concentration was examined for a selection of genes, and none was found to have higher expression at 20 µm compared with 10 µm AsIII (Supplemental Fig. S3).
The short-term gene responses seemed to be specific to AsIII. When expressed on a total RNA basis, we did not find large or consistent responses to AsIII of either the metabolic control gene HvGAPDH or the structural control gene HvTub. In addition, we know from previous work that the boron efflux transporter HvBOR2 is not responsive to AsIII and that boron-tolerant plants are not also AsIII tolerant, despite the similarity in structure of boric acid and AsIII and the fact that both are taken up through aquaglyceroporins.
DISCUSSION
Arsenic Accumulation and Thiol Synthesis
There are two important facts to emerge from this study. First, the rate of AsIII permeation through the plasma membrane of root cells greatly exceeds the rate at which arsenic and thiols accumulate in the cell. Second, thiol synthesis was linearly related to the AsIII concentration in the external solution.
The imbalance between the rate of AsIII permeation of membranes and the rate at which complexing thiols can be synthesized means that the free AsIII concentration in the cytoplasm is likely to be close to that in the external solution and that thiol synthesis will have little effect on toxicity. It is widely assumed that arsenic toxicity is mediated by AsIII binding to thiols in proteins and enzymes. However, there is little evidence to support this assumption. In studies with purified animal enzymes, most required at least 5 mm AsIII for 50% inhibition, some requiring hundreds of millimolar (Hu et al., 1998). Others were actually stimulated by AsIII concentrations between 0.1 and 100 mm (Hu et al., 1998). The enzyme considered most likely to be inhibited by low concentrations of AsIII is pyruvate dehydrogenase, through binding to the dithiol groups of its cofactor dihydrolipoic acid rather than to the enzyme itself. Reported concentrations for 50% inhibition of pyruvate dehydrogenase in vitro range from 6 to 120 µm (Hu et al., 1998; Petrick et al., 2001).
Over a range of AsIII treatment concentrations and growth periods, the ratio of thiol to arsenic remained close to 3:1. AsIII is known to spontaneously form stable complexes with excess GSH in a ratio of 3:1. A further consequence of the formation of these complexes is that, as the free AsIII concentration increases, the free GSH concentration would progressively decrease. GSH is known to be a key regulator both of the enzymes of the sulfate reduction pathway and of genes encoding these enzymes (Davidian and Kopriva 2010; Na and Salt 2011; Takahashi et al., 2011). Such a reduction in GSH also occurs under sulfur deprivation, which would explain the similarity in response of genes involved in sulfate assimilation when the cell is challenged with AsIII. Bolchi et al. (1999) found that the coordinate expression of both a high-affinity sulfate transporter and ATP sulfurylase was jointly regulated by Cys and glutathionine levels but not by tissue sulfate status, which helps explain the rapid up-regulation of HvST1 and ATP sulfurylase by AsIII in plants replete with sulfur (Fig. 5).
Sulfur Deficiency and Arsenic Toxicity
In addition to the up-regulation of sulfate transporter and sulfate assimilatory genes, there were morphological changes that point to a perceived sulfur deficiency during treatment with AsIII. Low concentrations of AsIII strongly stimulated root growth, which is a characteristic feature of sulfur deficiency (Clarkson et al., 1989), and at both low and toxic concentrations of AsIII, the ratio of root growth to shoot growth was similar to that in sulfur-deficient plants. Root development is under hormonal control by the levels of auxin, which increases under sulfur deficiency (López-Bucio et al., 2003), and cytokinin. There is now direct, albeit confusing, evidence for interactions between these hormones and gene expression under sulfur deficiency (Dan et al., 2007), which might lead to altered regulation of root growth and sulfate influx.
