Steady-state K+ uptake mechanisms differ between NH4+-grown barley and Arabidopsis. Sudden NH4+ withdrawal uncovers dramatic capacity and plasticity of K+ transport among the two model species.
Abstract
The role of potassium (K+) transporters in high- and low-affinity K+ uptake was examined in roots of intact barley (Hordeum vulgare) and Arabidopsis (Arabidopsis thaliana) plants by use of 42K radiotracing, electrophysiology, pharmacology, and mutant analysis. Comparisons were made between results from barley and five genotypes of Arabidopsis, including single and double knockout mutants for the high-affinity transporter, AtHAK5, and the Shaker-type channel, AtAKT1. In Arabidopsis, steady-state K+ influx at low external K+ concentration ([K+]ext = 22.5 µm) was predominantly mediated by AtAKT1 when high-affinity transport was inhibited by ammonium, whereas in barley, by contrast, K+ channels could not operate below 100 µm. Withdrawal of ammonium resulted in an immediate and dramatic stimulation of K+ influx in barley, indicating a shift from active to passive K+ uptake at low [K+]ext and yielding fluxes as high as 36 µmol g (root fresh weight)−1 h−1 at 5 mm [K+]ext, among the highest transporter-mediated K+ fluxes hitherto reported. This ammonium-withdrawal effect was also established in all Arabidopsis lines (the wild types, atakt1, athak5, and athak5 atakt1) at low [K+]ext, revealing the concerted involvement of several transport systems. The ammonium-withdrawal effect coincided with a suppression of K+ efflux and a significant hyperpolarization of the plasma membrane in all genotypes except athak5 atakt1, could be sustained over 24 h, and resulted in increased tissue K+ accumulation. We discuss key differences and similarities in K+ acquisition between two important model systems and reveal novel aspects of K+ transport in planta.
Potassium (K+), a major macronutrient in plants, is the most abundant intracellular cation (constituting up to 10% of plant dry weight) and is critical to such cellular functions as osmotic balance, enzyme activation, and electrical regulation (Leigh and Wyn-Jones, 1984; Maathuis and Sanders, 1996; Britto and Kronzucker, 2008). Understanding the mechanisms of K+ acquisition in plants has long been of scientific and practical importance and is increasingly urgent in light of major ecological and agricultural problems, such as soil K+ level decline (Ashley et al., 2006, and refs. therein) and sodium (Na+) and ammonium (NH4+) toxicities (Britto and Kronzucker, 2002; Kronzucker et al., 2006; ten Hoopen et al., 2010).
Since the pioneering work of Epstein et al. (1963), which described the acquisition of K+ by plants as the sum of activities of two transport systems with distinct substrate-binding affinities, major advances in the molecular and thermodynamic characterization of each system have been made (for review, see Maathuis and Sanders, 1996; Véry and Sentenac, 2003; Britto and Kronzucker, 2008; Szczerba et al., 2009). Generally, Epstein’s mechanism 1, or the high-affinity transport system (HATS), has been described as a saturable system that catalyzes the thermodynamically active uptake (i.e. ATP-dependent transport against an electrochemical gradient) of K+ from external concentrations ([K+]ext) of less than 1 mm (Kochian and Lucas, 1982; Maathuis and Sanders, 1994). Mechanism 2, or the low-affinity transport system (LATS), catalyzes a flux proportional to [K+]ext and is proposed to predominate above 1 mm [K+]ext (Epstein et al., 1963; Kochian et al., 1985; Maathuis and Sanders, 1996). Although several molecular candidates have been suggested to encode HATS and LATS proteins (Szczerba et al., 2009), it is believed that the majority of high-affinity transport is catalyzed by secondary active transporters of the HAK/KUP/KT family (e.g. AtHAK5 from Arabidopsis [Arabidopsis thaliana] and HvHAK1 from barley [Hordeum vulgare]), which operate via a proton (H+)/K+ symport mechanism (Gierth and Mäser, 2007). Low-affinity transport, by contrast, occurs via Shaker-like K+ channels (e.g. AtAKT1 from Arabidopsis and HvAKT1 from barley), which facilitate passive diffusion down the electrochemical gradient for K+ (Véry and Sentenac, 2003; Chérel, 2004). Other key transporters implicated in K+ uptake include nonselective cation channels (NSCCs; Demidchik et al., 2002) and HKT/TRK-type transporters (Rubio et al., 1995). An unidentified system in Arabidopsis, independent of AtAKT1 and AtHAK5 and reportedly operating only at high (millimolar) [K+]ext, has been the subject of considerable recent interest (Pyo et al., 2010; Rubio et al., 2010; Caballero et al., 2012; see also Hirsch et al., 1998); however, little is known about its molecular and physiological characterization.
The nutritional and molecular regulation of HAK/KUP/KT transporters and Shaker-like K+ channels has been extensively investigated in the model system Arabidopsis and has led to some blurring of distinctions between the traditional concepts of HATS and LATS (Spalding et al., 1999; Xu et al., 2006; Lee et al., 2007; Qi et al., 2008; Geiger et al., 2009; Honsbein et al., 2009; Pyo et al., 2010; Rubio et al., 2010; for review, see Alemán et al., 2011). For instance, although HAK/KUP/KT transporters appear to dominate the HATS (Gierth and Mäser, 2007), some members have been demonstrated to operate at [K+]ext as high as 20 mm, indicating a dual affinity (Fu and Luan, 1998; Kim et al., 1998). By contrast, although generally ascribed to the LATS (Maathuis and Sanders, 1996), AtAKT1 can operate at [K+]ext as low as 10 µm, given favorable thermodynamic conditions, and particularly if the HATS is suppressed by NH4+ (Hirsch et al., 1998; Spalding et al., 1999), which specifically inhibits HAK/KUP/KT transporters (Qi et al., 2008). Further blurring the distinction between HATS and LATS is the response of K+ uptake mechanisms to K+ limitation (Hampton et al., 2004). For instance, HAK/KUP/KT expression (Ahn et al., 2004; Gierth et al., 2005) and high-affinity K+ influx (Glass, 1976; Kochian and Lucas, 1982; Siddiqi and Glass, 1986) have both been shown to be up-regulated by K+ starvation. On the other hand, while AtAKT1 expression appears to be independent of K+ availability (Lagarde et al., 1996; Gierth et al., 2005), the posttranslational regulation of AtAKT1 (including phosphorylation/dephosphorylation networks and channel heteromerization) has shown that channel-mediated K+ uptake can increase in response to low-K+ conditions (Li et al., 2006; Xu et al., 2006; Lee et al., 2007; Geiger et al., 2009; Grefen et al., 2010; Jeanguenin et al., 2011).
While much recent work on K+ uptake has occurred in Arabidopsis, leading to the development of a sophisticated model (Alemán et al., 2011), little attention has been given to the question of how general this model might be, most importantly with respect to crop species. This is naturally the result of the vast catalog of well-characterized Arabidopsis mutant lines (e.g. The Arabidopsis Information Resource [http://www.arabidopsis.org] and the Arabidopsis Biological Resource Center [http://abrc.osu.edu]), which have no equivalent in crop species such as rice (Oryza sativa; Goff et al., 2002; Yu et al., 2002; see also Amrutha et al., 2007), barley (Mayer et al., 2012), and wheat (Triticum aestivum; Brenchley et al., 2012). Examinations into the molecular identity and contribution of K+ uptake systems in roots of barley have been limited to high-affinity concentrations (0.1–1 mm), with particular focus on the AtHAK5 homolog, HvHAK1, and its regulation (Santa-María et al., 2000; Vallejo et al., 2005; Fulgenzi et al., 2008). This work indicates that, like Arabidopsis (Hirsch et al., 1998; Spalding et al., 1999), high-affinity K+ uptake in roots of barley is dictated by NH4+-sensitive and -insensitive systems, linked to HvHAK1 and, most likely, HvAKT1, respectively (Santa-María et al., 2000). However, it has not been explored whether, under high NH4+ conditions, K+ channels can operate in roots of barley at very low [K+]ext (e.g. 10 µm), as they do in Arabidopsis (Hirsch et al., 1998); this information can help address some long-standing speculation on the role of K+ channels under nutrient deficiency (Kochian and Lucas, 1993). Moreover, the relative apportionment of K+ channels and secondary active transporters with respect to high- and low-affinity uptake in roots of barley has not been explored to the same extent as in Arabidopsis (Rubio et al., 2008, 2010). Lastly, although the inhibition of high-affinity K+ influx by external NH4+ supply is well documented (Vale et al., 1987; Spalding et al., 1999; Santa-María et al., 2000; Qi et al., 2008), as are its effects on membrane polarization (Ullrich et al., 1984; Wang et al., 1994; Britto et al., 2001), little is known about the recovery of K+ influx and the thermodynamic response following NH4+ removal under high- and low-affinity systems. As one of the chief aspects of NH4+ toxicity in higher plants, understanding the inhibitory role of this nitrogen source in K+ transport is of particular importance (Britto and Kronzucker, 2002).
Here, we address these gaps in understanding by providing, to our knowledge, the first in-depth physiological examination of the contribution of K+ channels and high-affinity transporters to K+ acquisition in barley. In particular, we posed the following questions. What is the relative apportionment of K+ channels and high-affinity transporters to high- and low-affinity K+ uptake in the presence of NH4+ in barley, and how does it differ from the Arabidopsis model? What are the maximal rates of high- and low-affinity K+ fluxes in planta? How does K+ uptake respond to NH4+ withdrawal, and what mechanisms underlie this response? With the use of 42K+ radiolabeling, coupled with mutant and electrophysiological analyses, we show that K+ acquisition at low (22.5 µm) [K+]ext, in the presence of high (millimolar) NH4+, is fundamentally different in the two model systems, chiefly in that K+ channels operate at such low [K+]ext in Arabidopsis but not in barley. However, we show that with sudden withdrawal of external NH4+, dramatic shifts in thermodynamic gradients and K+ fluxes can occur, revealing novel aspects of transport capacity and plasticity. We also provide, to our knowledge, the first in planta 42K examination of the athak5 atakt1 double mutant, revealing novel aspects of an uptake system as yet unidentified by genetic means.
