Abstract
Drug trials in neonates and children with pulmonary hypertensive vascular disease pose unique but not insurmountable challenges. Childhood is defined by growth and development. Both may influence disease and outcomes of drug trials. The developing pulmonary vascular bed and airways may be subjected to maldevelopment, maladaptation, growth arrest, or dysregulation that influence the disease phenotype. Drug therapy is influenced by developmental changes in renal and hepatic blood flow, as well as in metabolic systems such as cytochrome P450. Drugs may affect children differently from adults, with different clearance, therapeutic levels and toxicities. Toxicity may not be manifested until the child reaches physical, endocrine and neurodevelopmental maturity. Adverse effects may be revealed in the next generation, should the development of ova or spermatozoa be affected. Consideration of safe, age-appropriate tablets and liquid formulations is an obvious but often neglected prerequisite to any pediatric drug trial. In designing a clinical trial, precise phenotyping and genotyping of disease is required to ensure appropriate and accurate inclusion and exclusion criteria. We need to explore physiologically based pharmacokinetic modeling and simulations together with statistical techniques to reduce sample size requirements. Clinical endpoints such as exercise capacity, using traditional classifications and testing cannot be applied routinely to children. Many lack the necessary neurodevelopmental skills and equipment may not be appropriate for use in children. Selection of endpoints appropriate to encompass the developmental spectrum from neonate to adolescent is particularly challenging. One possible solution is the development of composite outcome scores that include age and a developmentally specific functional classification, growth and development scores, exercise data, biomarkers and hemodynamics with repeated evaluation throughout the period of growth and development. In addition, although potentially costly, we recommend long-term continuation of blinded dose ranging after completion of the short-term, double-blind, placebo-controlled trial for side-effect surveillance, which should include neurodevelopmental and peripubertal monitoring. The search for robust evidence to guide safe therapy of children and neonates with pulmonary hypertensive vascular disease is a crucial and necessary goal.
Keywords: drug toxicity, pediatrics, pulmonary vascular disease, pulmonary arterial hypertension, pulmonary hypertension with increased pulmonary vascular resistance
Pulmonary hypertensive vascular disease (PHVD) is defined as pulmonary hypertension with an increased pulmonary vascular resistance (PVR). PHVD is a condition with many etiologies that occurs in patients of all ages from the fetus to the elderly. PHVD affects children as frequently and as severely as adults. Idiopathic pulmonary arterial hypertension (IPAH) is less common in childhood, but pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD) occurs more often.[1,2] The hallmark of PHVD is an increase in PVR which results ultimately in right ventricular (RV) failure and death. Current medical therapies may prolong life and reduce symptoms, but are not curative.
PHVD in children includes developmental abnormalities of the pulmonary vascular bed and is often a more heterogeneous condition than in adults.[3] However, IPAH, heritable PAH, PAH associated with CHD, pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease appear to involve the same biological pathways and have the same histological features in children as in adults. It would seem logical to infer that, for the most part, therapies will be equally efficacious in both adults and children, but this requires further study. Indeed, to date, adult PAH therapies have been used “off-label” in children and appear to have similar efficacy. Some PHVDs are unique to childhood, such as persistent pulmonary hypertension of the newborn (PPHN), bronchopulmonary dysplasia, lung hypoplasia and alveolar capillary dysplasia. Other PHVDs are more common in childhood, such as PHVD associated with unusual congenital cardiac and lung diseases and PHVD associated with chromosomal and genetic disorders. The pathophysiology of these diseases may be different from PHVD in adults and may involve pulmonary vascular maldevelopment, growth arrest, or deranged repair after in utero or neonatal injury. Indeed, abnormal pulmonary vascular reactivity may be determined by events as early as conception as suggested by the increased vascular reactivity of children conceived by assisted reproductive technology.[3]
