Abstract
Nitric oxide (NO) is a diffusible gas with diverse roles in human physiology and disease. Significant progress in the understanding of its biological effects has taken place in recent years. This has led to a better understanding of the pathobiology of pulmonary hypertension (PH) and the development of new therapies. This article provides an overview of the NO physiology and its role in the pathobiology of lung diseases, particularly PH. We also discuss current and emerging specific treatments that target NO signaling pathways in PH.
Keywords: pulmonary hypertension, nitric oxide, physiopathology and therapeutics
Pulmonary arterial hypertension (PAH) is a progressive disease that has poor prognosis since it may lead to right ventricular failure and death.[1] The disease is characterized by excessive pulmonary vasoconstriction and abnormal vascular remodeling that result in loss of vascular cross-sectional area and increase in right ventricular afterload.[2] One of the proposed mechanisms involved in the pathogenesis of the disease is an imbalance in vasoactive mediators with reduced levels of the vasodilatory and antiproliferative nitric oxide (NO).[3]
Since the breakthrough discovery in 1987 that the endothelium-derived relaxing factor was nitric oxide (NO),[4,5] this colorless and odorless free-radical gas became increasingly recognized as a key factor in human physiology and disease.[6,7] NO is an autocrine and paracrine signaling molecule whose functions are diverse and involve smooth muscle relaxation, platelet inhibition, central and autonomic neurotransmission, tumor cell lysis, bacterial killing, and stimulation of hormonal release.[7–10] This review will focus on the role of NO in physiology and pathobiology of lung diseases, particularly pulmonary hypertension (PH), and the current and emerging pulmonary hypertension (PH)-specific treatments based on NO signaling.
NITRIC OXIDE PHYSIOLOGY
NO is an endogenously synthesized, diffusible, lipophilic gas that is produced by a group of enzymes known as nitric oxide synthases (NOS). Their role is to convert the amino acid L-arginine to L-citrulline and NO.[6] For their activity, NOS require oxygen, reduced nicotinamide-adenine dinucleotide phosphate (NADPH), and other cofactors such as flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), calmodulin, and tetrahydrobiopterin (BH4).[11–13] NOS is active as a homodimer and contains a reductase and an oxygenase domain. The oxygenase domain is the active site of NO synthesis with binding sites for heme, L-arginine, and BH4[14] (Fig. 1). Three NOS isoforms (Types I, II, and III) have been identified. These NOS isoforms have important differences in expression and regulation as shown in Table 1.[15,16] In general, NOS I is expressed in neuronal cells and skeletal muscle; NOS II is found in epithelial, and smooth muscles cells as well as in neutrophils, macrophages, and fibroblasts; and NOS III is present in endothelial cells throughout the body.[17] NOS I and III are continuously expressed and regulated by Calcium/Calmodulin; meanwhile, NOS II is regulated at the transcription level. NOS II transcription is increased by cytokines (e.g., TNF-α, interferon-γ, and IL-1β), endotoxins, oxidants, and shear stress,[10,18] and is decreased by corticosteroids, retinoids, transforming growth factor beta, platelet-derived growth factor, insulin-like growth factor 1, and thrombin.[9,10,13,19] The initial clear distinction between the constitutive and inducible isoforms has been recently distorted and constitutive isoforms may be induced, and vice versa.[20,21]
Figure 1.
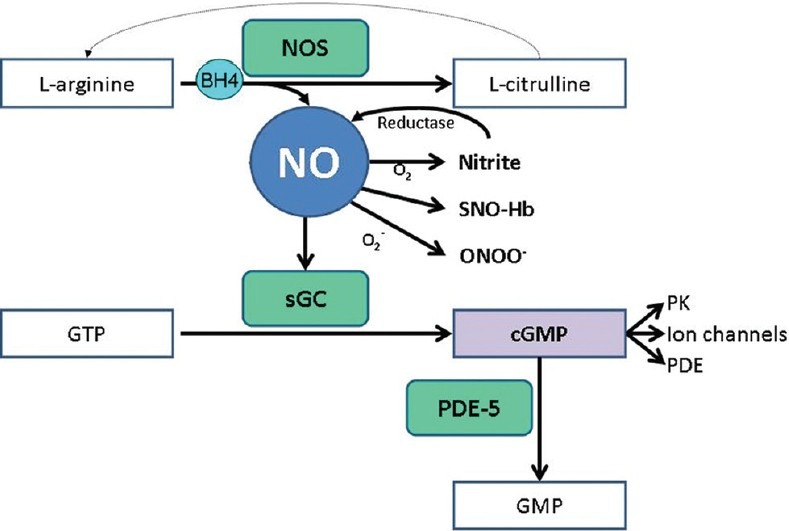
NO synthesis and signaling pathways. BH4, tetrahydrobiopterin; cGMP: Cyclic guanosine monophosphate; GMP: Guanosine monophosphate; GTP: Guanosine triphosphate; NO: Nitric oxide; NOS: Nitric oxide synthase; ONOO-, peroxynitrite; PK: Protein kinases; sGC: Soluble guanylate cyclase; SNO-Hb: S-nitrosothiol – hemoglobin. L-arginine can be regenerated from L-citrulline by two enzymes (argininosuccinate synthase and argininosuccinate lyase).
Table 1.
Characteristics of nitric oxide synthases isoforms
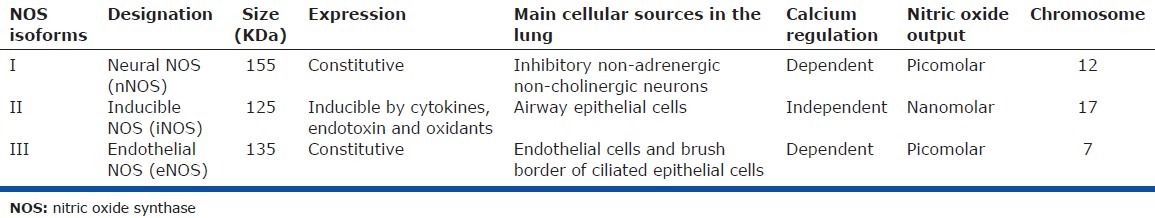
NOS I acts as a functional antagonist of acetylcholine and mediates inhibitory nonadrenergic non-cholinergic neural bronchodilation.[22] NOS II is involved in inflammation as it mediates the cytotoxic activity of activated macrophages and may be a contributing factor in the vasodilation observed in septic shock.[23] NOS III plays a role in the regulation of vascular flow[24] and may reduce plasma exudation in the airways[25] and regulate ciliary beating and mucociliary clearance.[26] NOS III is the predominant source of NO production in the pulmonary circulation.[14,20] Studies in transgenic mice and humans to assess the relative contribution of the three NOS isoforms showed that NOS II and III are the key regulators of the pulmonary circulation tone. NOS III is the key mediator of resting tone through endothelium-dependent pulmonary vasculature vasodilation,[27] while NOS II may mediate the pulmonary circulation's response to oxygen.[28] Targeted disruption of the NOS III gene in mice was associated with mild PH without evidence of pulmonary vascular remodeling,[29] which likely reflects compensation by the other NOS isoforms.