In sulfur-sufficient plants, exposure to AsIII increased the concentration of sulfur in roots and caused a temporary up-regulation of HvST1 and also, unexpectedly, a reduction in sulfate influx. Thus, there is a contradiction between the responses to sulfur deficiency and AsIII treatment, in that, while both induce expression of a sulfate transporter, both increase root sulfur concentrations, but only in the case of deficiency is there an observable increase in sulfate influx. However, for a number of reasons, supply of sulfur is unlikely to be a major factor in arsenic tolerance except at very low sulfur concentrations. The kinetic analysis of sulfate influx into barley roots demonstrated that under starvation conditions, sulfate could be taken up at a maximum rate of 380 nmol g–1 h–1, but that under sufficiency conditions, the rate fell to 50 nmol g–1 h–1. In the absence of sulfate efflux, this latter rate would increase the internal sulfur concentration by 1.2 mm per day, yet the total arsenic-stimulated increase in root sulfur concentrations over 7 d in the experiment described in Figure 2 was only 2.4 mm (equivalent to a net uptake of 16 nmol g–1 h–1 over this period). Clearly, a large proportion of the sulfate taken up is effluxed back into the bathing medium, so an increased demand for sulfur could easily be met by a moderate reduction in sulfate efflux rather than stimulation of influx or, alternatively, by a reduction in export from root to shoot as evidenced by the lower shoot sulfur concentrations in AsIII-treated plants. In sulfur-deficient plants where there was no sulfur in the external solution, the increase in root sulfur following addition of AsIII can only be explained by retranslocation of sulfur from shoot to root. It is possible that at least part of the reduction in shoot sulfur in sulfur-sufficient plants was also due to withdrawal of shoot sulfur to the roots.
Gene Expression
Based on what is known about regulation of sulfate uptake and reduction, the responses to both AsIII and sulfur deficiency appear to revolve around changes in the intracellular levels of Cys and GSH. Under sulfur deficiency, the depletion of sulfur would occur in parallel to a gradual decline in the free levels of these two thiol compounds, such that these compounds would be a proxy for cellular sulfur status. Hence, the greater toxicity of AsIII under sulfur-limiting conditions could be explained by an increase in the intensity of sulfur deficiency caused by binding of sulfur-containing compounds to AsIII. This is illustrated by the data in Figure 2, where, following the addition of 20 µm AsIII to sulfur-deficient plants, the root sulfur concentration increased from 2.8 to 4.0 mm by withdrawal of sulfur from the shoots. This 1.2-mm increase in sulfur would be fully absorbed by the thiol synthesis required to balance the 0.6 mm AsIII uptake (assuming two sulfhydryl groups per arsenic). In the case of AsIII, its high permeability and affinity for thiols could cause a rapid decrease in free thiol content, even in plants with abundant reserves of sulfur, thereby eliciting a sulfur deficiency response.
The largest changes in gene expression following AsIII treatment occurred in the first 6 h. Norton et al. (2008) also reported strong up-regulation of glutathione S-transferases in rice within 0.5 h of exposure to AsV, with peak expression occurring approximately 5 h after addition of AsV and then declining. These responses occur at a time when the total accumulation of arsenic must be relatively small; in the case of AsIII, this could be roughly estimated from the uptake curves to be around 50 µm after 2 h (Fig. 1). However, this concentration is comparable to the pools of free Cys and glutathione reported in barley roots of around 40 and 135 µm, respectively (Smith et al., 1997), and these pools would quickly diminish if thiol:arsenic (3:1) complexes spontaneously formed. Transcription of sulfate transporters and of several other enzymes of the sulfur reduction pathway is known to be regulated by Cys and GSH (Davidian and Kopriva 2010; Na and Salt 2011), so it seems reasonable to propose that reduction of the free concentrations of these compounds due to complexation with newly absorbed AsIII could trigger the gene responses observed.
There were strong similarities between sulfur deficiency and AsIII treatment for genes involved in uptake and reduction of sulfate. ST1, ATP sulfurylase, AP reductase, and sulfite reductase were all strongly up-regulated under both conditions. There were also AsIII-specific changes in gene expression; GSTu and GSTf were both strongly up-regulated with AsIII treatment, whereas they were strongly down-regulated under sulfur deficiency. MRP was also up-regulated with AsIII but not with sulfur deficiency. A comparison of the major changes in gene expression for AsIII and sulfur nutritional treatments is shown in Table I.
Table I. Summary of sulfate and AsIII responsive genes in roots of barley.
AsIII response refers to sulfur-sufficient plants. More details of the genes are given in Supplemental Table S3. Plus or minus symbols indicate increased or decreased expression. +, 2- to 5-fold; ++/– –, 5- to 10-fold; +++/– – –, more than 10-fold.
| Gene | Sulfur Deficiency Response | AsIII |
|---|---|---|
| ST1 | +++ | ++ |
| ATPS | ++ | +++ |
| APR | +++ | +++ |
| SIR | ++ | ++ |
| SAT | + | |
| OASTL | + | |
| GR | + | + |
| GSTf | – – | ++ |
| GSTu | – – – | +++ |
| MRP | ++ |
Gene responses to AsIII were quite different under high and low sulfur conditions. Under limiting sulfur conditions, most of the genes that were up-regulated following exposure to arsenic under high sulfur conditions were actually expressed at lower levels in the presence of arsenic, which may be a reflection of a general inhibition of metabolic processes, including transcription, due to high levels of uncomplexed AsIII in the cytoplasm.