Childhood is defined by growth and development. The lung and pulmonary vascular bed may continue to develop during at least the first eight years of life.[4] Renal and hepatic function and blood flow and metabolic systems such as cytochrome P450 enzyme activities change throughout early life.[5,6] Drugs, therefore, have the potential to affect children differently from adults, for example, accumulation of chloramphenicol because of immature glucuronidation causing the lethal gray baby syndrome.[5,7] Ambrisentan is cleared by glucuronidation.[8] Drugs such as carvedilol and sildenafil may be cleared faster in neonates and infants.[5] The adverse effects of drugs may have unique manifestations when given before the individual is fully grown or developed. The sequelae may affect children long term or lifelong. Genetics and comorbidities may interact with developmental changes, resulting in different drug clearance compared with adults. However, the collection of pharmacokinetic and drug clearance data in children has been limited to certain populations. Most of the data has been collected in neonates and children with critical illnesses who have indwelling lines that facilitate blood sampling. There is a paucity of drug clearance data in infants, in whom blood sampling is particularly challenging and in ambulatory children taking medication at home.[5]
Drugs used to treat all forms of PVHDs in children are given on an “off-label” basis and have been approved for use in adults first, with the exception of inhaled nitric oxide (iNO), which was approved for treatment of PPHN in 1999. The recent sildenafil trial was started years after we had started to give the drug to children with pulmonary hypertension.[9] Trials of pulmonary hypertension specific drugs have been designed mainly to study the drug effect in adults, although a few children > 12 years old have been included. This has been a realistic and pragmatic approach.
In developmental terms and to ensure inclusion of all individuals at risk of developmental adverse effects of drugs, the most appropriate approach is to consider a child as an individual who has not passed through puberty.
The specific therapeutic needs of children, as suggested by drug regulatory agencies in the United States of America and Europe, ought to drive investigation of safety and efficacy, whereas in practice pediatricians have explored whether drugs already on the market could be used to treat children. This is because, as pointed out by Rose and Della Pasqua, there is little consensus among pediatricians, drug regulatory agencies and the pharmaceutical industry in areas such as (1) accepted age-matched normal ranges for laboratory measurements, (2) requirements for the validation of clinical endpoints for the assessment of efficacy and safety and (3) standards for long-term safety monitoring and pharmacovigilance.[10]
In this manuscript, we shall focus on the differences between adults and children and try to answer the question, “How can we best evaluate a new drug for treating PHVD in children?” We shall discuss ethical issues, safety and long-term pharmacovigilance,[11] intrinsic pediatric specific issues to be considered in conducting clinical trials in children, how to evaluate clinical status and disease progression or clinical improvement in children with PHVD, the need for long-term monitoring and lastly, endpoints in clinical trials.
ETHICAL CONSIDERATIONS
A recent review of cancer drug research in adolescents concluded that clinicians often justify not involving adolescents in research discussions by referring to best interest arguments (adolescents’ incompetence, proxy consent from guardians and investigator integrity), although this is not in keeping with ethical principles or legal regulations.[12] It is assumed that all new drugs developed for use in patients with pulmonary vascular disease will undergo trials in children.
The issue of “informed consent” is challenging due to age and cultural inequalities. In contrast to most adults, children enter clinical trials through consent of their parents or guardians. In older children who have attained an appropriate developmental level, most ethics review boards will also require the “assent” of the children in addition to parental (or guardian) consent. In all but the youngest child, careful verbal and written explanations are required. International standards of children's rights are not uniformly applied throughout the world and will require careful scrutiny if drug trials are undertaken in countries where, for instance, child labor is a common occurrence. UNICEF estimates that 150 million children aged between five and 14 years are engaged in child labor.[13] A review of published pediatric randomized drug trials in developing countries suggested that in countries with a low or medium Human Development Index, institutional review board approval or safety monitoring was mentioned infrequently.[14]
SAFETY ISSUES AND LONG-TERM PHARMACOVIGILANCE
It is important to monitor specific features known to become abnormal in adults taking the drug and if laboratory and animal studies indicate risk. A trial of endothelin-receptor antagonists (ERAs), for example, would include close monitoring of liver function. Long-term monitoring of endocrine function and reproduction is indicated since these drugs are teratogens and their effect on fertility is uncertain. Pregnancy is contraindicated in women taking ERAs. With any drug, long-term toxicity could affect neurodevelopmental outcome, delayed growth, delayed or accelerated puberty, fertility and even the child's offspring. Ambrisentan is cleared by glucuronidation and pharmacokinetic data and clinical evaluation suggest that it is safe and efficacious in children over three years of age.[8] The glucuronidation system is not fully matured until two and a half years of age[15] and suggests that ambrisentan be avoided in neonates and infants until more data are available.