Vascular smooth muscle and endothelial cells have the ability to regenerate the NOS substrate L-arginine by synthesizing argininosuccinate from citrulline and aspartate, a process that requires two enzymes, argininosuccinate synthase and argininosuccinate lyase.[30–32] The first enzyme is co-induced with NOS II and the second is constitutively expressed in these cells.[33] Exogenous citrulline administration effectively stimulated NO production in vascular endothelial cells by means of regenerating arginine.[31]
Once NO is produced, it may act within the cell in which it is generated or freely diffuse into adjacent cells (e.g., vascular smooth muscle cells), acting as an intra- or intercellular messenger.[9,34] The NO intracellular diffusion may be limited because NO is readily oxidized to the more stable metabolic products nitrite (NO2–) and nitrate (NO3–)[11] and is scavenged predominantly by hemoglobin. Upon entering the cells, NO activates the intracellular soluble guanylate cyclase (sGC) to produce 3’,5’-cyclic guanosine monophosphate (cGMP), which mediates most of the physiological and pathological effects of NO (Fig. 1).[10,35]
Two main types of guanylate cyclase (GC) are known: the particulate-associated enzymes, which are transmembrane receptors that contain GC that is activated by atrial and brain natriuretic peptide; and the cytosolic or soluble type which is activated by NO.[36,37] NO appears to exert its effect by binding to the heme iron (ferrous state) of the sGC, stimulating the enzyme basal cyclase activity several hundred-fold.[13,34,38] Once cGMP is produced, it mediates physiologic responses through its effects in cGMP-gated ion channels, cGMP-regulated phosphodiesterases, or cGMP-dependent protein kinases.[34,39] A preferential activation of specific target proteins is believed to underlie the differential effects of cGMP in various cells.[40] cGMP-induced vasorelaxation of vascular smooth muscle is through several mechanisms including activation of K-channels which hyperpolarizes the cell membrane, inhibition of calcium influx, and reduction of myofilament calcium sensitivity.[14,39]
The cGMP signal is chiefly limited by phosphodiesterases (PDE) which degrade the cyclic nucleotide of GMP. PDE degrade both cyclic adenosine monophosphate (cAMP) and cGMP; however, PDE-5 is specific to cGMP, and indeed the enzyme requires the binding of cGMP for full activation.[41]
NOS inhibitors and NO generators have allowed for a better understanding of the NO signaling pathways. NOS inhibitors are analogs of L-arginine that act as a false substrate for this enzyme and inhibit both the constitutive and inducible forms of NOS. Of these, the most widely used include N-monomethyl-L-arginine (L-NMMA), N-nitro-L-arginine (L-NNA), and N-nitro-L-arginine methyl ester (L-NAME).[42] Another commonly utilized NOS inhibitor is L-aminoguanidine, a more selective inhibitor of NOS II.[8] NO generators are compounds that release NO such as diethylamine, sodium nitroprusside, and isosorbide dinitrite, among others.
The most important endogenous NOS inhibitor among the methylated arginines is the asymmetric dimethylarginine (ADMA).[43,44] ADMA is produced as a result of proteolysis of methylated proteins. The methylation of arginine is a post-translational modification via protein arginine methyltransferaes (PRMT). ADMA acts as a false substrate and competitively inhibits NOS activity, blocking the formation of endogenous NO.[44,45] ADMA undergoes clearance by the dimethylarginine dimethylaminohydrolase (DDAH) and it is also partially cleared by the kidneys.[46] Methylated arginines, like ADMA, may be responsible for the “L-arginine paradox.” At physiological state, NOS is saturated with arginine, thus an increase in arginine concentration in plasma or cytosol should have no effect in NO production.[31] However, elevating plasma arginine or citrulline levels enhance NO production, suggesting some form of competitive inhibition of NOS, such as ADMA, is present and can be overcome by increasing the arginine concentration.[45,47] Another explanation for the arginine paradox is the potential existence of a separated cellular pool of arginine allocated to NO synthesis, i.e., caveolar-localized arginine regeneration system, in which citrulline is recycled to arginine.[32] This hypothesis is supported by a study that revealed the colocalization in discrete cellular domains (caveolae) of enzymes involved in arginine regeneration and NO production.[31,32]
NITRIC OXIDE PHYSIOLOGY IN THE PULMONARY VASCULATURE
The specific role of NO produced in the pulmonary vasculature is still a matter of intense investigation. Data suggest that NO inhibits smooth muscle tone, proliferation, and migration.[48–50] NO causes relaxation of the vascular smooth muscle tone via the activation of cGMP.[48,49] In fact, endogenous NO plays a key role in decreasing the pulmonary artery resistance at the time of birth and in maintaining the dilation of the pulmonary vasculature.[39,49,51] The production of NO by NOS II in injured vascular smooth muscle cells may prevent vasospasm and inhibit cell proliferation by possibly inducing apoptosis.[52–55] In addition, NO in this setting may regulate the metabolism of vascular smooth muscle cells, favoring anaerobic glycolysis,[52] and lead to toxic effects on adjacent endothelial cells.[56]
NO inhibits vascular smooth muscle cell proliferation,[57] DNA synthesis,[58] and collagen production via activation of cGMP.[48,50,58,59] Furthermore, NO inhibits vascular smooth muscle cell migration independent of the effects on proliferation.[50] Higher levels of NO are required to inhibit proliferation rather than to produce vasodilation, suggesting a potential concomitant activation by cGMP of the cAMP kinase pathway which inhibits cellular proliferation.[60] NO generators can also inhibit proliferation in cells that lack sGC, suggesting that cGMP-independent mechanisms play a role. One of these potential mechanisms is the up regulation of Fas, a membrane protein that belongs to the TNF receptor family and induces apoptosis.[54] NO-induced apoptosis could be the result of a feedback control on calcium responses to growth factors, or deamination of purine and pyrimidine bases in DNA that leads to increased mutagenesis and DNA strand breaks.[61,62] Although NO has been reported to inhibit cell proliferation in endothelial cells,[63] other investigations have shown than either exogenous NO[64] or NO produced by NOS II in vascular smooth muscle cells may stimulate endothelial cell proliferation.[65]
Alternative NO pathways include the oxidation of NO to form nitrite or reaction with protein thiols to form S-nitrosothiols, molecules that can lead to vasodilation or can regulate protein function by post-translational modification.[66,67] NO is oxidized in the blood and tissues to form nitrite and nitrate.[13] Nitrate is produced by the reaction of NO with oxyhemoglobin, while nitrite is formed by oxidation of NO.[13] These molecules can be recycled to form NO by an allosterically controlled nitrite reductase reaction predominantly during hypoxia, thereby complementing the NOS pathway.[68–70] Deoxygenated hemoglobin has nitrite reductase activity, forming NO from nitrate (NO3–) and nitrite (NO2–),[69,71] a reaction that can explain the hypoxia-specific vasodilatory effect observed in some organs. The NOS pathway is oxygen dependent while the nitrate-nitrite-NO pathway is hypoxia activated.[69,70]
In addition, NO can be responsible for nitrosylation of a cysteine residue of the β-subunit of hemoglobin, resulting in S-nitrosylated-hemoglobin, a protein that exerts NO-like vasodilator effects.[39] This reaction is favored in the oxygenated state of hemoglobin, and, once desaturation of hemoglobin occurs, there is release of SNO to acceptor thiols potentially delivering NO to the systemic circulation.[72] S-nitrosylation and nitration enable the systemic transport of the NO signal, a process that would not be possible for NO due to its very short half-life[14] and strong affinity to bind hemoglobin.