RESULTS
The Relative Apportionment of K+ Channels and High-Affinity Transporters Differs between Barley and Arabidopsis under Steady-State Conditions
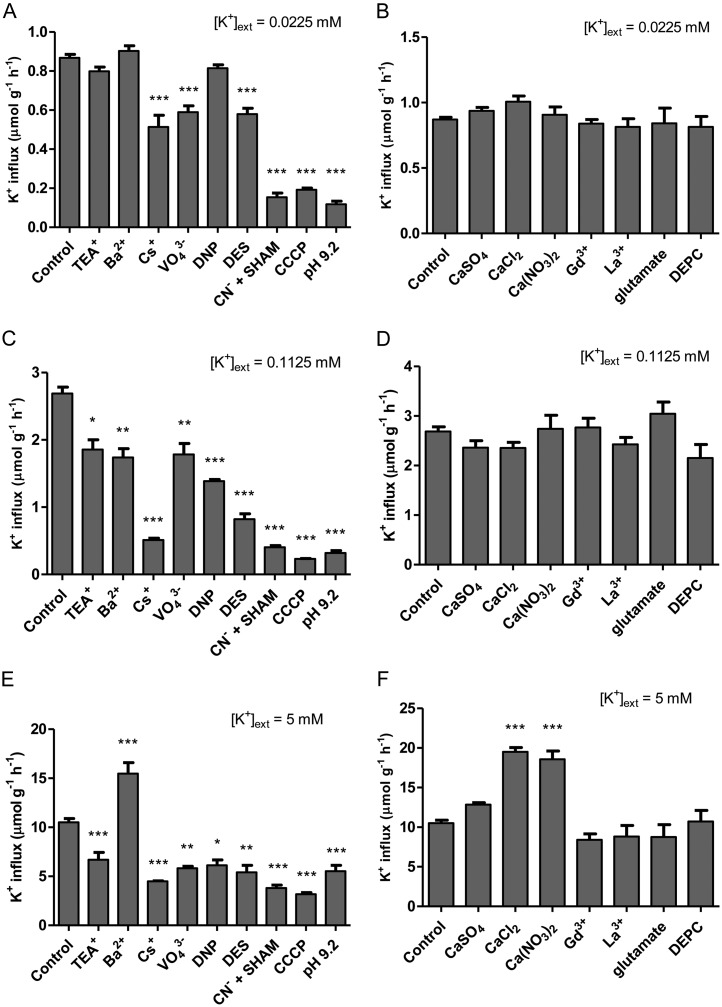
Figure 1 shows the results of an extensive pharmacological profiling of steady-state K+ influx, targeting either Shaker-like K+ channels and HAK/KUP/KT transporters (Fig. 1, A, C, and E) or NSCCs (Fig. 1, B, D, and F), in barley grown and tested under high (10 mm) NH4+ and three levels of [K+]ext: 0.0225 mm (low), 0.1125 mm (intermediate), and 5 mm (high). K+ influx was insensitive to the standard K+ channel inhibitors, tetraethyl ammonium (TEA+) and Ba2+ (White and Lemtiri-Chlieh, 1995; Bertl et al., 1997; Hille, 2001), under low-K+ conditions (Fig. 1A) but showed significant (P < 0.05) inhibition at intermediate K+ (Fig. 1C). Under high-K+ conditions, influx was suppressed by TEA+ and, surprisingly, stimulated by BaCl2 (Fig. 1E), as also observed with 5 mm CaCl2 and Ca(NO3)2 (Fig. 1F). Cs+, a potent inhibitor of both K+ channels and high-affinity transporters (Krol and Trebacz, 2000; White and Broadley, 2000), significantly suppressed K+ influx at low, intermediate, and high K+, by 41%, 81%, and 57%, respectively (Fig. 1, A, C, and E). Metabolic inhibitors vanadate (VO43−), 2,4-dinitrophenol (DNP), diethylstilbestrol (DES), cyanide (CN−) + salicylhydroxamic acid (SHAM), carbonyl cyanide m-chlorophenyl hydrazone (CCCP), and pH 9.2 (adjusted with NaOH) were all very effective in suppressing K+ influx under all [K+]ext conditions tested, except for DNP at low K+ (Fig. 1, A, C, and E). The NSCC inhibitors Ca2+, Gd3+, La3+, Glu, and diethylpyrocarbonate (DEPC; White and Lemtiri-Chlieh, 1995; Essah et al., 2003) had no effect on K+ influx at any [K+]ext tested (Fig. 1, B, D, and F). Note that counter-ion controls for VO43−, CN−, Glu, and pH 9.2 treatments were conducted with 10 mm NaCl and showed no response at any [K+]ext (data not shown).
Figure 1.
The effects of various pharmacological and nutritional treatments, targeting either Shaker-like K+ channels and HAK/KUP/KT transporters (A, C, and E) or NSCCs (B, D, and F), on steady-state K+ influx in intact roots of barley seedlings grown in full nutrient medium at low (A and B), intermediate (C and D), and high (E and F) [K+]ext and 10 mm [NH4+]ext. Fluxes are indicated on a root fresh weight basis. Asterisks denote different levels of significance between control and treatment pairs (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett’s multiple comparison post hoc test). Each treatment represents the mean of four to 69 replicates. Error bars indicate se.
Table I displays the results of a thermodynamic (Nernstian) analysis for barley based on compartmental analysis by 42K+ efflux and electrophysiology. Since physiological efflux was confirmed for low- and intermediate-K+ conditions (Coskun et al., 2010; see below), the methods of compartmental analysis (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995) were used to estimate cytosolic potassium concentration ([K+]cyt), along with unidirectional fluxes and cytosolic half-times of exchange. Based on estimates of [K+]cyt, equilibrium potentials for K+ (EK+) were calculated (see “Materials and Methods”) and were found to be more negative than measured membrane potentials (ΔΨm) for epidermal and cortical root cells at both K+ conditions (Table I); thus, thermodynamically active K+ uptake was predicted. Since physiological efflux was not found under high-K+ conditions (see below), neither compartmental nor subsequent thermodynamic analyses could be conducted under those conditions. We should note that EK+ is only an approximation, as it should use K+ activities rather than K+ concentrations. However, approximations of cytosolic K+ activity coefficients (γcyt) vary widely in the literature (e.g. 0.72 < γcyt < 1.29; Kielland, 1937; Robinson and Stokes, 1965; Ling, 1969; Palmer et al., 1978), reflecting a still surprisingly poor understanding of ion sequestration and interaction in the plant cell cytosol (Cheeseman, 2013). When K+ activities were estimated according to standard procedures (using γcyt = 0.75; Walker et al., 1996; Cuin et al., 2003), however, EK+ still remained negative of ΔΨm (data not shown); thus, the principal thermodynamic conclusions of our analysis still hold.
Table I. Steady-state K+ fluxes, compartmentation, and electrophysiology of intact barley seedling roots.
One-week-old barley seedlings were grown on full nutrient medium supplemented with 10 mm NH4+ and either 0.0225 or 0.1125 mm K+. ΔΨm measurements were taken from root epidermal and cortical cells 2 to 3 cm from the root tip. EK+ and predicted [K+]cyt were determined with the Nernst equation. Error values indicate se of six to eight replicates.
| [K+]ext | Influx | Efflux | Net Flux | Efflux-Influx Ratio | Half-Time | ΔΨm | EK+ |
[K+]cyt |
|
|---|---|---|---|---|---|---|---|---|---|
| Predicted | Measured | ||||||||
| mm | µmol g−1 h−1 | min | mV | mm | |||||
| 0.0225 | 0.52 ± 0.03 | 0.27 ± 0.02 | 0.25 ± 0.02 | 0.52 ± 0.03 | 32.90 ± 2.92 | −143.6 ± 2.7 | −153.8 | 5.57 | 8.24 ± 0.69 |
| 0.1125 | 1.89 ± 0.13 | 0.57 ± 0.05 | 1.32 ± 0.10 | 0.30 ± 0.01 | 32.50 ± 4.69 | −136.2 ± 3.0 | −144.1 | 20.95 | 28.39 ± 3.40 |
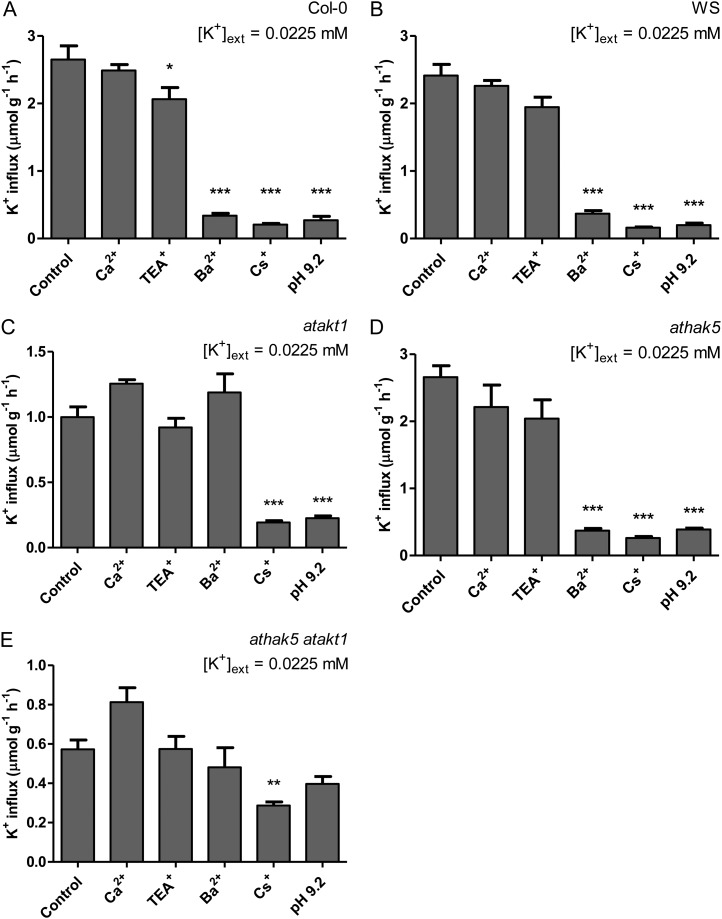
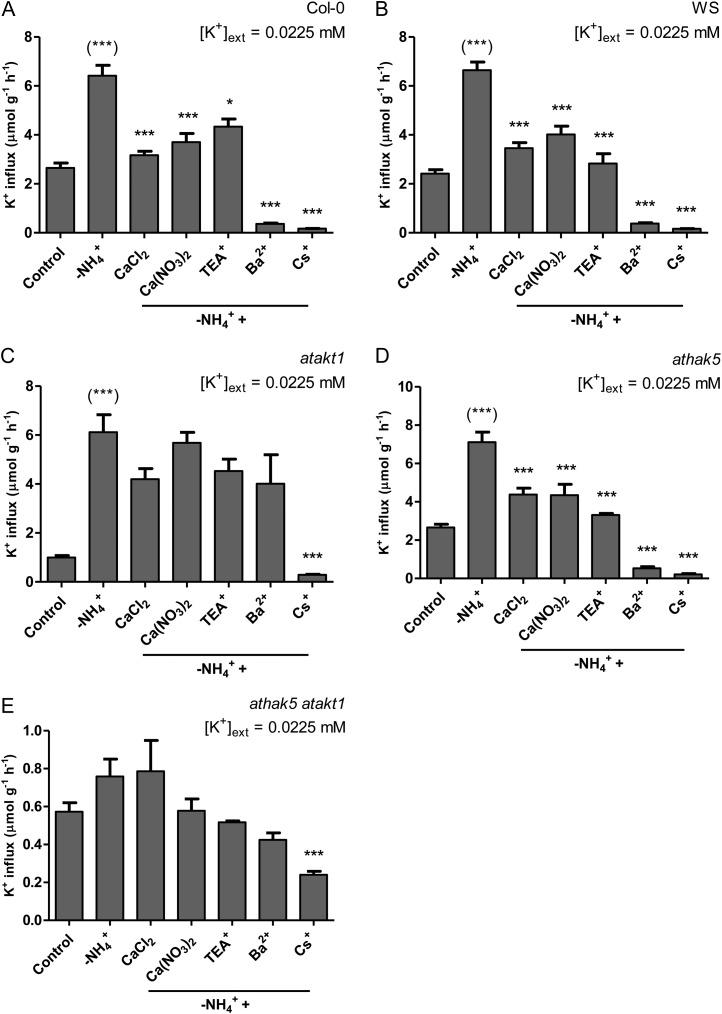
A selective pharmacological profiling of K+ influx in Arabidopsis (Columbia [Col-0] wild type, Wassilewskija [WS] wild type, atakt1, athak5, and athak5 atakt1) was conducted at low K+ and high NH4+ (Fig. 2). Because Arabidopsis exhibited NH4+ toxicity at much lower concentrations than barley (data not shown), NH4+ was provided only at 2 mm (compared with 10 mm in barley). Unlike barley, Arabidopsis wild-type lines generally displayed TEA+ and Ba2+ sensitivity under low-K+, high-NH4+ conditions (although it was not statistically significant in the case of TEA+ for WS; Fig. 2B). Consistent with their pharmacological targeting (see above), TEA+ and Ba2+ sensitivity was not found in the AtAKT1 knockout lines atakt1 and athak5 atakt1 (Fig. 2, C and E). Further confirmation of the involvement of AtAKT1 was provided by the dramatic decrease in steady-state influx in atakt1 and athak5 atakt1 lines compared with their respective wild types (59% and 78%, respectively), while influx for athak5 was essentially equal to the Col-0 wild type and displayed both TEA+ and Ba2+ sensitivity (albeit not statistically significant in the case of TEA+; Fig. 2D). Cs+ significantly suppressed steady-state influx in all lines, including athak5 atakt1 (Fig. 2, A–D), while pH 9.2 was about as effective as Cs+ in all cases except in athak5 atakt1 (Fig. 2, A–D). Ca2+ was ineffective at suppressing steady-state influx in all lines (Fig. 2, A–D).