None of the genes examined were expressed more highly at 20 µm AsIII compared with 10 µm AsIII, as most genes were expressed at significantly lower levels, and this is interpreted as being due to toxicity at the higher AsIII concentration. Expression levels of all of the genes of sulfate uptake and reduction that were up-regulated by AsIII returned to their original levels within 1 to 3 d, which suggests that the initial reaction was sufficient to return Cys and GSH to normal levels. However, expression of GSTs remained elevated, as also occurred in rice, where GST expression 7 d after exposure to AsV was still much higher than control levels (Norton et al., 2008). GSTs were strongly down-regulated by sulfur deficiency alone, but strongly up-regulated by AsIII even in sulfur-deficient plants. This result seems to indicate that expression of GSTs is not under control of reduced thiol concentrations, but responds directly to the presence of AsIII.
CONCLUSION
Plants such as barley respond to AsIII in a variety of ways. At low concentrations (e.g. less than about 10 μm), early growth was either unaffected or slightly stimulated. The responses are quite similar to sulfur deficiency even in plants with abundant resources of sulfur. At higher concentrations, toxicity was observed, and this correlates with AsIII concentrations required to inhibit pyruvate dehydrogenase, a central enzyme in energy metabolism that requires a dithiol cofactor for which AsIII has high affinity (Spuches et al., 2005). Sulfur-deficient plants were sensitive to AsIII even at concentrations that would not be expected to inhibit enzyme activity. In this case, the effect of AsIII was most probably due to the induction of thiol synthesis, which creates an increased demand for the already depleted sulfur reserves, such that the deficiency becomes too intense for growth. It is unclear the extent to which genetic and morphological changes are actually a response to AsIII rather than a coincidental response to induced changes in levels of metabolites controlling sulfur nutrition. Genes involved in sulfate uptake and reduction responded similarly to both sulfur deficiency and AsIII. On the other hand, genes involved in arsenic complexation and compartmentation responded only to AsIII and not to sulfur deficiency. The other interesting question is whether these responses are really a detoxification strategy, and if so, whether it is effective. The mismatch between the rates of AsIII permeation and thiol synthesis would seem to indicate that complexation of arsenic would have little impact on the free concentration of arsenic in the cell. In controlled hydroponic culture, it would not be possible to deplete arsenic around the root by uptake into the plant, and this would apply to plants such as rice when grown under flooded conditions. Under aerobic soil conditions, diffusion of arsenic species would be slower but would continue to be delivered to the root by bulk water movement caused by transpiration. Whether this would reduce arsenic uptake by an order of magnitude to match thiol synthesis is debatable.
MATERIALS AND METHODS
Plant Material
Seeds of barley (Hordeum vulgare) were surface sterilized with 1% (v/v) sodium hypochlorite for 10 min, rinsed with deionized water, and germinated on damp paper towel. After 3 d, the seedlings were mounted in 1-mL Eppendorf tubes from which the base had been excised and placed in holes in the lids of 400-mL plastic containers with over 350 mL of nutrient solution. Each container held five or six plants, and solutions were changed every 2 to 3 d. The containers were blackened to exclude light from the roots. The plants were grown on a 14h-h/10-h day/night cycle at 24°C/18°C at a light intensity of approximately 600 µmol m–2 s–1 at the level of the solution.
Treatment Solutions
The nutrient medium was a one-fifth-strength modified Hoagland solution as described by Elberse et al. (2003), in which the sulfur concentration was varied using mixtures of MgSO4 and MgCl2. AsIII was prepared freshly for each change of solution as a 5-mm stock of AsO3 in 10 mm NaOH.
Net growth rates following application of treatments were determined by weighing each plant at the beginning and end of the treatment period. Where net growth of roots and shoots is presented, the initial weights for each tissue were calculated based on average root-to-shoot ratios of similar plants harvested at the beginning of the treatment period.
Analytical Procedures
For analysis of sulfur and arsenic, plants were dried and digested in nitric/perchloric acids and analyzed by inductively coupled plasma atomic emission spectroscopy. Where shoot arsenic concentrations were low, they were analyzed by atomic fluorescence spectroscopy.