NITRIC OXIDE AND NITRIC OXIDE SYNTHASES IN THE LUNG
NO is produced endogenously in the human lung in the upper and lower respiratory tract and it is detectable in exhaled breath (6-8 ppb).[28,73] The origin of exhaled NO in the human lung likely depends upon all three isoforms of NOS (I-III) but predominantly derives from the airway epithelial expression of NOS II, the high-producer of NO. NO metabolites like nitrosothiol and nitrite (NO2–) are found in the bronchoalveolar lavage of human lungs.[8] NO is formed in high concentrations in the upper respiratory tract (nasopharynx and paranasal sinuses) and in lower quantities in the lower respiratory tract.[28,73] It is produced in a variety of cells including epithelial, endothelial, and smooth muscle cells, and also in inhibitory non-adrenergic non-cholinergic neurons, mastocytes, fibroblasts, macrophages, lymphocytes, and neutrophils.[6,7,28]
Immunohistochemical studies have identified the presence of all three isoforms of NOS, expressed in different cells of the human lung (Table 1).[6,10,12,28,74–76] Specifically, NOS I is located in inhibitory non-adrenergic non-cholinergic neurons; NOS II is expressed in the airway epithelium; and NOS III is found in endothelial cells.[8,21,76,77] Contrary to what occurs in other organs where NOS II needs to be induced, in the lungs this enzyme is continuously expressed in the airway epithelium at basal conditions.[21]
NO is involved in pulmonary neurotransmission, host defense, airway and vascular smooth muscle relaxation, mucociliary clearance, airway mucus secretion, inflammation, and cytotoxicity.[8,77] NO plays key roles in lung biology and has been implicated in the pathophysiology of several lung diseases such as asthma, cystic fibrosis, bronchopulmonary dysplasia, lymphangioleiomyomatosis, and adult respiratory distress syndrome in addition to pulmonary hypertension.[8,12,20,28,35,78–84]
Endogenous NO plays an important role in the regulation of airway function, having both beneficial and detrimental effects.[20] It leads to bronchial smooth muscle relaxation, potentially modulating the basal airway tone.[8] Inhaled NO decreased pulmonary airway resistance in pigs and NO generators relaxed human airway smooth muscle in vitro.[8] Exhaled NO is increased in inflammatory airway diseases such as asthma and bronchiectasis likely due to an increase in NOS II expression in the epithelial cells of these patients with some contribution from the constitutive NOS isoforms.[8,20,77] In asthma the fraction of exhaled NO is considered a surrogate for eosinophilic airway inflammation and steroid responsiveness.[20,85–87] A reduction in exhaled NO levels is observed in smokers possibly as a result of a dysregulation of NOS activity as cigarette smoke contains high levels of NO.[77,88]
NO is a key molecule in the oxidative metabolism since it can exert oxidant or antioxidant effects depending on the local tissue milieu. Hence, in an environment where the load of antioxidant is low, NO will have oxidant properties; however, when the oxidant load is high, NO plays an antioxidant role by scavenging free radicals and reactive oxygen species.[16,74] NO rapidly consumes superoxide (O2–) by forming peroxynitrite (ONOO–) which is a less reactive oxidant that can be further metabolized to products like nitrate (NO3–).[79] Some of these reactive oxygen species could be responsible for oxidative modification of cellular proteins such as oxidation of sGC, a reaction that can impair the specific activity of sGC and reduce the ability of NO to stimulate cGMP.[89] On the same lines, recombinant human superoxide dismutase decreases oxidative stress and increases eNOS activity and expression, stimulating NO production, and ultimately pulmonary vasodilatation.[90–92]
NITRIC OXIDE IN PULMONARY HYPERTENSION
NO is a potent pulmonary vasodilator that is produced locally in the lung and has effects on smooth muscle relaxation and proliferation. The close proximity of the airways and vessels in the lung allows NO produced in high levels in the upper[93] and lower[28] airways by NOS II to affect pulmonary vascular tone, in concert with the low NO levels that are produced by NOS III in the vascular endothelium.[35] NO is considered to be a selective pulmonary vasodilator because after exerting its vasodilator action, NO is scavenged by hemoglobin having minimal effects on systemic hemodynamics.[35]
Disruption of the NO pathway is a major contributor to the pathobiology of PH. NO in exhaled breath and NO biochemical reaction products in bronchoalveolar lavage are lower in lungs of patients with PAH than controls and their level is inversely related to the degree of PH.[45,94] NO in exhaled breath of individuals with idiopathic PAH is significantly lower than subject with PH associated with other causes or healthy nonsmoking controls.[95] In fact, patients with PH associated with other causes had similar levels of exhaled NO than healthy controls.[95]
Early data demonstrated a reduced expression of NOS III, measured by immunostaining, in the vascular endothelium of pulmonary arteries in patients with PAH. This reduced expression inversely correlated with the severity of the morphological arterial changes.[96] More recently, however, other investigators showed increased or unaltered NOS III immunostaining in PH.[97,98] Moreover, there is evidence of high NOS III expression in plexiform lesions in PAH.[99] A unifying hypothesis suggested that the activity of NOS III may be reduced rather that its expression.[100] Other NOS isoforms besides NOS III may contribute to the low exhaled NO observed in idiopathic PAH.[94]
NO has also been implicated in the important role that bone morphogenetic protein receptor II (BMPRII) has in the pathogenesis of PAH.[101] In the lung, BMPRII is highly expressed in endothelial cells and its activation promotes proliferation, migration, and survival of these cells.[102] BMPRII levels are markedly reduced in patients with heritable or idiopathic PAH, promoting endothelial dysfunction and apoptosis.[102] These effects have been recently attributed to a decrease in NOS III activity, since BMPRII ligands failed to stimulate NOS III dependent protein kinase activation in pulmonary artery endothelial cells from patients with mutation in the BMPR2 gene.[103]
PAH patients treated with epoprostenol had a three-fold higher exhaled NO than PH patients not receiving this treatment and two-fold higher than healthy controls. Interestingly, exhaled NO increased at 24 hours in those patients treated with this prostacyclin analog.[95] These data suggest that prostacyclin analogs may in part improve PH through effects on NO. In support of this, previous work has shown that nebulized epoprostenol increased the exhaled NO in patients with PAH associated with congenital heart disease.[104] Similarly, inhaled iloprost also led to an increase in NO concomitant with a decrease in pulmonary artery pressure in a patient with PAH associated with scleroderma.[105] Other PAH-specific therapies that do not directly target the NO pathway may also improve the fraction of exhaled nitric oxide suggesting a crosstalk between different signaling pathways.[95,106,107]
The NO signaling pathways have been found to be affected in PH at different levels. Endogenous NOS III inhibitors may be involved in the pathogenesis of PH. These include the symmetric and asymmetric dimethylarginines (ADMA). Animal models of hypoxic PH showed increased ADMA levels and decrease in activity of dimethylarginine dimethylaminohydrolase (DDAH), the endothelial enzyme that metabolizes ADMA.[108] Higher serum levels of ADMA are increased in patients with idiopathic PAH[109,110] and chronic thromboembolic pulmonary hypertension,[111] and correlate with disease severity and survival. ADMA levels increase predominantly due to a reduction in the expression and function DDAH.[110]
Arginase II, an enzyme that is part of the urea cycle and breaks down arginine to ornithine, can decrease the substrate available to NOS for NO synthesis. Idiopathic and PAH associated with sickle cell disease patients have been found to have higher levels of Arginase II and lower levels of L-arginine than healthy controls.[112] In the absence of L-arginine or BH4, NOS III may become “uncoupled,”[14] resulting in the generation of the free radical superoxide.