Figure 2.
The effects of various pharmacological and nutritional treatments on steady-state K+ influx in intact roots of Arabidopsis Col-0 wild type (A), WS wild type (B), atakt1 (C), athak5 (D), and athak5 atakt1 (E) grown in full nutrient medium at low [K+]ext and 2 mm [NH4+]ext. Fluxes are indicated on a root fresh weight basis. Asterisks denote different levels of significance between control and treatment pairs (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett’s multiple comparison post hoc test). Each treatment represents the mean of three to 14 replicates. Error bars indicate se.
Mechanism of K+ Efflux in Barley Differs Based on [K+]ext
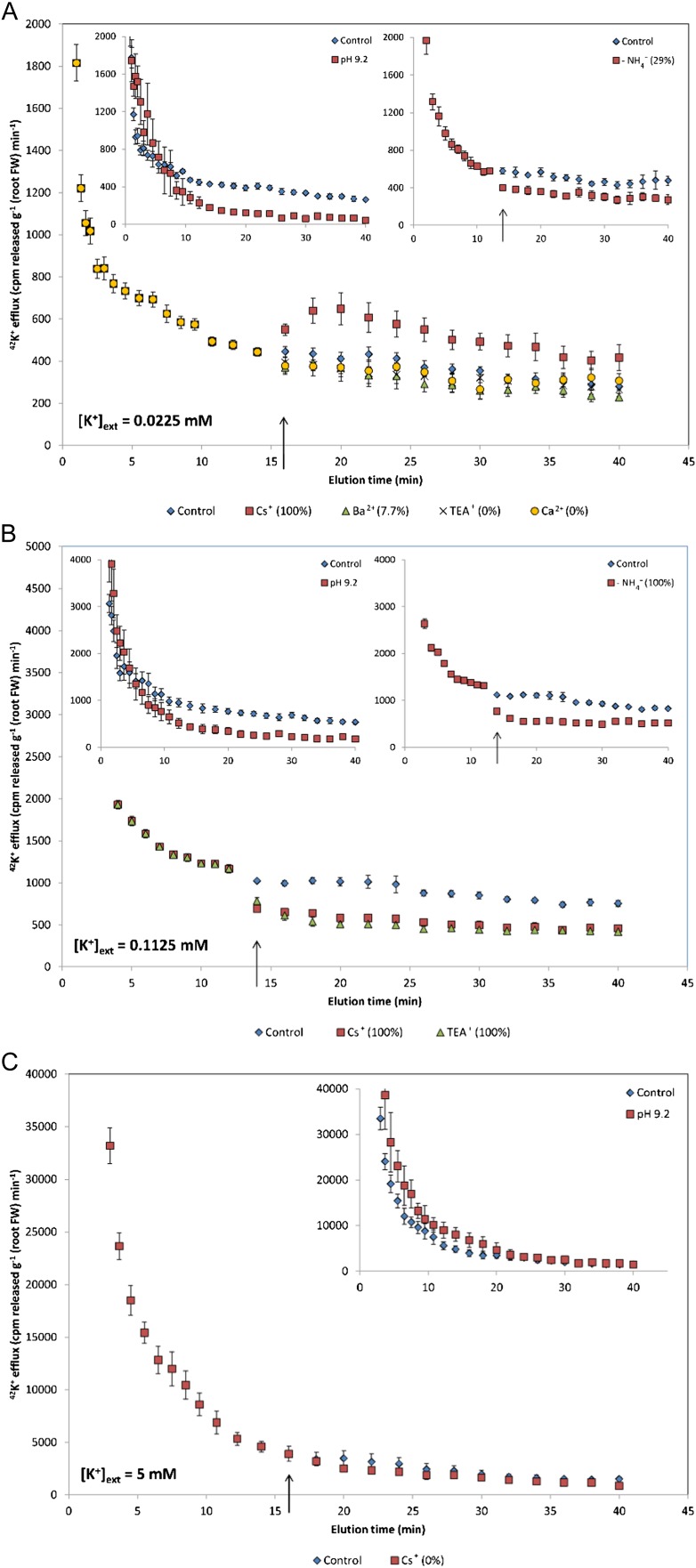
Figure 3 illustrates the pharmacological profiling of steady-state K+ efflux in barley under high NH4+ and varying [K+]ext. Interestingly, at low (22.5 µm) [K+]ext, application of 10 mm Cs+ resulted in an immediate and significant stimulation in K+ efflux (Fig. 3A), unlike TEA+, Ba2+, and Ca2+, which had no effect. By contrast, at intermediate (112.5 µm) [K+]ext, Cs+ was effective in inhibiting efflux, as was TEA+ (Fig. 3B). At high (5 mm) [K+]ext, Cs+ showed no effect (Fig. 3C), consistent with the cessation of physiological efflux, which was confirmed by comparing steady-state efflux to pH 9.2 treatments, where roots had been exposed to alkalinity during tracer uptake and elution periods (see “Materials and Methods”). Since pH 9.2 was effective in suppressing influx at all [K+]ext tested (Fig. 1, A, C, and E), its application during tracer uptake would have inhibited intracellular accumulation. Thus, the significant suppression of tracer release during the slowly exchanging phase in the low- and intermediate-K+ conditions, and lack thereof at high K+ (Fig. 3, insets), with pH 9.2 indicates the cytosolic origin of released tracer at low and intermediate K+ and the lack thereof at high K+. Sudden withdrawal of external NH4+ results in a thermodynamic shift (at low and intermediate K+) and significant stimulations in K+ influx (see below), but it was also found to inhibit K+ efflux at both [K+]ext and to cause no effect at high K+ (Fig. 3, insets), confirming the proposed dual nature of efflux under high- and low-affinity conditions.
Figure 3.
Responses of 42K+ efflux from roots of intact barley seedlings to sudden application (see arrows) of various pharmacological and nutritional treatments. Plants were grown in full nutrient medium at low (A), intermediate (B), and high (C) [K+]ext and 10 mm [NH4+]ext. Insets show responses of K+ efflux to sudden (see arrows) withdrawal of external NH4+ and/or alkalinity during radiotracer uptake and elution periods. Numbers in parentheses indicate percentages of treated points differing significantly from the control (Student’s t test; P < 0.05). In the insets, axis labels are as in the main figures. Each plot represents the mean of three to seven replicates. Error bars indicate se. FW, Fresh weight. [See online article for color version of this figure.]
NH4+ Withdrawal Results In Thermodynamic Shifts and Significant K+ Influx
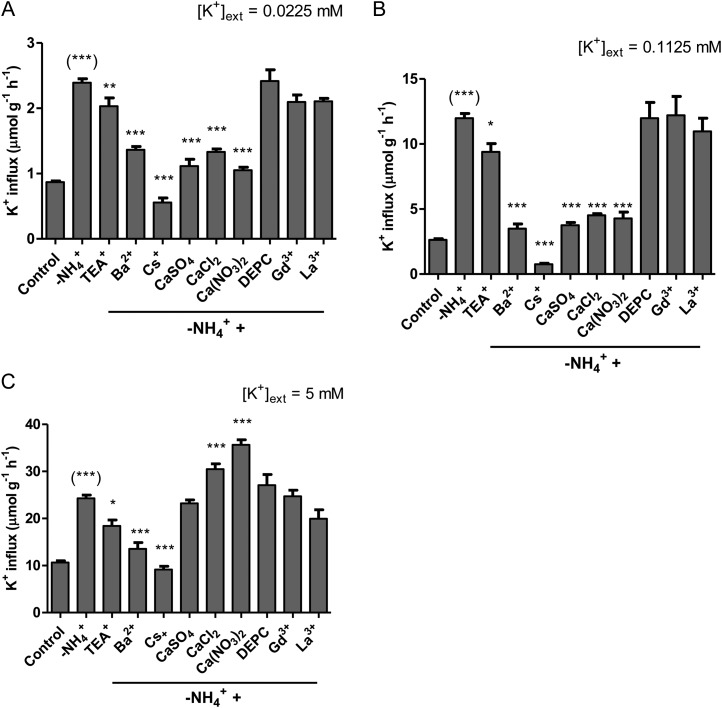
K+ influx was stimulated by sudden withdrawal (5-min pretreatment) of NH4+, by 176%, 355%, and 131% [corresponding to 2.4 ± 0.1, 12.0 ± 0.4, and 24.3 ± 0.7 µmol g (root fresh weight)−1 h−1, respectively], at low-, intermediate-, and high-K+ conditions, respectively (Fig. 4). NH4+ withdrawal also led to immediate and significant hyperpolarizations of root epidermal and cortical ΔΨm (59.1 ± 13.2, 53.1 ± 8.3, and 31.4 ± 4.9 mV at low, intermediate, and high K+, respectively), corresponding to ΔΨm much more negative than EK+ and, thus, thermodynamic shifts (active to passive influx) at low and intermediate K+ (Table II; compare with Table I).
Figure 4.
The effects of various pharmacological and nutritional treatments on K+ influx stimulated due to NH4+ withdrawal in intact roots of barley seedlings grown in full nutrient medium at low (A), intermediate (B), and high (C) [K+]ext and 10 mm [NH4+]ext. Fluxes are indicated on a root fresh weight basis. Asterisks denote different levels of significance between –NH4+ and treatment pairs (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett’s multiple comparison post hoc test). Asterisks in parentheses denote levels of significance between control and –NH4+ pairs (Student’s t test). Each treatment represents the mean of four to 69 replicates. Error bars indicate se.
Table II. Electrophysiological responses of barley and Arabidopsis root cells to sudden NH4+ withdrawal with or without Ca(NO3)2.