By definition, childhood is a period of growth and development and yet few PHVD-targeted drug studies have evaluated the effects of treatment on long-term outcome outside of functional capacity and hemodynamics. The exceptions are the neurodevelopmental outcomes included in trials of iNO in preterm and term neonates.[11,16,17] Long-term follow-up in the pediatric sildenafil monotherapy trial revealed unsuspected differences in dose response between children and the outcome seen in short-term studies in adults. The long-term outcome in children was based on the dose the child was randomized to at the start of the 16-week randomized controlled trial (RCT).[9] At three years of age, the mortality was higher in the group randomized to high dose, regardless of whether the drug was up-titrated in the blinded extension to the high-dose group. Although the reasons for this are yet to be fully elucidated, these data demonstrate the importance of long-term blinded data in efficacy and safety assessments and potential different responses of children compared with adults. Unfortunately, there are no blinded dose long-term data for adult sildenafil monotherapy as the majority were treated with the high dose once they entered the open-label extension study (regardless of their dose in the 12-week RCT) anticipating that the high dose would be most efficacious (which was not shown by the 12-week RCT study).
The importance of long-term follow-up is illustrated by the furosemide ototoxicity story. In the mid-1990s, it was found that neonates with birth asphyxia, including some with PPHN, had late-onset progressive sensorineural hearing loss first detected at two to four years of age. Further studies suggested that neonates with PPHN and congenital diaphragmatic hernia who were treated with extracorporeal membrane oxygenation (ECMO) were found to have the same late-onset progressive sensorineural hearing loss.[18] Among the children who underwent the Norwood procedure for hypoplastic left heart syndrome between 2002 and 2007, sensorineural hearing loss occurred in 28%.[19] Robertson et al. suggested that the sensorineural deafness was related to the use of bolus doses of furosemide and particularly the rate of furosemide administration.[19] Preliminary results from follow-up studies suggest that in the later cohorts of children, furosemide infusions were given slowly and the incidence of sensorineural deafness had decreased. This story illustrates the vulnerability of developing systems to unique injury with late onset years after the event and that the adverse effect may be related not only to the drug directly, but also to other factors related to administration and preparation. Careful and prolonged follow-up is required to avoid missing important adverse events. If detection is delayed, we shall have missed the opportunity and would be unable to prevent further exposure to children in a timely fashion. The treatment of children with recombinant growth hormone, particularly at high doses, has been associated with adult-onset cancers and cerebrovascular hemorrhage,[20,21] illustrating the importance of long-term follow-up of patients first treated as children.