CLINICAL IMPLICATIONS IN PH: DIAGNOSIS AND PROGNOSIS
Although the vascular endothelium produces large amounts of NO, very little is exhaled as a result of the marked affinity of NO to hemoglobin in the pulmonary circulation.[28] In spite of this potential limitation, NO can be measured in exhaled breath and its concentration is inversely related to the exhalation flow. For this reason, the fraction of exhaled NO is measured at a constant flow (usually 50 mL/s). Using this approach, the fraction of exhaled NO is a reliable surrogate of the maximal flux of NO from the large airway compartment.[113] This method does not measure the steady-state mean distal airway/alveolar concentration of NO. There is no simple surrogate for measuring this parameter since its determination requires the measurement of the fraction of exhaled NO at multiple expiratory flow rates and the application of a modified “slope-intercept” algorithm.[20,114]
Authors have investigated whether exhaled NO could serve as a noninvasive marker of severity of disease and response to therapy in PH. The value of repetitive exhaled NO measurements was studied in 17 PAH patients over two years. NO levels at entry were inversely correlated with the number of months from the PAH diagnosis, suggesting a global decrease in NO over longer periods with the disease.[106] Lower exhaled NO levels at entry were associated with higher pulmonary artery pressures and less decrease over time. NO at the beginning of the study was not associated with survival; nevertheless, its level increased over time in the PAH individuals who survived to complete the study when compared to those who died, and correlated with changes in pulmonary artery pressures.[106] Thus, exhaled NO could be a useful marker of disease severity and response to therapy.[94,95,106]
Inhaled NO is frequently used for acute vasodilator challenge during right heart catheterization in patients with PAH. A positive pulmonary vasodilator test (decrease in mean pulmonary artery pressure of at least 10 mmHg to an absolute value less than 40 mmHg without decrease in cardiac output) indicates the patient that can potentially benefit from long-term calcium-channel blockers.[115–118] NO vasodilator challenge in several types of PH can also provide prognostic information, as responders may have better outcome, independent of the treatment administered.[119–122]
CLINICAL IMPLICATIONS IN PH: THERAPY
Inhibiting phosphodiesterases-5
Therapies that manipulate the downstream NO signaling have revolutionized the treatment of PH (Fig. 2). Sildenafil and tadalafil are FDA approved PDE-5 inhibitors for the treatment of PAH in adults.[123–125] The FDA recently placed a safety warning on the prescription of sildenafil, not recommending its use in pediatric patients due to increase mortality with increasing doses in this age group.[125] This modification was based on the results of the Sildenafil Citrate in Treatment-Naive Children with Pulmonary Arterial Hypertension (STARTS-2) trial.[126] This extension study, designed to assess the safety and tolerability of long-term treatment with oral sildenafil monotherapy in children (aged 1-17 years) with PAH, showed a higher mortality risk in patients randomized to high-dose sildenafil.[126]
Figure 2.
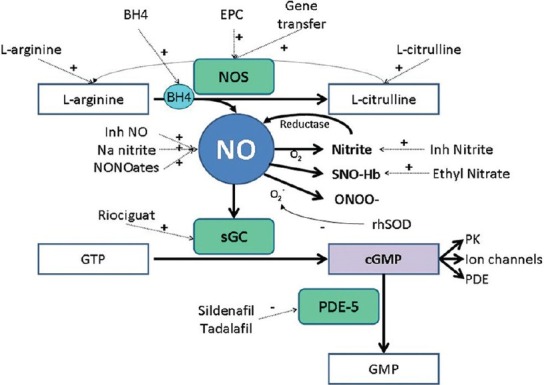
Therapeutic strategies to increase NO effect. BH4, tetrahydrobiopterin; cGMP: Cyclic guanosine monophosphate; EPC: Endothelial progenitor cells; GMP: Guanosine monophosphate; GTP: Guanosine triphosphate; HMG coA red, hydroxyl-methylglutaryl-CoA reductase; Inh, inhaled; NO: Nitric oxide; NOS: Nitric oxide synthase; ONOO-, peroxynitrite; PDE: Phosphodiesterases; PK: Protein kinases; rhSOD: Recombinant human superoxide dismutase; sGC: Soluble guanylate cyclase; SNO-Hb: S-nitrosothiol – hemoglobin. L-arginine can be regenerated from L-citrulline by two enzymes (argininosuccinate synthase and argininosuccinate lyase).
PDE-5 inhibitors prevent the normal hydrolysis of cGMP, prolonging the NO effects on tissues. PDE-5 is mainly expressed in the pulmonary vascular bed,[127] thus its inhibition has primarily pulmonary-specific effects.[100,128] Furthermore, PDE5 is upregulated in the lung[127] and the hypertrophied right ventricular myocardium[129] of patients with PAH. Therefore, PDE-5 inhibitors are an ideal treatment for PAH because they decrease the right ventricular afterload and improve right ventricular inotropy, without relevant systemic hemodynamic effects.[129]
Using nitric oxide as an inhaled gas
Inhaled NO was first shown to selectively reduce pulmonary vascular resistance in a lamb model;[130] subsequently, multiple studies have confirmed this finding in humans.[118,131,132] Continuous inhaled NO had beneficial effects in patients with PAH or PH associated with COPD;[132–134] however, high cost and technical difficulties for its delivery and avoidance of the toxic effects of NO oxidative products have prevented its widespread use.[133] During continuous administration of NO, NO oxidative products (NO2) can build up and cause airway hyperactivity at low concentrations[135] and pulmonary edema at higher concentrations.[136] Methemoglobin is also formed as NO reacts with oxyhemoglobin. Furthermore, mechanisms should be in place to avoid abrupt cessation of inhaled NO as this may lead to rebound PAH with deleterious effects.[137,138] Nonetheless, two studies that evaluated the long-term use (one and three months, respectively) of inhaled NO in PAH, chronic thromboembolic PH, and PH due to COPD reported no significant increase in methemoglobin, withdrawal syndrome, change in oxygenation, pulmonary function, or systemic hemodynamics.[132,134]
Currently, inhaled NO is FDA approved for the treatment of term and near-term (> 34 week gestation) neonates with hypoxic respiratory failure with clinical and echocardiographic evidence of pulmonary hypertension.[81,82,139] Extended administration (weeks) of inhaled NO has been studied in premature infants and does not appear to improve survival or prevent bronchopulmonary dysplasia.[81–83,140,141] At present, inhaled NO is undergoing clinical investigations to evaluate its utility as a treatment for bronchopulmonary dysplasia (NCT01503801). There are two ongoing studies using extended administration of inhaled NO in adults with PH. The PHiano study (NCT01265888, Geno LLC) is an open label, dose-escalation, Phase II study using a NO delivery system (NITROsyl) in patients with PAH or PH secondary to idiopathic pulmonary fibrosis. The second study (NCT01457781, INO Therapeutics) is a randomized, double blind, placebo control, Phase II study of inhaled NO versus placebo as add-on therapy in subjects with PAH. NO is delivered by a special device (INOpulse DS).