One-week-old barley seedlings and 5-week-old Arabidopsis plants were grown on full nutrient medium supplemented with high NH4+ (2 mm in Arabidopsis, 10 mm in barley) and 0.0225, 0.1125, or 5 mm K+. ΔΨm measurements were taken from root epidermal and cortical cells 2 to 3 cm from the root tip, and the AWE ± Ca(NO3)2 (5 mm in barley) was measured. Letters denote significantly different means (P < 0.05; one-way ANOVA with Bonferroni post hoc test), and asterisks denote different levels of significance between control and treatment pairs (**0.001 < P < 0.01; Student’s t test); ns, not significant. Error values indicate se of four replicates. n.d., Not determined.
| Species | Genotype | [K+]ext | ΔΨm |
||
|---|---|---|---|---|---|
| Control | AWE | AWE ± Ca(NO3)2 | |||
| mm | mV | ||||
| Barley | Metcalfe (wild type) | 0.0225 | −143.6 ± 2.7a | −202.6 ± 21.7b | −251.4 ± 13.0c |
| 0.1125 | −136.2 ± 3.0a | −189.3 ± 8.9b | −208.5 ± 10.0b | ||
| 5 | −133.2 ± 2.2a | −164.6 ± 5.1b | −149.0 ± 8.0b | ||
| Arabidopsis | Col-0 | 0.0225 | −165.0 ± 13.7 | −231.0 ± 8.1** | n.d. |
| WS | −137.6 ± 7.2 | −172.9 ± 21.5ns | n.d. | ||
| atakt1 | −139.2 ± 3.5 | −192.4 ± 8.3** | n.d. | ||
| athak5 | −106.1 ± 8.6 | −156.6 ± 22.2ns | n.d. | ||
| athak5 atakt1 | −258.0 ± 22.9 | −265.3 ± 20.8ns | n.d. | ||
This ammonium withdrawal effect (AWE) in barley was also found in Arabidopsis wild-type lines, resulting in 142% and 175% increases in K+ influx for Col-0 and WS, respectively (Fig. 5, A and B). Interestingly, AWE was also consistently observed in the knockout lines atakt1 (513%), athak5 (167%), and athak5 atakt1 (32% [albeit not statistically significant]; Fig. 5, C–E). As in barley, NH4+ withdrawal also consistently resulted in hyperpolarizations of the plasma membrane of root epidermal and cortical cells in all lines except athak5 atakt1 (albeit not statistically significant in the cases of WS and athak5; Table II).
Figure 5.
The effects of various pharmacological and nutritional treatments on K+ influx stimulated due to NH4+ withdrawal in intact roots of Arabidopsis Col-0 wild type (A), WS wild type (B), atakt1 (C), athak5 (D), and athak5 atakt1 (E) grown in full nutrient medium at low [K+]ext and 2 mm [NH4+]ext. Fluxes are indicated on a root fresh weight basis. Asterisks denote different levels of significance between –NH4+ and treatment pairs (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett’s multiple comparison post hoc test). Asterisks in parentheses denote levels of significance between control and –NH4+ pairs (Student’s t test). Each treatment represents the mean of four to 14 replicates. Error bars indicate se.
Ca2+ Sensitivity of the AWE Depends on [K+]ext and Genotype
The results of a selective pharmacological profiling of AWE in barley can be seen in Figure 4. Under the three levels of [K+]ext, AWE displayed similar inhibitory responses to TEA+, Ba2+, and Cs+ (i.e. TEA+ < Ba2+ < Cs+). Interestingly, AWE also displayed significant (P < 0.01) inhibition by 5 mm Ca2+ (regardless of the counter ion) at low and intermediate K+ (Fig. 4, A and B) but not at high K+ (Fig. 4C). To the contrary, AWE at high K+ was stimulated by Cl− and NO3− (supplied as Ca2+ salts), with the greatest stimulation observed with NO3− [36 µmol g (root fresh weight)−1 h−1]. AWE showed no response to the NSCC inhibitors DEPC, Gd3+, and La3+ at any [K+]ext (Fig. 4).
The differential response of AWE to Ca(NO3)2 under varying [K+]ext (i.e. inhibition at low and intermediate K+, stimulation at high K+) was also explored electrophysiologically. As shown in Table II, switching from (NH4)2SO4 to equimolar Ca(NO3)2 resulted in further hyperpolarizations of the plasma membrane of root epidermal and cortical cells at low and intermediate K+ compared with NH4+ withdrawal alone. By contrast, at high K+, this resulted in less hyperpolarization compared with NH4+ withdrawal alone.
Similar to barley (at low K+), AWE in Arabidopsis wild-type lines showed comparable responses to TEA+, Ba2+, Cs+, and Ca2+ (Fig. 5, A and B). This was also observed in athak5 (Fig. 5D). As expected, AWE in atakt1 and athak5 atakt1 no longer displayed sensitivity to the channel inhibitors TEA+ and Ba2+ but remained significantly Cs+ sensitive (Fig. 5, C and E). Surprisingly, however, the Ca2+ sensitivity of AWE was lost in atakt1 and athak5 atakt1 (Fig. 5, C and E).
AWE over 24 h Reveals Peaks in K+ Influx and Leads to Tissue K+ Accumulation
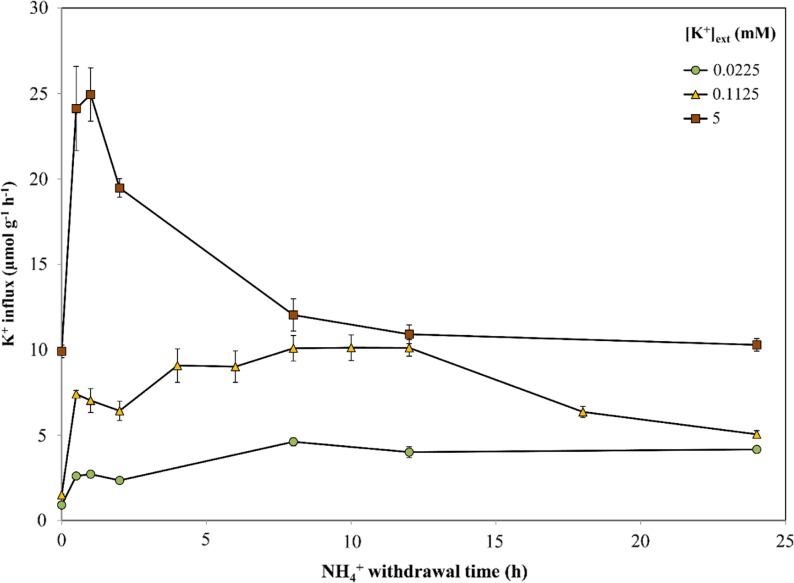
Figure 6 illustrates the sustainability of AWE (i.e. elevated K+ influx after ammonium withdrawal) and its magnitude over 24 h at all three K+ levels in barley. At low K+, AWE was not only sustained over 24 h but continued to rise, reaching 4.2 µmol g (root fresh weight)−1 h−1 (a 4.6-fold increase from control). At intermediate K+, AWE plateaued between 8 and 12 h, reaching 10 µmol g (root fresh weight)−1 h−1 (a 6.8-fold increase from control), before dropping to approximately 5 µmol g (root fresh weight)−1 h−1 by 24 h. At high K+, NH4+ withdrawal peaked at 25 µmol g (root fresh weight)−1 h−1 by 1 h before leveling off at approximately 10 µmol g (root fresh weight)−1 h−1 by the end of the 24-h period.
Figure 6.
Responses of K+ influx to the duration of NH4+ withdrawal in roots of intact barley seedlings grown under full nutrient medium, various [K+]ext levels, and 10 mm [NH4+]ext. Fluxes are indicated on a root fresh weight basis. Each data point represents the mean of four replicates. Error bars indicate se. [See online article for color version of this figure.]
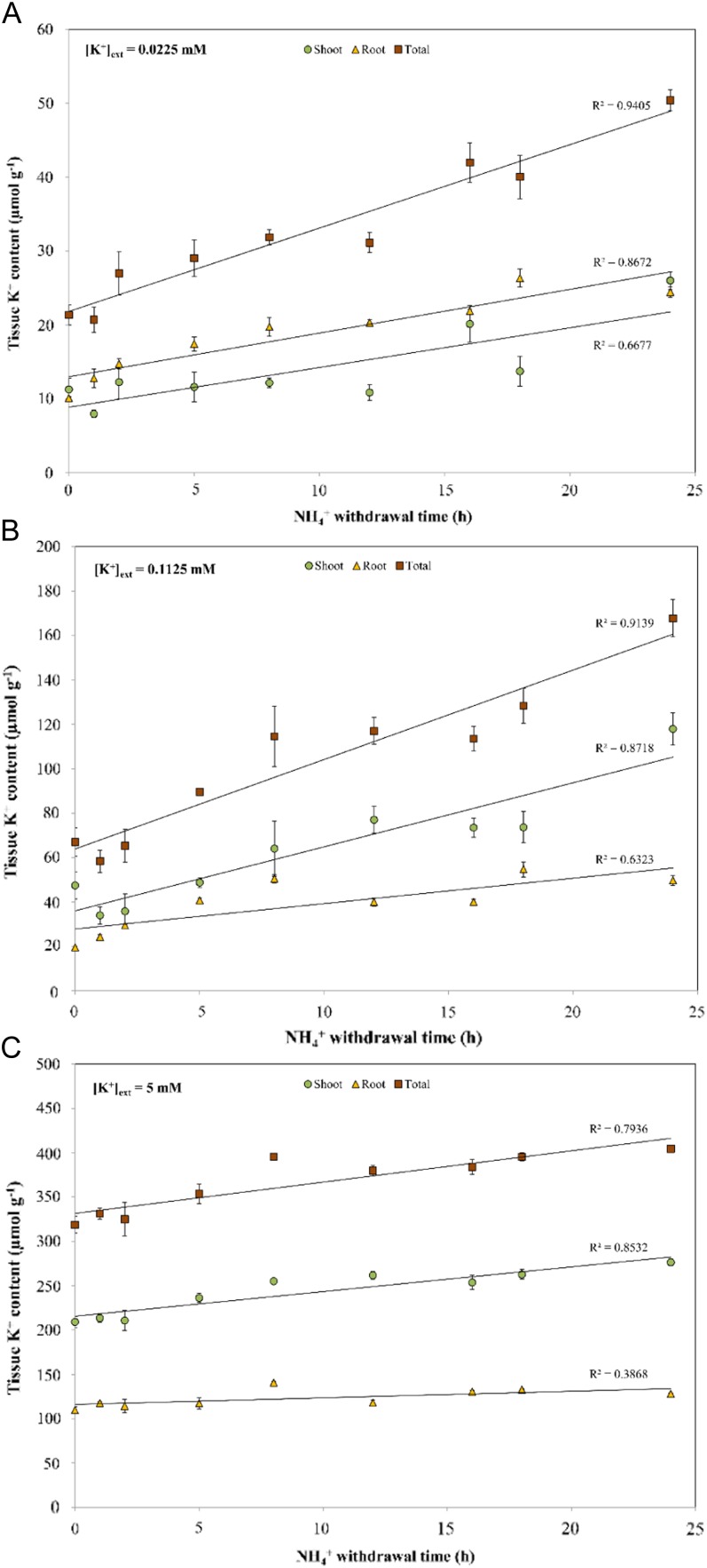
Despite variations in the sustainability of AWE (Fig. 6), NH4+ withdrawal consistently resulted in increased tissue (shoot, root, and total) K+ accumulation over 24 h at all K+ levels tested. By the end of 24 h, NH4+ withdrawal resulted in 136%, 150%, and 27% increases in total tissue K+ levels under low-, intermediate-, and high-K+ conditions, respectively (Fig. 7). Interestingly, NH4+ withdrawal over 24 h also resulted in increased tissue accumulation of Na+ (Supplemental Fig. S1).
Figure 7.
Responses of tissue K+ content to the duration of NH4+ withdrawal in barley seedlings grown under full nutrient medium at low (A), intermediate (B), and high (C) [K+]ext and 10 mm [NH4+]ext. Content measurements are indicated on a root fresh weight basis. Each data point represents the mean of four replicates. Error bars indicate se. [See online article for color version of this figure.]