Soluble thiols were extracted and analyzed based on the method described in Wawrzynski et al. (2006). Approximately 100 mg fresh weight of root or shoot material was ground in 1 mL 0.1 m HCl/1 mm EDTA and then centrifuged at 12,000g for 5 min. An aliquot of the supernatant (200 µL) was mixed with 120 mm K2HPO4/6 mm EDTA (pH 7.8, 700 µL) and 6 mm 5,5′-dithio-2-nitrobenzoic acid (100 µL), and the absorbance was read at 412 nm. Correction was made for the background absorbance of the sample by subtracting the absorbance of a duplicate sample without 5,5′-dithio-2-nitrobenzoic acid. Thiol content is expressed as Cys equivalents.
Uptake Kinetics for Sulfate
The concentration dependence of sulfate uptake was determined using 35SO4. Plants were germinated for 3 d and then grown with 0 or 400 µM sulfur for 7 d. Uptake rates were measured over 20 min in whole seedlings in one-fifth-strength Hoagland solution, followed by a 2-min rinse in unlabeled solution. Roots were then excised, blotted, and radioactivity determined by liquid scintillation counting.
Gene Expression
Plants were germinated for 3 d and then grown with 0 or 400 µM sulfur in one-fifth-strength Hoagland solution for 7 d. Depending on the experiment, seedlings were treated with 10 or 20 µM AsIII for 3, 6, 12, or 24 h. Roots were harvested and ground in liquid N2. RNA was extracted from at least four replicate root samples using TRIzol (Invitrogen) following the manufacturer’s protocol. RNA was transcribed using the Omniscript RT Kit (Qiagen) with a polydT primer (Invitrogen). Details of the accession numbers and the primers for all of the genes examined are given in Supplemental Table S3.
Real-time PCR was performed on a Corbett Rotor Gene 6000 (Corbett Research). Expression was normalized against HvGAPDH using primers described in Burton et al. (2004), who evaluated six potential control genes for barley and found that under a range of treatments, GAPDH and tubulin were most stably expressed. Preliminary experiments tested the geometric mean of HvGAPDH and HvTub, but the values were similar to that of HvGAPDH alone.
Statistics
Differences between treatments were evaluated using parametric pairwise comparisons at P < 0.05.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ST1, U52867; ATPS, BQ740179; AP red, BF257518; SiR, BQ472077; OAStl, BE422221; SAT, CB883876; yECS, BJ465541; GS, DQ291128; PCS, AL510072; GSTF, VI AF430069; GSTU, AF109194; GRII, AB277097; MRP, AU252331; and GaPDH, X60343.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sulfate uptake kinetics for sulfur-deficient and -sufficient plants.
Supplemental Figure S2. Effect of sulfate concentration on growth of barley.
Supplemental Figure S3. Time course of expression of selected genes of sulfate reduction and thiol synthesis in sulfur-sufficient barley roots following exposure to 10 μm AsIII.
Supplemental Table S1. Ratios of root growth to shoot growth for sulfur-deficient plants and plants treated with AsIII.
Supplemental Table S2. Sulfur content of shoots of barley seedlings treated with AsIII.
Supplemental Table S3. Gene names, accession numbers, and primer sequences for genes encoding enzymes and transporters.