Nitric oxide donors
Plasma levels of nitrate and nitrite are low in diseases that have endothelial dysfunction like PAH.[142] Inhaled nitrite could be converted to NO and selectively dilate the pulmonary circulation.[143] The vasodilatory effects of these inorganic anions (nitrite and nitrate) are less potent than the organic nitrates (nitroglycerin) and nitrites (amyl-nitrite);[69] however, the inorganic compounds do not induce significant systemic vasodilator effects and tachyphylaxis as the organic molecules.[69] Nebulized sodium nitrite has been shown to ameliorate PH induced by hypoxia in animals and humans, with a longer duration of action than that of inhaled NO,[143,144] supporting that nitrites are NO donors particularly during hypoxia as result of the nitrite reductase action of deoxyhemogobin.[69,143] Ethyl nitrate is an organic nitrate given by inhalation that forms S-nitrosothiols and has shown to improve pulmonary hemodynamics in a model of hypoxia-induced PH without altering systemic vascular resistance or increasing methemoglobin levels.[145] Its low potency when compared with inhaled NO can be markedly enhanced both in vivo and in vitro by the addition of the thiol glutathione.[145]
Efforts in trying to find better stability and prolonged half-life in NO delivery led to the discovery of diazeniumdiolates (diethylenetriamine/NO) that can form a group of adducts called NONOates that are complexes of NO with nucleophiles that spontaneously and nonenzymatically release NO when dissolved in aqueous neutral PH solutions. This process prolongs the half-life of NO release up to 20 hours.[146,147] Nebulized NO donors have been shown to reduce PVR in hypoxia-induced PAH in piglets,[148] monocrotaline rat model,[147] and ARDS patients[149] with no systemic adverse effects or toxic reaction products of NO.
Other therapies based on the nitric oxide pathway
Several promising therapies which affect the signaling pathway of NO are under active investigation (Fig. 1). Soluble guanylate cyclase stimulators increase cGMP independently of NO. One of these stimulators, Riociguat, increased the activity of soluble guanylate cyclase 73-fold in vivo with partial reduction in pulmonary pressures, RV hypertrophy, and pulmonary artery muscularization in animal models of PH.[150] This medication showed promising results in a Phase II study in patients with PAH and chronic thromboembolic PH.[151] We are awaiting the results of two Phase III studies evaluating safety and clinical effectiveness of riociguat in PAH (NCT00810693) and chronic thromboembolic PH (NCT00855465).[152]
L-arginine replacement aims at providing excess substrate for the NOS enzyme and stimulating the NO production. L-arginine attenuated PAH in different animal models of PAH[153–155] and in patients with PAH associated with sickle cell disease, idiopathic PAH, and chronic thromboembolic PH.[156,157] L-citrulline, a urea cycle intermediate, is metabolized to L-arginine in pulmonary vascular endothelial cells. Oral supplementation increased NO synthesis and ameliorated chronic hypoxia-induced PH in newborn piglets.[158] In children undergoing cardiopulmonary bypass, the oral supplementation of L-citrulline safely increased plasma citrulline and arginine concentrations, and more importantly PH did not occur in those with elevated citrulline levels.[159] The safety and effectiveness of intravenous L-citrulline, in children undergoing cardiopulmonary bypass for surgical repair of a congenital heart defect, is currently being tested (NCT01120964).
NO production by NOS depends on the de novo biosynthesis of the enzyme cofactor tetrahydrobiopterin (BH4).[160,161] BH4 stabilizes the NOS dimmer assembly and the favorable spin state of the Fe (II)-O2 heme intermediate preventing “uncoupling” of the enzyme and formation of reactive oxygen products.[14,162,163] The augmentation of tetrahydrobiopterin showed promising results in a pilot study in patients with PAH or chronic thromboembolic PAH.[164] Another promising therapy is the recombinant human superoxide dismutase (rhSOD) that scavenges superoxide anion and increases the bioavailability of NO.[165] In a lamb model of persistent PH, rhSOD administered through the endotracheal tube as a bolus enhanced the effects of inhaled NO on pulmonary vasculature.[165]
Attractive new potential therapies for PAH include the NOS III enhancers, the delivery of autologous endothelial progenitor cells,[166] or the enzyme NOS II and III using adenoviral-mediated transfer or adult stem cell-based ex vivo gene therapy.[167] Bone marrow-derived endothelial-like progenitor cells prevented the development or progression of PH in a monocrotaline rat model of PH.[168] The engraftment of these progenitor cells in the pulmonary vasculature may restore the microvascular structure and function.[168] Meanwhile, animals receiving endothelial-like progenitor cells or mesenchymal stem cells transduced with NOS III had reversal of established PH and improved survival.[168,169] Adenoviral gene transfer of NOS III produced an increase in the NOS III expression and activity with attenuation in the hypoxia-induced increase in pulmonary artery pressure.[170] Similarly, NOS II gene transfer increased pulmonary NO production with reduction in hypoxia-induced PH and vascular remodeling in rats.[171]
CONCLUSIONS
Nitric oxide mediates diverse key signaling functions in human physiology and disease. Major progress in the understanding of NO signaling pathway has led to the approval of PAH-specific treatments and the ongoing discovery and development of promising new therapies. Molecules in the NO pathway also have the potential to be used as biomarkers of disease severity, outcomes, or response to therapy in pulmonary hypertension.
Footnotes
Source of Support: Dr. Adriano R. Tonelli is supported by CTSA KL2 Grant # RR024990 from the National Center for Research Resources (NCRR). Dr. Raed A. Dweik is supported by the following grants: HL081064, HL107147, HL095181, and RR026231 from the National Institutes of Health (NIH), and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD). Dr. Metin Aytekin is supported by 0826095H from the American Heart Association (AHA)
Conflict of Interest: None declared.