DISCUSSION
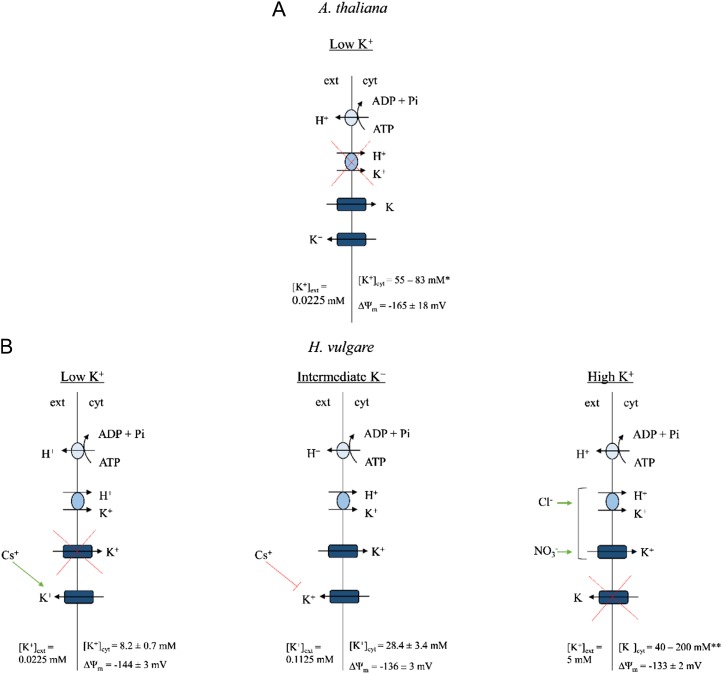
Although the genetic identities of K+ channels and high-affinity transporters, and their contributions to high- and low-affinity K+ uptake, have been examined extensively in Arabidopsis, few studies have placed these fundamental discoveries into the context of agriculturally important species such as barley. In this work, both barley and Arabidopsis were grown at high external ammonium concentration ([NH4+]ext of 10 and 2 mm, respectively; the difference is a reflection of each species’ unique sensitivity to NH4+; Britto and Kronzucker, 2002; ten Hoopen et al., 2010) to inhibit high-affinity transporters and thereby isolate K+ channel functioning (Hirsch et al., 1998; Spalding et al., 1999; Santa-María et al., 2000). We should also note that each species was grown for different lengths of time in different media (see “Materials and Methods”), reflecting their different developmental programs and nutritional preferences. Although differences in growth regime and developmental stage are important to consider, both species displayed severely reduced high-affinity K+ uptake due to NH4+ (see below), and this set the stage for critical evaluations and comparisons of K+ transporter functioning. At the lowest [K+]ext tested (0.0225 mm), fundamental differences in the apparent mechanisms of steady-state K+ uptake were observed between the two species (Fig. 8). In both the wild type and athak5 mutants of Arabidopsis, K+ influx was blocked by the classic channel inhibitors TEA+ and Ba2+ (Fig. 2, A, B, and D), indicating the involvement of the Shaker-like K+ channel AtAKT1 at very low [K+]ext in this species (and confirming the results of Hirsch et al. [1998] and Spalding et al. [1999]). Moreover, the AtAKT1 knockout lines atakt1 and athak5 atakt1 showed significantly lower (59% and 78%) influx than their respective wild types. By contrast, in barley, neither TEA+ nor Ba2+ affected K+ influx at this [K+]ext (Fig. 1A), indicating that it does not appear to involve (TEA+- or Ba2+-sensitive) channels; this is consistent with our thermodynamic (Nernstian) analysis based on compartmental and electrophysiological data, which predicted influx to be an active process (Table I). Consistent with this analysis, influx in barley was significantly suppressed by the K+ transporter inhibitor Cs+, the metabolic inhibitors VO43−, DES, CN− + SHAM, and CCCP, and pH 9.2 (which collapses the electrochemical potential difference for protons). Thus, it appears that steady-state potassium influx at low K+ and high NH4+, while reduced, is mediated by high-affinity transporters in this species, most likely including HvHAK1 (Santa-María et al., 1997). We should note, however, that pH 9.2 also inhibited steady-state influx in athak5 (Fig. 2D), indicating the possibility of alkalinity-induced AtAKT1 inhibition (Fuchs et al., 2005).
Figure 8.
Schematic overview of K+ uptake in plant roots under steady-state conditions (i.e. in the presence of high [millimolar] [NH4+]ext). Under low (0.0225 mm) [K+]ext, K+ uptake is predominantly mediated by K+ channels (AtAKT1) in Arabidopsis (Col-0 wild type; A), whereas in barley (B), K+ channels do not operate and uptake is likely mediated by high-affinity transporters (HvHAK1), albeit at a residual capacity due to NH4+-induced inhibition. Above intermediate K+ levels ([K+]ext = 0.1125 mm), K+ channels do operate in barley, with further Cl−- and NO3−-induced stimulations of K+ uptake observed at high K+ ([K+]ext = 5 mm). In barley, K+ efflux is likely channel mediated at low and intermediate K+, although with varying sensitivities to Cs+, whereas K+ efflux is likely inoperative at high K+. K+ efflux in Arabidopsis is also likely channel mediated (Maathuis and Sanders, 1993). [K+]cyt and resting ΔΨm values, when measured, are also listed. Asterisks refer to references as follows: *see Maathuis and Sanders (1993) and Halperin and Lynch (2003); **see Leigh and Wyn-Jones (1984), Walker et al. (1996), and Kronzucker et al. (2006). [See online article for color version of this figure.]
Interestingly, the Cs+ sensitivity of K+ influx in athak5 atakt1 double mutants at low K+ suggests that a genetically unidentified uptake system is operative, albeit at a relatively minor capacity. This is in contrast to other reports suggesting that “unknown systems” only operate at high [K+]ext (more than 0.5 mm; Pyo et al., 2010; Rubio et al., 2010). Thus, at least two distinct unknown systems appear to operate in Arabidopsis; moreover, the pharmacological profile of athak5 atakt1 in our study did not match that of others, particularly with respect to Ca2+ and Ba2+ sensitivity (Caballero et al., 2012). This raises the question of whether similar mechanisms are at play in barley at low-K+, high-NH4+ conditions and provides avenues for future research.
At [K+]ext above 0.1 mm, by contrast, K+ channels do appear to participate in K+ uptake in barley, since, at the intermediate [K+]ext tested (0.1125 mm), we observed significant inhibition of K+ influx by TEA+ and Ba2+ (Fig. 1C). Although thermodynamic analyses suggested K+ uptake at intermediate K+ to be an active process (Table I), this apparent contradiction most likely reflects some methodological discrepancies. Pharmacological testing of K+ influx and compartmental analysis of 42K+ efflux take the entire root into account and thus reflect an average of different cell types along the root axis. On the other hand, electrophysiological measurements of ΔΨm are, by nature, single-cell measurements and thus do not necessarily represent the whole root. In fact, membrane polarization is known to follow the longitudinal axis of the root, with the most polarized cells located near the root tip (Hirsch et al., 1998). As our ΔΨm measurements were made 2 to 3 cm from the root tip (see “Materials and Methods”), it is likely that we measured from cells not polarized enough to conduct passive K+ uptake. Thus, at intermediate [K+]ext, what we observe may be a mixed population of cells: some conducting channel-mediated K+ uptake, others engaging high-affinity transporters. At 5 mm [K+]ext, where channel-mediated K+ uptake is proposed to dominate and high-affinity transporters are assumed to be largely irrelevant (Maathuis and Sanders, 1996; Rubio et al., 2010), we did indeed find TEA+ sensitivity; however, oddly, we also observed stimulations in steady-state K+ influx with BaCl2 application (Fig. 1E). Similarly, applications of CaCl2 and Ca(NO3)2 also stimulated K+ influx (Fig. 1F), while they produced no effect at low and intermediate [K+]ext (Fig. 1, B and D, respectively). This may be the result of anion effects specific to the LATS, as demonstrated in earlier reports (Epstein et al., 1963; Kochian et al., 1985). Kochian et al. (1985) showed that Cl− stimulated low-affinity K+ uptake (possibly via coupled transport), unlike SO42−, H2PO4−, and (in contrast to this study) NO3−. The apparent contradiction with respect to NO3− could be attributed to a variety of differences in experimental procedures, including species, growth medium, and influx protocol. Surprisingly, further elucidation of the mechanism underlying this effect has not occurred since then; however, this falls out of the scope of our study. What is important to note is that three distinct uptake scenarios appear to occur in barley roots grown under high NH4+ and varying [K+]ext: at low K+, an active process dominates, attributable to residual HvHAK1 activity and/or unknown systems; at intermediate K+, a mixed population of channel (HvAKT1)- and high-affinity transporter (HvHAK1)-mediated K+ uptake occurs; and at high K+, channel (HvAKT1)-mediated K+ uptake appears to dominate, but with unique Cl−- and NO3−-induced stimulations (Fig. 8).
Consistent across all [K+]ext tested in barley is the significant reduction of K+ influx by metabolic inhibitors (Fig. 1, A, C, and E). Typically, the HATS is reported to be more sensitive to metabolic inhibitors compared with the LATS (Malhotra and Glass, 1995); however, the vast majority of metabolic inhibitors was most effective in suppressing influx at the intermediate K+ level and not at the lowest level. Interestingly, influx at intermediate K+ was most strongly inhibited by Cs+ and was the most stimulated in response to NH4+ withdrawal. Perhaps this is indicative of the mixed population of channels and high-affinity transporters operating under these conditions and reflects a highly dynamic transport capacity. Also evident is the lack of NSCC involvement at all [K+]ext tested, as Ca2+, Gd3+, La3+, Glu, and DEPC had no effect on K+ influx (Fig. 1, B, D, and F; Essah et al., 2003). It is rather surprising that, if NSCCs catalyze high rates of cation fluxes, as has been proposed (White and Davenport, 2002; Essah et al., 2003; Kronzucker and Britto, 2011, and refs. therein), we were unable to observe their activity physiologically. However, the presence of significant concentrations of Ca2+ (1–5 mm), reflective of common soil conditions (Garciadeblás et al., 2003; Zhang et al., 2010), may have reduced putative K+ currents through NSCCs considerably (White and Davenport, 2002). Similarly, Na+-K+ cotransport does not appear to be operative, as no effect of 10 mm NaCl on K+ influx was observed (data not shown), ruling out the involvement of HKT/TRK-type transporters (Rubio et al., 1995). It is worth highlighting here that pharmacological profiling, like any experimental method, is not without its caveats. Although a traditionally useful tool for gaining insight into the structure and function of membrane transporters, particularly in planta, the lack of specificity of several blockers/chemical treatments (White and Broadley, 2000; Coskun et al., 2012), as well as the need to employ relatively high concentrations at times, can potentially have secondary (“pleiotropic”) effects. This by no means invalidates the use of pharmacology but simply speaks to the importance of a multipronged approach in such studies.