Glossary
- AsIII
arsenite
- AsV
arsenate
- PC
phytochelatin
- GSH
reduced glutathione
References
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP. (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker PM, Schat H, Jos RV, Verkleij VAC, Ernst WHO. (2003) Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol 157: 33–38 [DOI] [PubMed] [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. (1999) Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by l-cysteine. Plant Mol Biol 39: 527–537 [DOI] [PubMed] [Google Scholar]
- Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB. (2004) The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol 134: 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Saker LR, Purves JV. (1989) Depression of nitrate and ammonium transport in barley plants with diminished sulfate status. Evidence of co-regulation of nitrogen and sulfate intake. J Exp Bot 40: 953–963 [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18: 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan H, Yang G, Zheng Z-L. (2007) A negative regulatory role for auxin in sulphate deficiency response in Arabidopsis thaliana. Plant Mol Biol 63: 221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidian J-C, Kopriva S. (2010) Regulation of sulfate uptake and assimilation—the same or not the same? Mol Plant 3: 314–325 [DOI] [PubMed] [Google Scholar]
- Dong R, Formentin E, Losseso C, Carimi F, Benedetti P, Terzi M, Schiavo F. (2005) Molecular cloning and characterization of a phytochelatin synthase gene, PvPCS1, from Pteris vittata L. J Ind Microbiol Biot 32: 527–533 [DOI] [PubMed] [Google Scholar]
- Duan G-L, Zhu Y-G, Tong Y-P, Cai C, Kneer R. (2005) Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol 138: 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberse IAM, van Damme JMM, van Tienderen PH. (2003) Plasticity of growth characteristics in wild barley (Hordeum spontaneum) in response to nutrient limitation. J Ecol 91: 371–382 [Google Scholar]
- Gasic K, Korban SS. (2007) Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 225: 1277–1285 [DOI] [PubMed] [Google Scholar]
- Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, Goldsbrough PB, Cobbett CS. (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss S, Wachter A, Bogs J, Cobbett C, Rausch T. (2003) Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J Exp Bot 54: 1833–1839 [DOI] [PubMed] [Google Scholar]
- Hu Y, Su L, Snow ET. (1998) Arsenic toxicity is enzyme specific and its affects on ligation are not caused by the direct inhibition of DNA repair enzymes. Mutat Res 408: 203–218 [DOI] [PubMed] [Google Scholar]
- Klein M, Burla B, Martinoia E. (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett 580: 1112–1122 [DOI] [PubMed] [Google Scholar]
- Léonard A, Lauwerys RR. (1980) Carcinogenicity, teratogenicity and mutagenicity of arsenic. Mutat Res 75: 49–62 [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen G, Tian Y. (2008) Arsenic tolerance, uptake and translocation by seedlings of three rice cultivars. Acta Ecol Sin 28: 3228–3235 [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao F-J. (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105: 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N. (1993) ATP-dependent glutathione S-conjugate ‘export’ pump in the vacuolar membrane of plants. Nature 364: 247–249 [Google Scholar]
- Meharg A, Hartley-Whitaker J. (2002) Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species. New Phytol 154: 29–43 [Google Scholar]
- Meharg AA, Jardine L. (2003) Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol 157: 39–44 [DOI] [PubMed] [Google Scholar]
- Na G, Salt DE. (2011) The role of sulfur assimilation and sulfur-containing compounds in trace element homeostasis in plants. Environ Exp Bot 72: 18–25 [Google Scholar]
- Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian J-C, Sacchi GA. (2006) Heavy metal stress and sulfate uptake in maize roots. Plant Physiol 141: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton GJ, Lou-Hing DE, Meharg AA, Price AH. (2008) Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59: 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Jagadish B, Mash EA, Aposhian HV. (2001) Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14: 651–656 [DOI] [PubMed] [Google Scholar]
- Raab A, Feldmann J, Meharg AA. (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Schat H, Meharg AA, Feldmann J. (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168: 551–558 [DOI] [PubMed] [Google Scholar]
- Ramos J, Naya L, Gay M, Abián J, Becana M. (2008) Functional characterization of an unusual phytochelatin synthase, LjPCS3, of Lotus japonicus. Plant Physiol 148: 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E. (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Härtling S, Tanneberg H. (2008) The identification and quantification of arsenic-induced phytochelatins—comparison between plants with varying As sensitivities. Plant Soil 303: 275–287 [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher AR, Warrilow AGS. (1997) Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J 12: 875–884 [DOI] [PubMed] [Google Scholar]
- Spuches AM, Kruszyna HG, Rich AM, Wilcox DE. (2005) Thermodynamics of the As(III)-thiol interaction: arsenite and monomethylarsenite complexes with glutathione, dihydrolipoic acid, and other thiol ligands. Inorg Chem 44: 2964–2972 [DOI] [PubMed] [Google Scholar]
- Sung DY, Lee D, Harris H, Raab A, Feldmann J, Meharg A, Kumabe B, Komives EA, Schroeder JI. (2007) Identification of an arsenic tolerant double mutant with a thiol-mediated component and increased arsenic tolerance in phyA mutants. Plant J 49: 1064–1075 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62: 157–184 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA. (1999) AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA 96: 7110–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Duan GL, Williams PN, Zhu YG. (2008) Influence of phosphorus starvation on OsACR2.1 expression and arsenic metabolism in rice seedlings. Plant Soil 313: 129–139 [Google Scholar]
- Wawrzynski A, Kopera E, Wawrzynska A, Kaminska J, Bal W, Sirko A. (2006) Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. J Exp Bot 57: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Zhao FJ. (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176: 590–599 [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP. (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159: 403–410 [DOI] [PubMed] [Google Scholar]