REFERENCES
- 1.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension.A national prospective study. Ann Intern Med. 1987;107:216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S3–9. doi: 10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: Pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–55. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C, Xie QW. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt HH, Walter U. NO at work. Cell. 1994;78:919–25. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 8.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–51. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 10.Dweik RA, Erzurum SC. Effects of Nitric Oxide and Cyclic GMP on Smooth Muscle Proliferation. In: Moss J, editor. LAM and Other Diseases Characterized by Smooth Muscle Proliferation. New York: Marcel Dekker Inc; 1999. pp. 333–49. [Google Scholar]
- 11.Stuehr DJ, Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- 12.Dweik RA, Laskowski D, Ozkan M, Farver C, Erzurum SC. High levels of exhaled nitric oxide (NO) and NO synthase III expression in lesional smooth muscle in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2001;24:414–8. doi: 10.1165/ajrcmb.24.4.4127. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 14.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–85. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 15.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–30. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 16.Dweik RA. Nitric oxide, hypoxia, and superoxide: The good, the bad, and the ugly! Thorax. 2005;60:265–7. doi: 10.1136/thx.2004.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–76. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 18.Newby AC, Southgate KM, Assender JW. Inhibition of vascular smooth muscle cell proliferation by endothelium-dependent vasodilators. Herz. 1992;17:291–9. [PubMed] [Google Scholar]
- 19.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–8. [PubMed] [Google Scholar]
- 20.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, et al. Exhaled nitric oxide in pulmonary diseases: A comprehensive review. Chest. 2010;138:682–92. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 21.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92:7809–13. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward JK, Belvisi MG, Fox AJ, Miura M, Tadjkarimi S, Yacoub MH, et al. Modulation of cholinergic neural bronchoconstriction by endogenous nitric oxide and vasoactive intestinal peptide in human airways in vitro. J Clin Invest. 1993;92:736–42. doi: 10.1172/JCI116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 24.Shaul PW. Regulation of endothelial nitric oxide synthase: Location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 25.Bernareggi M, Mitchell JA, Barnes PJ, Belvisi MG. Dual action of nitric oxide on airway plasma leakage. Am J Respir Crit Care Med. 1997;155:869–74. doi: 10.1164/ajrccm.155.3.9117019. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 27.Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr, Huang PL, et al. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–8. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 28.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, et al. Nitric oxide synthesis in the lung.Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101:660–6. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, et al. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990;87:8612–6. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–97. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 32.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol. 2003;206:2083–7. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 33.Hattori Y, Campbell EB, Gross SS. Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle.Role in the regeneration or arginine for nitric oxide synthesis. J Biol Chem. 1994;269:9405–8. [PubMed] [Google Scholar]
- 34.Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: Regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–75. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 35.Dweik RA. Pulmonary hypertension and the search for the selective pulmonary vasodilator. Lancet. 2002;360:886–7. doi: 10.1016/S0140-6736(02)11067-1. [DOI] [PubMed] [Google Scholar]
- 36.Garbers DL. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 37.McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med. 1996;211:1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- 38.Murad F. Regulation of cytosolic guanylyl cyclase by nitric oxide: The NO-cyclic GMP signal transduction system. Adv Pharmacol. 1994;26:19–33. doi: 10.1016/s1054-3589(08)60049-6. [DOI] [PubMed] [Google Scholar]
- 39.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91:7583–7. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–38. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 41.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–91. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 42.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulau P, Zakrzewicz D, Kitowska K, Leiper J, Gunther A, Grimminger F, et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol. 2007;292:L18–24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- 44.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler. 2003;4:33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 45.Dweik RA. The lung in the balance: Arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L15–7. doi: 10.1152/ajplung.00322.2006. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG, NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264:10205–9. [PubMed] [Google Scholar]
- 47.Sy BMC, Dweik EE, Dweik RA. Arginine and nitric oxide. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 571–81. [Google Scholar]
- 48.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–7. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomae KR, Nakayama DK, Billiar TR, Simmons RL, Pitt BR, Davies P. The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res. 1995;59:337–43. doi: 10.1006/jsre.1995.1173. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996;78:225–30. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 51.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–7. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 52.Geng Y, Hansson GK, Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992;71:1268–76. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- 53.Stein CS, Fabry Z, Murphy S, Hart MN. Involvement of nitric oxide in IFN-gamma-mediated reduction of microvessel smooth muscle cell proliferation. Mol Immunol. 1995;32:965–73. doi: 10.1016/0161-5890(95)00062-j. [DOI] [PubMed] [Google Scholar]
- 54.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, et al. Nitric oxide induces upregulation of Fas and apoptosis in vascular smooth muscle. Hypertension. 1996;27:823–6. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 55.Schini VB, Vanhoutte PM. Role of the L-arginine-nitric oxide pathway in vascular smooth muscle. Eur Heart J. 1993;14:16–21. [PubMed] [Google Scholar]
- 56.Thomae KR, Joshi PC, Davies P, Pitt BR, Billiar TR, Simmons RL, et al. Nitric oxide produced by cytokine-activated pulmonary artery smooth muscle cells is cytotoxic to cocultured endothelium. Surgery. 1996;119:61–6. doi: 10.1016/s0039-6060(96)80215-7. [DOI] [PubMed] [Google Scholar]
- 57.Nunokawa Y, Tanaka S. Interferon-gamma inhibits proliferation of rat vascular smooth muscle cells by nitric oxide generation. Biochem Biophys Res Commun. 1992;188:409–15. doi: 10.1016/0006-291x(92)92400-r. [DOI] [PubMed] [Google Scholar]
- 58.Nakaki T, Nakayama M, Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990;189:347–53. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- 59.Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76:305–9. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- 60.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994;267:C1405–13. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 61.Clementi E, Sciorati C, Nistico G. Growth factor-induced Ca2+ responses are differentially modulated by nitric oxide via activation of a cyclic GMP-dependent pathway. Mol Pharmacol. 1995;48:1068–77. [PubMed] [Google Scholar]
- 62.Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992;89:3030–4. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W, Ando J, Korenaga R, Toyo-oka T, Kamiya A. Exogenous nitric oxide inhibits proliferation of cultured vascular endothelial cells. Biochem Biophys Res Commun. 1994;203:1160–7. doi: 10.1006/bbrc.1994.2304. [DOI] [PubMed] [Google Scholar]