As with influx, efflux analysis in barley demonstrates that distinct mechanisms are at play at each of the three [K+]ext tested (Fig. 8). At low K+, where influx was determined to be solely active, we see the perplexing result of efflux stimulation upon Cs+ application (Fig. 3A). Testing of the involvement of Cs+-induced depolarizations in this phenomenon (Nocito et al., 2002) yielded negative results (data not shown). We should note that instances of cellular K+/Cs+ exchange have been documented in the animal literature (Beaugé et al., 1973; Guerin and Wallon, 1979); however, to our knowledge, no precedence exists in the plant literature. Moreover, this does not explain its isolated incidence at low [K+]ext; thus, the mechanism remains unknown. In stark contrast with these observations, both Cs+ and TEA+ significantly inhibited K+ efflux at intermediate K+ levels (Fig. 3B). This effect has also been observed at a similar [K+]ext (0.1 mm) under an NO3− background (Coskun et al., 2010) and is consistent with other reports of the Cs+ and TEA+ sensitivities of channel-mediated ion fluxes (Krol and Trebacz, 2000; White and Broadley, 2000). At the highest [K+]ext tested (5 mm), a third scenario emerged, one in which physiological efflux was ruled out, as was previously shown under low-affinity conditions with NO3−-grown barley (Coskun et al., 2010). While an examination of the mechanisms of K+ efflux was beyond the scope of this study, physiological-efflux data at low and intermediate [K+]ext lend themselves to compartmental analysis (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995). In these cases, we have strong evidence that efflux at low and intermediate K+ is occurring from the cytosol and across the plasma membrane, allowing us to confidently estimate [K+]cyt (Table I). The variability of these results also confirms some of the earlier work on barley by Kronzucker et al. (2003), which investigated the heterostasis of [K+]cyt, in particular its suppression on high-NH4+ medium. The lack of physiological efflux at high K+ prevents us from making such estimates. Future studies will examine the differences in K+ efflux between NO3−- and NH4+-grown plants and may help provide insight into the mechanisms of NH4+ toxicity.
Withdrawal of NH4+ resulted in immediate and dramatic stimulations of K+ influx, as high as 4.5-fold in barley and 6-fold in Arabidopsis. We should note that there have been a few reports of K+ uptake stimulation upon NH4+ withdrawal in both barley (Santa-María et al., 2000) and Arabidopsis (Rubio et al., 2010); however, little attention was directed to this phenomenon. In addition, the magnitude of unidirectional fluxes measured was minuscule in one study [approximately 1 µmol g (root fresh weight)−1 h−1; Santa-María et al., 2000] and not measured in the other (Rubio et al., 2010). By contrast, some of the fluxes recorded in this study at high K+ in barley [25–36 µmol g (root fresh weight)−1 h−1] are among the highest bona fide transmembrane fluxes of K+ reported. Although some published rates of unidirectional Na+ and NH4+ fluxes under toxicity conditions are larger [e.g. 60–600 µmol g (root fresh weight)−1 h−1; Lazof and Cheeseman, 1986; Britto et al., 2001; Essah et al., 2003; Horie et al., 2007; Szczerba et al., 2008], these values have come into question, particularly with respect to the improbably high energy costs of such fluxes (Britto and Kronzucker, 2009). In this study, these powerful stimulations of K+ influx might be explained by the significant hyperpolarizations observed upon NH4+ withdrawal (Table II). At low and intermediate K+ levels, these electrical changes translate into thermodynamic shifts from active to passive K+ uptake in barley, the latter driven by a powerful downhill gradient; this was confirmed by use of the channel-blocking agents TEA+, Ba2+, and Cs+ (Figs. 4 and 5). Interestingly, AWE was observed in both atakt1 and athak5 knockout lines (Fig. 5, C and D) and was substantial in both cases (513% and 167% of control values, respectively), suggesting a sizable participation by both transporters. This result demonstrates that AtHAK5 can operate under thermodynamically passive conditions, lending support to the idea that some HAK/KUP/KT transporters have a dual-affinity nature (Fu and Luan, 1998; Kim et al., 1998). However, it is not clear whether it would function under these conditions as an H+/K+ symporter (Gierth and Mäser, 2007) or engage a channel-like mechanism (Fu and Luan, 1998). Further experimentation is required to address this possibility. The fact that AWE was minor in athak5 atakt1 double mutants suggests that AtAKT1 and AtHAK5 are the major contributors, and any contribution from unknown systems is small (Fig. 5E), as it is under steady-state conditions (Fig. 2E). Lastly, the inhibition of K+ efflux upon NH4+ withdrawal at low and intermediate K+ (Fig. 3, A and B, insets) is further evidence for a shift in thermodynamic gradients; under these conditions, it is likely that physiological K+ efflux has shut down entirely.
Pharmacological profiling of AWE across all [K+]ext clearly implicated channel (AKT1) involvement; however, the response of AWE to external Ca2+ revealed some unusual results. At low and intermediate K+, AWE was all but suppressed by Ca2+ in barley (Fig. 4, A and B). This effect was not observed at high K+, however: CaSO4 had no effect, while CaCl2 and Ca(NO3)2 both stimulated AWE, by 26% and 47%, respectively (Fig. 4C). The Ca2+ sensitivity of AWE was also observed in Arabidopsis wild-type lines and athak5 mutants at low K+ (Fig. 5, A, B, and D). Surprisingly, it was not observed in AtAKT1 knockout lines (Fig. 5, C and E), suggesting that the Ca2+ sensitivity of AWE is linked to the AtAKT1 channel. To our knowledge, evidence of AtAKT1 blockage by external Ca2+ is sparse; in one case, though, a weak inhibition of inward-rectifying K+ channels in the plasma membrane of rye roots by high concentrations of Ca2+ was observed (White and Lemtiri-Chlieh, 1995), which provides evidence that K+ channels, such as AtAKT1, may be sensitive to extracellular Ca2+ under some conditions. An alternative hypothesis, that Ca2+ coprovision coincides with an increased Ca2+ influx, resulting in less hyperpolarization and thus a reduced K+ flux, was ruled out in two ways. First, the Ca2+ channel inhibitor verapamil (Lee and Tsien, 1983; compare with White, 1998) was supplied alongside Ca2+ during NH4+ withdrawal, with no effects observed (data not shown). Second, when ΔΨm was measured during Ca(NO3)2 coprovision with NH4+ withdrawal, not only was the hyperpolarization undiminished, but, in fact, even greater hyperpolarization was measured (Table II). Surprisingly, however, at high K+, membrane hyperpolarization was reduced, even though Ca(NO3)2 provision upon NH4+ withdrawal resulted in even greater K+ influx than NH4+ withdrawal alone (Table II). Thus, it appears that AWE and its stimulation or inhibition do not solely depend on membrane polarization but involve other processes, such as channel gating (as with Ca2+ effects) or coupling to anion transport (as in the case of Cl− and NO3− effects). This is further confirmed by the fact that the greatest AWE occurred in barley at intermediate K+ (355% increase from control) while the concomitant hyperpolarizations were no greater than that seen at other K+ levels (Table II). Also, the largest relative AWE in Arabidopsis was seen in atakt1 (513%), but it, too, showed hyperpolarization no greater than in any other line (Table II).
A 24-h time course revealed the upper limits of AWE on K+ influx, both in terms of magnitude and sustainability. At high K+, influx peaked at approximately 25 µmol g (root fresh weight)−1 h−1 by 1 h, in contrast with low and intermediate K+, at which the fluxes continued to rise over 24 and 8 h, respectively (Fig. 6). Nevertheless, all time courses resulted in increased accumulation of tissue K+, demonstrating the nutritional significance of this effect (Fig. 7). Interestingly, at low and intermediate K+, NH4+ withdrawal also resulted in significant tissue accumulation of Na+ (Supplemental Fig. S1), particularly in the roots. This may reflect the ability of Na+ to replace K+ in some of its cellular roles (Subbarao et al., 2003), since these growth conditions (low and intermediate [K+]ext, high [NH4+]ext) are toxic for barley and result in extremely low tissue K+ levels (Fig. 7). Some reports suggest that Na+ and K+ could share similar uptake mechanisms, such as K+ channels (for review, see Kronzucker and Britto, 2011); thus, situations where stimulated channel activity is induced could account for these findings. While out of the scope of this work, it would be interesting to measure Na+ fluxes in such a scenario, especially in the toxic range, to better understand the mechanisms of Na+ transport.
We have demonstrated here that the Arabidopsis model of K+ acquisition is not universally applicable. Although K+ channels appear to be the sole means of K+ acquisition under low-K+, high-NH4+ conditions in Arabidopsis, this is not the case for barley. Our study provides new physiological evidence of three distinct modes of K+ influx and efflux in NH4+-grown barley, operating at 0.0225, 0.1125, and 5 mm [K+]ext. Figure 8 illustrates these key findings. Moreover, we demonstrate that NH4+ withdrawal can reveal a very high capacity and plasticity among K+ transporters. This work provides a framework of characteristics, including nutritional and pharmacological profiles, by which discoveries in molecular genetics, particularly in the emerging field of cereal genomics, can be gauged.
MATERIALS AND METHODS
Plant Culture
Seeds of barley (Hordeum vulgare ‘Metcalfe’) were surface sterilized for 15 min in 1% sodium hypochlorite and germinated under acid-washed sand for 3 d prior to placement in 14-L vessels containing aerated modified Johnson’s nutrient solution. All solutions were composed of 5 mm (NH4)2SO4, 0.5 mm NaH2PO4, 0.25 mm MgSO4, 0.2 mm CaSO4, 25 µm H3BO3, 20 µm FeEDTA, 6.25 µm CaCl2, 2 µm ZnSO4, 0.5 µm MnSO4, 0.5 µm CuSO4, and 0.125 µm Na2MoO4 (pH adjusted to 6.2–6.3, using 1 m NaOH). K+ was supplied at 0.0225 (low), 0.1125 (intermediate), or 5 mm (high) as K2SO4. Solutions were completely exchanged on days 5 (for all K+ conditions) and 6 (for low- and intermediate-K+ conditions) following germination to ensure that plants remained at a nutritional steady state for experimentation on day 7. Plants were grown in a climate-controlled, walk-in growth chamber under fluorescent lights, with an irradiation of 200 µmol photons m−2 s−1 at plant height, for 16 h d−1 (Philips Silhouette High Output F54T5/850HO; Philips Electronics). Daytime temperature was 20°C, nighttime temperature was 15°C, and relative humidity was approximately 70%.
Seeds of Arabidopsis (Arabidopsis thaliana) wild-type ecotypes Col-0 (N1092) and WS (N1601) and transfer DNA insertion lines atakt1-1 (CS3762; WS ecotype), athak5-1 (SALK_014177; Col-0 ecotype), and athak5 atakt1 (Col-0 ecotype; Rubio et al., 2010) were surface sterilized for 5 min with 70% ethanol, followed by 10 min with a 1% sodium hypochlorite-0.05% SDS mixture, and allowed to stratify in a 0.1% agar solution in the dark for 3 d at 4°C, prior to germination on acid-washed sand for 4 d. Seedlings were placed in 14-L vessels containing aerated nutrient solution composed of 5 mm K2SO4, 1 mm Ca(NO3)2, 1 mm NaH2PO4, 0.5 mm MgSO4, 0.25 mm CaSO4, 25 µm H3BO3, 20 µm FeEDTA, 2 µm ZnSO4, 0.5 µm MnCl2, 0.5 µm CuSO4, and 0.5 µm Na2MoO4 (pH 6.0, with 1 m NaOH). Solutions were completely exchanged once per week for 3 weeks. During the final (5th) week of growth, [K+]ext was reduced to 0.0225 mm and Ca(NO3)2 was replaced with an equimolar (1 mm) amount of (NH4)2SO4. Nutrient solutions were completely exchanged every other day during the final week of growth and experimented with on day 35. Such a growth regime was particularly important for the proper growth of atakt1 and athak5 atakt1 mutants, as germination and growth were severely hindered on low-K+, high-NH4+ medium (data not shown; Rubio et al., 2010). Plants were grown in a climate-controlled chamber under fluorescent lights, with an irradiation of 200 µmol photons m−2 s−1 at plant height, for 12 h d−1 (Philips F96T8/TL841/HO/PLUS; Philips Electronics). Daytime temperature was 20°C, nighttime temperature was 15°C, and relative humidity was approximately 70%.
Direct Influx
Unidirectional K+ influx in roots of intact barley and Arabidopsis was measured as described in detail elsewhere (Coskun et al., 2010). In brief, roots of replicate units of three to four plants were preequilibrated for 5 or 10 min (see below) in a solution either identical to the growth solution (control) or in growth solution supplemented with a chemical treatment (see below). Roots were then immersed for 5 min in a solution identical to the preequilibration solution but containing 42K (half-life = 12.36 h), received as 42K2CO3 from the McMaster University Nuclear Reactor. Labeled plants were transferred to nonradioactive growth solution for 5 s to reduce tracer carryover and further desorbed of radioactivity from the extracellular space for 5 min in fresh growth solution. Based on the half-times of cytosolic exchange and efflux-influx ratios reported in Table I, K+ influx was underestimated by no more than 5% due to efflux during desorption (Britto and Kronzucker, 2001). Immediately following desorption, roots were detached from shoots and spun in a low-speed centrifuge for 30 s to remove surface solution prior to weighing. Radioactivity in root tissues was counted (shoot counts were not detectable) and corrected for isotopic decay using one of two γ counters (Perkin-Elmer Wallac 1480 Wizard 3′ and Packard Instrument Quantum Cobra Series II, model 5003). Throughout, K+ influx is expressed in terms of µmol g (root fresh weight)−1 h−1.
Compartmental Analysis by Tracer Efflux
42K+ efflux from roots on intact barley was examined as described previously (Coskun et al., 2010) and based on the method of compartmental analysis (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995). In brief, roots of replicate units of five to 10 seedlings were immersed for 1 h in aerated nutrient medium identical to growth conditions but containing 42K (see above). Labeled seedlings were secured in glass efflux funnels, and roots were eluted of radioactivity with successive 20-mL aliquots of aerated, nonradioactive growth solution. The desorption series was timed as follows, from first to final eluate: 15 s (four times), 20 s (three times), 30 s (two times), 40 s (once), 50 s (once), 1 min (five times), 1.25 min (once), 1.5 min (once), 1.75 min (once), 2 min (13 times), for a total of 40 min of elution. The first 19 eluates (14 min into the elution series) were identical to the growth solution, and the final 13 eluates contained either growth solution (control) or a chemical treatment (see below).
Immediately following elution, plant organs were harvested as described in the preceding section, and radioactivity from eluates, roots, and shoots was counted and corrected for isotopic decay, as described above. For comparison charts of 42K efflux, the specific activities of all replicates were normalized to the arbitrary value of 2 × 105 cpm µmol−1. Throughout, 42K+ efflux is expressed in terms of cpm released g (root fresh weight)−1 min−1.
For the results in Table I, tracer efflux and retention data were used to estimate unidirectional and net fluxes, and cytosolic half-times of exchange and pool sizes, according to the methods of compartmental analysis (Kronzucker et al., 1995). In brief, linear regression of the function ln Φco(t)* = ln Φco(i)* − kt [where Φco(t)* is tracer efflux at elution time t, Φco(i)* is initial tracer efflux, and k is the rate constant describing the exponential decline in radioactive tracer efflux, found from the slope of the tracer release rate; Fig. 3] was used to resolve the kinetics of the slowest exchanging (cytosolic) phase in these experiments (Kronzucker et al., 1995). The cytosolic origin of the slowest exchanging phase was confirmed for the low- and intermediate-K+ conditions, and ruled out for the high-K+ condition, by means of pharmacological testing (Fig. 3; Coskun et al., 2010); thus, compartmental analysis could not be performed at high K+. Based on literature precedents demonstrating the relatively long half-times of vacuolar K+ exchange compared with that of the cytosol (hours versus minutes; Poole, 1971; Walker and Pitman, 1976; Behl and Jeschke, 1982; Memon et al., 1985; Hajibagheri et al., 1988; Siddiqi et al., 1991; White et al., 1991), we could assume that the vast majority of intracellular tracer released was from the cytosolic pool after only 1 h of loading. Chemical efflux, Φco, was determined from Φco(i)*, divided by the specific activity of the cytosol (SAcyt) at the end of the labeling period. SAcyt was estimated by using external specific activity (SAext), labeling time t, and the rate constant k, which are related in the exponential rise function SAcyt = SAext (1 – e–kt) (Walker and Pitman, 1976). Net flux, Φnet, was found using total plant (root and shoot) 42K retention after desorption, and influx, Φoc, was calculated from the sum of Φco and Φnet. Note that shoot accumulation of radiotracer is routinely dealt with in compartmental analysis of intact seedlings (Jeschke and Jambor, 1981; Siddiqi et al., 1991); thus, the parameters listed in Table I can be accurately estimated. [K+]cyt was determined using the flux turnover equation, [K+]cyt = Ω Φoc k−1, where Ω is a proportionality constant correcting for cytosolic volume being approximately 5% of total tissue (Lee and Clarkson, 1986; Siddiqi et al., 1991).
Pharmacological/Chemical Treatments
For K+ influx experiments, the following agents were used to test for the involvement of different uptake mechanisms: 10 mm TEACl, 5 mm BaCl2, 10 mm CsCl, 10 mm Na3VO4, 100 µm DNP (1% ethanol), 50 µm DES (1% ethanol), 1 mm NaCN + SHAM, 10 µm CCCP (1% ethanol), pH 9.2 (adjusted with 1 m NaOH), 5 mm CaSO4, 5 mm CaCl2 (1 mm in the case of Arabidopsis; see “Results”), 5 mm Ca(NO3)2 (1 mm for Arabidopsis), 50 µm GdCl3, 50 µm LaCl3, 10 mm sodium Glu, and 100 µm DEPC. All treatments involved a 5-min pretreatment, except for DNP, DES, CN− + SHAM, CCCP, and DEPC, which took 10 min. It should be noted that no effect of 1% ethanol (a vehicle for DNP, DES, and CCCP) on K+ influx was found (data not shown). Inhibitors were added in the presence of a complete growth medium, including NH4+, except when it was withdrawn for AWE experiments (see “Results”).
For K+ efflux experiments, eluates were supplemented with 10 mm CsCl, 10 mm TEACl, 5 mm BaCl2, or 5 mm CaSO4. Other treatments involved growth solution without (NH4)2SO4. A small subset of experiments also involved the loading and elution solutions altered to pH 9.2 (with 1 m NaOH).
Electrophysiology
Measurements of ΔΨm from epidermal and cortical root cells of barley and Arabidopsis (aged 7–8 d and 35–37 d, respectively) were conducted as described previously (Schulze et al., 2012). In brief, roots were immersed in a Plexiglas chamber filled with nutrient solution and installed onto the stage of an inverted light microscope (Leica DME; Leica Microsystems). Microelectrodes (tip diameter < 1 µm), made from borosilicate glass (i.d. = 0.75 mm, o.d. = 1.00 mm; World Precision Instruments) and produced using an electrode puller (P-30; Sutter Instrument), were filled with 3 m KCl solution (pH 2). Both impaling and reference electrodes were prepared in this manner. ΔΨm measurements were made in a region 2 to 3 cm from the root tip, with the use of an electrometer (Duo 773; World Precision Instruments), and recorded on an oscilloscope (TDS2002B; Tektronix). Once steady readings were obtained, treatment solution was perfused through Tygon tubing via a peristaltic pump at a rate of approximately 7.5 mL min−1. Treatments included growth solution with (NH4)2SO4 excluded with or without an equimolar amount of Ca(NO3)2.
Table I displays the results of a thermodynamic (Nernstian) analysis. EK+ was estimated using the Nernst equation:
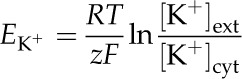 |
where R is the universal gas constant (8.314 J K−1 mol−1), T is ambient temperature (293.15 K), z is the ionic charge of the species (+1 for K+), F is the Faraday constant (96,485 C mol−1), and [K+]ext and [K+]cyt are as defined previously (for activity coefficients, see “Results”).
Tissue Content
Tissue K+ and Na+ contents for barley (aged 7 d) were determined by methods described previously for steady-state and non-steady-state conditions (Britto et al., 2010; Coskun et al., 2012). In brief, replicate units of three to five seedlings had their roots incubated for 5 min in 10 mm CaSO4 to release extracellular K+/Na+ (steady state) or were treated in growth solution with NH4+ removed for various time points spanning 24 h (see “Results”; non steady state) prior to CaSO4 desorption. From there, plant organs were harvested as above (see “Direct Influx”), oven dried for 3 d at 80°C to 90°C, and pulverized and digested in 30% HNO3 for an additional 3 d. K+ and Na+ concentrations of tissue digests were measured using a dual-channel flame photometer (model 2655-10; Cole-Parmer Instrument). Tissue ion concentration is expressed in µmol g (root fresh weight)−1.
Statistics
For influx experiments, each treatment was replicated a minimum of four times and tested for statistical significance with the use of a one-way ANOVA with Dunnett’s multiple comparison post hoc test (with steady-state or NH4+ withdrawal-induced flux as a control; see “Results”). For efflux analyses, each treatment was replicated a minimum of three times. Significance testing of each treatment was conducted by matching each pair of data points for a given elution time between treatment and control traces and running Student’s t test (Coskun et al., 2010). The percentages of paired points that were found to be significantly different (P < 0.05) are shown in Figure 3 (Coskun et al., 2010).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Response of tissue Na+ content to duration of NH4+ withdrawal in barley seedlings grown under full nutrient medium at low, intermediate, and high [K+]ext and 10 mm [NH4+]ext.
Acknowledgments
We thank R. Pasuta and M. Butler (McMaster University Nuclear Reactor) for the provision of 42K, F. Rubio (Centro de Edafología y Biología Aplicada del Segura-Consejo Superior de Investigaciones Científicas) for his kind donation of athak5 atakt1 seed material, and A. Walsh (University of Toronto) for his assistance with experimentation.