- 64.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuo K, Inoue T, Morimoto S, Nakahashi T, Yasuda O, Kitano S, et al. Nitric oxide mediates cytotoxicity and basic fibroblast growth factor release in cultured vascular smooth muscle cells. A possible mechanism of neovascularization in atherosclerotic plaques. J Clin Invest. 1995;95:669–76. doi: 10.1172/JCI117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 67.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 68.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 69.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 70.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–47. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 73.Tsujino I, Miyamoto K, Nishimura M, Shinano H, Makita H, Saito S, et al. Production of nitric oxide (NO) in intrathoracic airways of normal humans. Am J Respir Crit Care Med. 1996;154:1370–4. doi: 10.1164/ajrccm.154.5.8912750. [DOI] [PubMed] [Google Scholar]
- 74.Dweik RA, Erzurum SC. Regulation of nitric oxide (NO) synthases and gas phase NO by oxygen. In: Marczin N, Kharitonov SA, Yacoub MH, Barnes PJ, editors. Disease markers in exhaled breath. New York: Marcel Dekker Inc; 2003. pp. 235–46. [Google Scholar]
- 75.Dweik RA, Guo FH, Uetani K, Erzurum SC. Nitric oxide synthase in the human airway epithelium. Zhongguo Yao Li Xue Bao. 1997;18:550–2. [PubMed] [Google Scholar]
- 76.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, et al. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–7. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 77.Barnes PJ, Belvisi MG. Nitric oxide and lung disease. Thorax. 1993;48:1034–43. doi: 10.1136/thx.48.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dweik RA. The promise and reality of nitric oxide in the diagnosis and treatment of lung disease. Cleve Clin J Med. 2001;68:486. doi: 10.3949/ccjm.68.6.486. 8, 90, 93. [DOI] [PubMed] [Google Scholar]
- 79.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol. 2003;95:436–40. doi: 10.1152/japplphysiol.01127.2002. [DOI] [PubMed] [Google Scholar]
- 81.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–64. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 82.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 83.Stark AR. Inhaled NO for preterm infants-getting to yes? N Engl J Med. 2006;355:404–6. doi: 10.1056/NEJMe068129. [DOI] [PubMed] [Google Scholar]
- 84.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–9. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–82. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 86.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: An observational study in adults with asthma. Clin Exp Allergy. 2005;35:1175–9. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 87.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: A predictor of steroid response. Am J Respir Crit Care Med. 2005;172:453–9. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 88.Ischiropoulos H, Mendiguren I, Fisher D, Fisher AB, Thom SR. Role of neutrophils and nitric oxide in lung alveolar injury from smoke inhalation. Am J Respir Crit Care Med. 1994;150:337–41. doi: 10.1164/ajrccm.150.2.8049812. [DOI] [PubMed] [Google Scholar]
- 89.Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med. 2001;31:1360–7. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 90.Firth AL, Yuan JX. Bringing down the ROS: A new therapeutic approach for PPHN. Am J Physiol Lung Cell Mol Physiol. 2008;295:L976–8. doi: 10.1152/ajplung.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L979–87. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robbins CG, Horowitz S, Merritt TA, Kheiter A, Tierney J, Narula P, et al. Recombinant human superoxide dismutase reduces lung injury caused by inhaled nitric oxide and hyperoxia. Am J Physiol. 1997;272:L903–7. doi: 10.1152/ajplung.1997.272.5.L903. [DOI] [PubMed] [Google Scholar]
- 93.Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, et al. High nitric oxide production in human paranasal sinuses. Nat Med. 1995;1:370–3. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 94.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–23. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 95.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung. 2001;179:233–43. doi: 10.1007/s004080000064. [DOI] [PubMed] [Google Scholar]
- 96.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 97.Xue C, Johns RA. Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:1642–4. doi: 10.1056/NEJM199512143332416. [DOI] [PubMed] [Google Scholar]
- 98.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–32. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 99.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, et al. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol. 1998;185:313–8. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 100.Hagan G, Pepke-Zaba J. Pulmonary hypertension, nitric oxide and nitric oxide-releasing compounds. Expert Rev Respir Med. 2011;5:163–71. doi: 10.1586/ers.11.5. [DOI] [PubMed] [Google Scholar]
- 101.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–5. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–8. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 103.Gangopahyay A, Oran M, Bauer EM, Wertz JW, Comhair SA, Erzurum SC, et al. Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J Biol Chem. 2011;286:33134–40. doi: 10.1074/jbc.M111.274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forrest IA, Small T, Corris PA. Effect of nebulized epoprostenol (prostacyclin) on exhaled nitric oxide in patients with pulmonary hypertension due to congenital heart disease and in normal controls. Clin Sci (Lond) 1999;97:99–102. [PubMed] [Google Scholar]
- 105.Rolla G, Colagrande P, Brussino L, Bucca C, Bertero MT, Caligaris-Cappio F. Exhaled nitric oxide and pulmonary response to iloprost in systemic sclerosis with pulmonary hypertension. Lancet. 1998;351:1491–2. doi: 10.1016/S0140-6736(05)78874-7. [DOI] [PubMed] [Google Scholar]
- 106.Machado RF, Londhe Nerkar MV, Dweik RA, Hammel J, Janocha A, Pyle J, et al. Nitric oxide and pulmonary arterial pressures in pulmonary hypertension. Free Radic Biol Med. 2004;37:1010–7. doi: 10.1016/j.freeradbiomed.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 107.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–73. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Millatt LJ, Whitley GS, Li D, Leiper JM, Siragy HM, Carey RM, et al. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–8. doi: 10.1161/01.CIR.0000089087.25930.FF. [DOI] [PubMed] [Google Scholar]
- 109.Kielstein JT, Bode-Boger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–8. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 110.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–7. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 111.Skoro-Sajer N, Mittermayer F, Panzenboeck A, Bonderman D, Sadushi R, Hitsch R, et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2007;176:1154–60. doi: 10.1164/rccm.200702-278OC. [DOI] [PubMed] [Google Scholar]
- 112.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–8. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 113.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 114.Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol. 2007;102:417–25. doi: 10.1152/japplphysiol.00533.2006. [DOI] [PubMed] [Google Scholar]
- 115.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 116.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 117.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med. 2010;104:481–96. doi: 10.1016/j.rmed.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 119.Weir EK, Rubin LJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. The acute administration of vasodilators in primary pulmonary hypertension.Experience from the National Institutes of Health Registry on Primary Pulmonary Hypertension. Am Rev Respir Dis. 1989;140:1623–30. doi: 10.1164/ajrccm/140.6.1623. [DOI] [PubMed] [Google Scholar]
- 120.Post MC, Janssens S, Van de Werf F, Budts W. Responsiveness to inhaled nitric oxide is a predictor for mid-term survival in adult patients with congenital heart defects and pulmonary arterial hypertension. Eur Heart J. 2004;25:1651–6. doi: 10.1016/j.ehj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Skoro-Sajer N, Hack N, Sadushi-Kolici R, Bonderman D, Jakowitsch J, Klepetko W, et al. Pulmonary vascular reactivity and prognosis in patients with chronic thromboembolic pulmonary hypertension: A pilot study. Circulation. 2009;119:298–305. doi: 10.1161/CIRCULATIONAHA.108.794610. [DOI] [PubMed] [Google Scholar]
- 122.Krasuski RA, Devendra GP, Hart SA, Wang A, Harrison JK, Bashore TM. Response to inhaled nitric oxide predicts survival in patients with pulmonary hypertension. J Card Fail. 2011;17:265–71. doi: 10.1016/j.cardfail.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 123.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 124.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 125.Label approved for REVATIO, NDA # 021845. FDA, 2012. [Last accessed on 2012 April 10]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda .
- 126.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 127.Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172:105–13. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 128.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation. 2002;105:2398–403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- 129.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–48. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 130.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide.A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–47. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 131.Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338:1173–4. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- 132.Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, et al. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax. 2003;58:289–93. doi: 10.1136/thorax.58.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: An ambulatory delivery system and initial clinical tests. Chest. 1996;109:1545–9. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 134.Perez-Penate GM, Julia-Serda G, Ojeda-Betancort N, García-Quintana A, Pulido-Duque J, Rodríguez-Pérez A, et al. Long-term inhaled nitric oxide plus phosphodiesterase 5 inhibitors for severe pulmonary hypertension. J Heart Lung Transplant. 2008;27:1326–32. doi: 10.1016/j.healun.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 135.Frampton MW, Morrow PE, Cox C, Gibb FR, Speers DM, Utell MJ. Effects of nitrogen dioxide exposure on pulmonary function and airway reactivity in normal humans. Am Rev Respir Dis. 1991;143:522–7. doi: 10.1164/ajrccm/143.3.522. [DOI] [PubMed] [Google Scholar]
- 136.Clutton-Brock J. Two cases of poisoning by contamination of nitrous oxide with higher oxides of nitrogen during anaesthesia. Br J Anaesth. 1967;39:388–92. doi: 10.1093/bja/39.5.388. [DOI] [PubMed] [Google Scholar]
- 137.Miller OI, Tang SF, Keech A, Celermajer DS. Rebound pulmonary hypertension on withdrawal from inhaled nitric oxide. Lancet. 1995;346:51–2. doi: 10.1016/s0140-6736(95)92681-x. [DOI] [PubMed] [Google Scholar]
- 138.Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000;161:1443–9. doi: 10.1164/ajrccm.161.5.9806138. [DOI] [PubMed] [Google Scholar]
- 139.Label for INOMAX, NDA # 020845. U.S. Food and Drug Administration. 2010. [Last accessed on 2012, April 10]. Accessed from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 140.Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): A randomised controlled trial. Lancet. 2010;376:346–54. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 141.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 142.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 143.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–7. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 144.Ingram TE, Pinder AG, Bailey DM, Fraser AG, James PE. Low-dose sodium nitrite vasodilates hypoxic human pulmonary vasculature by a means that is not dependent on a simultaneous elevation in plasma nitrite. Am J Physiol Heart Circ Physiol. 2010;298:H331–9. doi: 10.1152/ajpheart.00583.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brandler MD, Powell SC, Craig DM, Quick G, McMahon TJ, Goldberg RN, et al. A novel inhaled organic nitrate that affects pulmonary vascular tone in a piglet model of hypoxia-induced pulmonary hypertension. Pediatr Res. 2005;58:531–6. doi: 10.1203/01.PDR.0000179399.64025.37. [DOI] [PubMed] [Google Scholar]
- 146.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–93. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 147.Hampl V, Tristani-Firouzi M, Hutsell TC, Archer SL. Nebulized nitric oxide/nucleophile adduct reduces chronic pulmonary hypertension. Cardiovasc Res. 1996;31:55–62. [PubMed] [Google Scholar]
- 148.Young KC, Ladino J, Navarrete C, Dabrowska K, Hehre D, Bancalari E, et al. The effect of a nebulized NO donor, DPTA/NO, on acute hypoxic pulmonary hypertension in newborn piglets. Biol Neonate. 2004;85:195–202. doi: 10.1159/000075815. [DOI] [PubMed] [Google Scholar]
- 149.Lam CF, Van Heerden PV, Blott J, Roberts B, Ilett KF. The selective pulmonary vasodilatory effect of inhaled DETA/NO, a novel nitric oxide donor, in ARDS-a pilot human trial. J Crit Care. 2004;19:48–53. doi: 10.1016/j.jcrc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 150.Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Müller D, Schlüter KD, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32:881–91. doi: 10.1183/09031936.00114407. [DOI] [PubMed] [Google Scholar]
- 151.Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: A phase II study. Eur Respir J. 2010;36:792–9. doi: 10.1183/09031936.00182909. [DOI] [PubMed] [Google Scholar]
- 152.Schermuly RT, Janssen W, Weissmann N, Stasch JP, Grimminger F, Ghofrani HA. Riociguat for the treatment of pulmonary hypertension. Expert Opin Investig Drugs. 2011;20:567–76. doi: 10.1517/13543784.2011.565048. [DOI] [PubMed] [Google Scholar]
- 153.Ou ZJ, Wei W, Huang DD, Luo W, Luo D, Wang ZP, et al. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab. 2010;298:E1131–9. doi: 10.1152/ajpendo.00107.2010. [DOI] [PubMed] [Google Scholar]
- 154.Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. L-Arginine promotes angiogenesis in the chronically hypoxic lung: A novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1042–50. doi: 10.1152/ajplung.90327.2008. [DOI] [PubMed] [Google Scholar]
- 155.Mitani Y, Maruyama K, Sakurai M. Prolonged administration of L-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 1997;96:689–97. [PubMed] [Google Scholar]
- 156.Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, Kyotani S, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163:887–91. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 157.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, et al. Arginine therapy: A new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–9. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 158.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L506–11. doi: 10.1152/ajplung.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, et al. Nitric oxide precursors and congenital heart surgery: A randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 160.Nakayama DK, Geller DA, Di Silvio M, Bloomgarden G, Davies P, Pitt BR, et al. Tetrahydrobiopterin synthesis and inducible nitric oxide production in pulmonary artery smooth muscle. Am J Physiol. 1994;266:L455–60. doi: 10.1152/ajplung.1994.266.4.L455. [DOI] [PubMed] [Google Scholar]
- 161.Gross SS, Levi R. Tetrahydrobiopterin synthesis.An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992;267:25722–9. [PubMed] [Google Scholar]
- 162.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: A paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–50. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 163.Venema RC, Ju H, Zou R, Ryan JW, Venema VJ. Subunit interactions of endothelial nitric-oxide synthase.Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem. 1997;272:1276–82. doi: 10.1074/jbc.272.2.1276. [DOI] [PubMed] [Google Scholar]
- 164.Robbins IM, Hemnes AR, Gibbs JS, Christman BW, Howard L, Meehan S, et al. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res. 2011;37:26–34. doi: 10.3109/01902148.2010.512972. [DOI] [PubMed] [Google Scholar]
- 165.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–9. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 166.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: A pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–71. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 167.Deng W, Bivalacqua TJ, Champion HC, Hellstrom WJ, Murthy SN, Kadowitz PJ. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the lung for pulmonary hypertension. Methods Mol Biol. 2010;610:309–21. doi: 10.1007/978-1-60327-029-8_18. [DOI] [PubMed] [Google Scholar]
- 168.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: Efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–50. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 169.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–5. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 170.Janssens SP, Bloch KD, Nong Z, Gerard RD, Zoldhelyi P, Collen D. Adenoviral-mediated transfer of the human endothelial nitric oxide synthase gene reduces acute hypoxic pulmonary vasoconstriction in rats. J Clin Invest. 1996;98:317–24. doi: 10.1172/JCI118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Budts W, Pokreisz P, Nong Z, Van Pelt N, Gillijns H, Gerard R, et al. Aerosol gene transfer with inducible nitric oxide synthase reduces hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 2000;102:2880–5. doi: 10.1161/01.cir.102.23.2880. [DOI] [PubMed] [Google Scholar]