Glossary
- K+
potassium
- Na+
sodium
- NH4+
ammonium
- [NH4+]ext
external ammonium concentration
- HATS
high-affinity transport system
- [K+]ext
external potassium concentration
- LATS
low-affinity transport system
- NSCC
nonselective cation channel
- VO43−
vanadate
- DNP
2,4-dinitrophenol
- DES
diethylstilbestrol
- CN−
cyanide
- SHAM
salicylhydroxamic acid
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- DEPC
diethylpyrocarbonate
- [K+]cyt
cytosolic potassium concentration
- EK+
equilibrium potentials for K+
- ΔΨm
membrane potential
- γcyt
cytosolic K+ activity coefficient
- Col-0
Columbia
- WS
Wassilewskija
- AWE
ammonium withdrawal effect
- H+
proton
- TEA+
tetraethyl ammonium
References
- Ahn SJ, Shin R, Schachtman DP. (2004) Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 134: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemán F, Nieves-Cordones M, Martínez V, Rubio F. (2011) Root K(+) acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol 52: 1603–1612 [DOI] [PubMed] [Google Scholar]
- Amrutha RN, Sekhar PN, Varshney RK, Kishor PBK. (2007) Genome-wide analysis and identification of genes related to potassium transporter families in rice (Oryza sativa L.). Plant Sci 172: 708–721 [Google Scholar]
- Ashley MK, Grant M, Grabov A. (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57: 425–436 [DOI] [PubMed] [Google Scholar]
- Beaugé LA, Medici A, Sjodin RA. (1973) The influence of external caesium ions on potassium efflux in frog skeletal muscle. J Physiol 228: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl R, Jeschke WD. (1982) Potassium fluxes in excised barley roots. J Exp Bot 33: 584–600 [Google Scholar]
- Bertl A, Reid JD, Sentenac H, Slayman CL. (1997) Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot (Spec No) 48: 405–413 [DOI] [PubMed] [Google Scholar]
- Brenchley R, Spannagl M, Pfeifer M, Barker GLA, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, et al. (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491: 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ. (2010) 42K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytol 186: 373–384 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2001) Can unidirectional influx be measured in higher plants? A mathematical approach using parameters from efflux analysis. New Phytol 150: 37–47 [Google Scholar]
- Britto DT, Kronzucker HJ. (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159: 567–584 [Google Scholar]
- Britto DT, Kronzucker HJ. (2008) Cellular mechanisms of potassium transport in plants. Physiol Plant 133: 637–650 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2009) Ussing’s conundrum and the search for transport mechanisms in plants. New Phytol 183: 243–246 [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ. (2001) Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98: 4255–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero F, Botella MA, Rubio L, Fernández JA, Martínez V, Rubio F. (2012) A Ca(2+)-sensitive system mediates low-affinity K(+) uptake in the absence of AKT1 in Arabidopsis plants. Plant Cell Physiol 53: 2047–2059 [DOI] [PubMed] [Google Scholar]
- Cheeseman JM. (2013) The integration of activity in saline environments: problems and perspectives. Funct Plant Biol (in press) [DOI] [PubMed] [Google Scholar]
- Chérel I. (2004) Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot 55: 337–351 [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Jean YK, Schulze LM, Becker A, Kronzucker HJ. (2012) Silver ions disrupt K+ homeostasis and cellular integrity in intact barley (Hordeum vulgare L.) roots. J Exp Bot 63: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Kronzucker HJ. (2010) Regulation and mechanism of potassium release from barley roots: an in planta 42K+ analysis. New Phytol 188: 1028–1038 [DOI] [PubMed] [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh RA. (2003) Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot 54: 657–661 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53: 67–107 [DOI] [PubMed] [Google Scholar]
- Epstein E, Rains DW, Elzam OE. (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA 49: 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M. (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HH, Luan S. (1998) AtKuP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell 10: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs I, Stölzle S, Ivashikina N, Hedrich R. (2005) Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 221: 212–221 [DOI] [PubMed] [Google Scholar]
- Fulgenzi FR, Peralta ML, Mangano S, Danna CH, Vallejo AJ, Puigdomenech P, Santa-María GE. (2008) The ionic environment controls the contribution of the barley HvHAK1 transporter to potassium acquisition. Plant Physiol 147: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, Anschuetz U, Dreyer I, Kudla J, Hedrich R. (2009) Heteromeric AtKC1·AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem 284: 21288–21295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Mäser P. (2007) Potassium transporters in plants: involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581: 2348–2356 [DOI] [PubMed] [Google Scholar]
- Gierth M, Mäser P, Schroeder JI. (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM. (1976) Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol 58: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan TH, Presting G, Wang RL, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100 [DOI] [PubMed] [Google Scholar]
- Grefen C, Chen Z, Honsbein A, Donald N, Hills A, Blatt MR. (2010) A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 22: 3076–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M, Wallon G. (1979) The reversible replacement of internal potassium by caesium in isolated turtle heart. J Physiol 293: 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajibagheri MA, Flowers TJ, Collins JC, Yeo AR. (1988) A comparison of the methods of x-ray-microanalysis, compartmental analysis and longitudinal ion profiles to estimate cytoplasmic ion concentrations in 2 maize varieties. J Exp Bot 39: 279–290 [Google Scholar]
- Halperin SJ, Lynch JP. (2003) Effects of salinity on cytosolic Na+ and K+ in root hairs of Arabidopsis thaliana: in vivo measurements using the fluorescent dyes SBFI and PBFI. J Exp Bot 54: 2035–2043 [DOI] [PubMed] [Google Scholar]
- Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ. (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136: 3824–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001) Ion Channels of Excitable Membranes, Ed 3. Sinauer Associates, Sunderland, MA [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Honsbein A, Sokolovski S, Grefen C, Campanoni P, Pratelli R, Paneque M, Chen ZH, Johansson I, Blatt MR. (2009) A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21: 2859–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI. (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26: 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanguenin L, Alcon C, Duby G, Boeglin M, Chérel I, Gaillard I, Zimmermann S, Sentenac H, Véry AA. (2011) AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J 67: 570–582 [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Jambor W. (1981) Determination of unidirectional sodium fluxes in roots of intact sunflower seedlings. J Exp Bot 32: 1257–1272 [Google Scholar]
- Kielland J. (1937) Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc 59: 1675–1678 [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. (1998) AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10: 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. (1982) Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiol 70: 1723–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. (1993) Can K+ channels do it all? Plant Cell 5: 720–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Xin-Zhi J, Lucas WJ. (1985) Potassium transport in corn roots. IV. Characterization of the linear component. Plant Physiol 79: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E, Trebacz K. (2000) Ways of ion channel gating in plant cells. Ann Bot (Lond) 86: 449–469 [Google Scholar]
- Kronzucker HJ, Britto DT. (2011) Sodium transport in plants: a critical review. New Phytol 189: 54–81 [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. (1995) Analysis of 13NH4+ efflux in spruce roots: a test case for phase identification in compartmental analysis. Plant Physiol 109: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT. (2003) Cytosolic potassium homeostasis revisited: 42K-tracer analysis reveals set-point variations in [K+]. Planta 217: 540–546 [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Moazami-Goudarzi M, Britto DT. (2006) The cytosolic Na+:K+ ratio does not explain salinity-induced growth impairment in barley: a dual-tracer study using 42K+ and 24Na+. Plant Cell Environ 29: 2228–2237 [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Lazof D, Cheeseman JM. (1986) Sodium transport and compartmentation in Spergularia marina: partial characterization of a functional symplasm. Plant Physiol 81: 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Tsien RW. (1983) Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature 302: 790–794 [DOI] [PubMed] [Google Scholar]
- Lee RB, Clarkson DT. (1986) Nitrogen-13 studies of nitrate fluxes in barley roots. 1. Compartmental analysis from measurements of 13N efflux. J Exp Bot 37: 1753–1767 [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci USA 104: 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RA, Wyn-Jones RG. (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97: 1–13 [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GN. (1969) Measurement of potassium ion activity in the cytoplasm of living cells. Nature 221: 386–387 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. (1993) Energization of potassium uptake in Arabidopsis thaliana. Planta 191: 302–307 [Google Scholar]
- Maathuis FJM, Sanders D. (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci USA 91: 9272–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. (1996) Mechanisms of potassium absorption by higher plant roots. Physiol Plant 96: 158–168 [Google Scholar]
- Malhotra B, Glass ADM. (1995) Potassium fluxes in Chlamydomonas reinhardtii. I. Kinetics and electrical potentials. Plant Physiol 108: 1527–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Waugh R, Brown JW, Schulman A, Langridge P, Platzer M, Fincher GB, Muehlbauer GJ, Sato K, Close TJ, et al (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716 [DOI] [PubMed] [Google Scholar]
- Memon AR, Saccomani M, Glass ADM. (1985) Efficiency of potassium utilization by barley varieties: the role of subcellular compartmentation. J Exp Bot 36: 1860–1876 [Google Scholar]
- Nocito FF, Sacchi GA, Cocucci M. (2002) Membrane depolarization induces K+ efflux from subapical maize root segments. New Phytol 154: 45–51 [Google Scholar]
- Palmer LG, Century TJ, Civan MM. (1978) Activity coefficients of intracellular Na+ and K+ during development of frog oocytes. J Membr Biol 40: 25–38 [DOI] [PubMed] [Google Scholar]
- Poole RJ. (1971) Effect of sodium on potassium fluxes at the cell membrane and vacuole membrane of red beet. Plant Physiol 47: 731–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo YJ, Gierth M, Schroeder JI, Cho MH. (2010) High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol 153: 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP. (2008) The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J Exp Bot 59: 595–607 [DOI] [PubMed] [Google Scholar]
- Robinson RA, Stokes RH (1965) Electrolyte Solutions, Ed 2. Butterworth, London [Google Scholar]
- Rubio F, Alemán F, Nieves-Cordones M, Martínez V. (2010) Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K uptake. Physiol Plant 139: 220–228 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270: 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rubio F, Nieves-Cordones M, Alemán F, Martínez V. (2008) Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol Plant 134: 598–608 [DOI] [PubMed] [Google Scholar]
- Santa-María GE, Danna CH, Czibener C. (2000) High-affinity potassium transport in barley roots: ammonium-sensitive and -insensitive pathways. Plant Physiol 123: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9: 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze LM, Britto DT, Li M, Kronzucker HJ. (2012) A pharmacological analysis of high-affinity sodium transport in barley (Hordeum vulgare L.): a 24Na+/42K+ study. J Exp Bot 63: 2479–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM. (1986) A model for the regulation of K+ influx, and tissue potassium concentrations by negative feedback effects upon plasmalemma influx. Plant Physiol 81: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ. (1991) Studies of the uptake of nitrate in barley. 3. Compartmentation of NO3−. J Exp Bot 42: 1455–1463 [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. J Gen Physiol 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao GV, Ito O, Berry WL, Wheeler RM. (2003) Sodium: a functional plant nutrient. Crit Rev Plant Sci 22: 391–416 [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ. (2008) Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J Exp Bot 59: 303–313 [DOI] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. (2009) K+ transport in plants: physiology and molecular biology. J Plant Physiol 166: 447–466 [DOI] [PubMed] [Google Scholar]
- ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61: 2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A. (1984) Ammonium uptake in Lemna gibba G1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant 61: 369–376 [Google Scholar]
- Vale FR, Jackson WA, Volk RJ. (1987) Potassium influx into maize root systems: influence of root potassium concentration and ambient ammonium. Plant Physiol 84: 1416–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AJ, Peralta ML, Santa-Maria GE. (2005) Expression of potassium-transporter coding genes, and kinetics of rubidium uptake, along a longitudinal root axis. Plant Cell Environ 28: 850–862 [Google Scholar]
- Véry AA, Sentenac H. (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. (1996) Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA 93: 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA, Pitman MG (1976) Measurement of fluxes across membranes. In U Luettge, MG Pitman, eds, Encyclopedia of Plant Physiology, Vol 2. Springer-Verlag, Berlin, pp 93–126 [Google Scholar]
- Wang MY, Glass ADM, Shaff JE, Kochian LV. (1994) Ammonium uptake by rice roots. III. Electrophysiology. Plant Physiol 104: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. (1998) Calcium channels in the plasma membrane of root cells. Ann Bot (Lond) 81: 173–183 [Google Scholar]
- White PJ, Broadley MR. (2000) Mechanisms of caesium uptake by plants. New Phytol 147: 241–256 [Google Scholar]
- White PJ, Davenport RJ. (2002) The voltage-independent cation channel in the plasma membrane of wheat roots is permeable to divalent cations and may be involved in cytosolic Ca2+ homeostasis. Plant Physiol 130: 1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Earnshaw MJ, Clarkson DT. (1991) Effects of growth and assay temperatures on unidirectional K+ fluxes in roots of rye (Secale cereale). J Exp Bot 42: 1031–1041 [Google Scholar]
- White PJ, Lemtiri-Chlieh F. (1995) Potassium currents across the plasma membrane of protoplasts derived from rye roots: a patch-clamp study. J Exp Bot 46: 497–511 [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yu J, Hu SN, Wang J, Wong GKS, Li SG, Liu B, Deng YJ, Dai L, Zhou Y, Zhang XQ, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92 [DOI] [PubMed] [Google Scholar]
- Zhang JL, Flowers TJ, Wang SM. (2010) Mechanisms of sodium uptake by roots of higher plants. Plant Soil 236: 45–60 [Google Scholar]