INTRINSIC PEDIATRIC-SPECIFIC ISSUES TO BE CONSIDERED IN CONDUCTING CLINICAL TRIALS IN CHILDREN
Diagnostic category of PHVD and patient phenotype
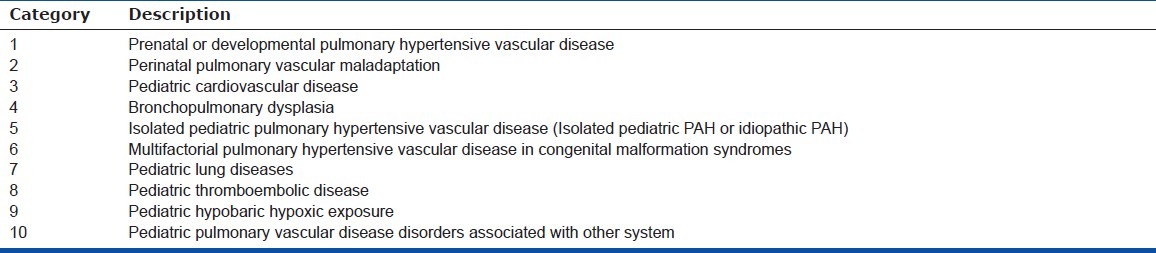
The phenotype and genetic associations of PHVD differ in children from adults as outlined in the Panama Classification[22] (Fig. 1, Table 1). For example, it has frequently been observed that children with Down syndrome are more prone to PHVD. They comprise a significant portion of patients at most pediatric pulmonary hypertension centers and so it is important to acknowledge the association. However, there are no trials which have evaluated whether there are treatment differences to targeted therapy in Down syndrome. In part, this is due to the small number of patients at any one center, a problem in all pediatric trials. However, it seems clear that the development of national and international databases of children with PHVD with common entry criteria and which use a diagnostic classification reflective of childhood PHVD is critical. The results of a number of such databases have been published recently.[2,23–27.
Figure 1.
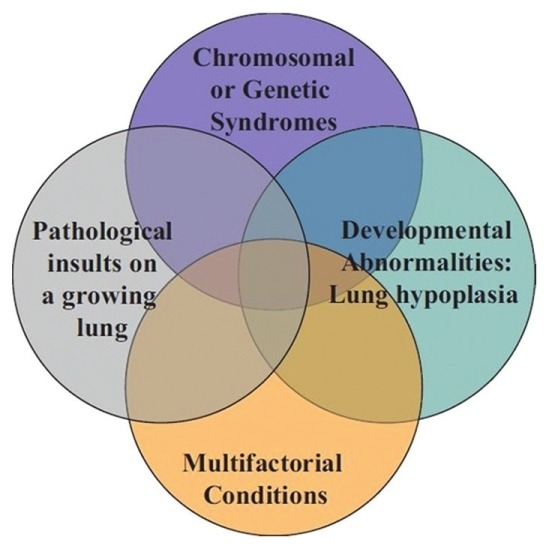
Venn diagram illustrating the heterogeneity and multifactorial elements in pediatric pulmonary hypertensive vascular disease. Originally from reference[85] with permission.
Table 1.
The broad schema of 10 basic categories of Pediatric Pulmonary Hypertensive Vascular Disease
Clinical trials with small sample sizes
It is difficult to recruit large numbers of children with PHVD into a clinical trial. Classical sample size calculations often lead to unrealistic expectations of the number of children who can be recruited. It is beyond our scope to discuss the statistical and epidemiological aspects of small sample approaches. However, Offringa and van der Lee[28] have described 12 possible approaches: (1) use of one-sided instead of two-sided hypothesis testing, (2) inflation of the minimal clinically relevant difference, (3) composite outcomes, (4) surrogate outcomes, (5) improved reproducibility of outcome measurements, (6) repeated measurements, (7) the crossover design, (8) matching or stratification, (9) analysis of covariance instead of simple comparison of outcomes in two groups, (10) response-adaptive design, (11) conducting an underpowered trial for a later meta-analysis, and (12) the prospective meta-analysis approach.[28]
It is important to maximize the information obtained from the few children who are enrolled. One intriguing concept is “N-of-1” or single-subject trials. These have created much interest and are a potential way to maximize data in rare diseases with few subjects. The goal of an “N-of-1” trial is to determine the best intervention for an individual using objective data driven criteria.[29] “N-of-1” trials challenge the dogma of RCTs that treatment effects can be generalized to all patients in a trial population. “N-of-1” trials suggest that certain individuals may benefit from treatment shown to be inferior in a population trial. In “N-of-1” trials, multiple, randomized, even blinded, crossover trials can be carried out in a single subject to measure efficacy in a specific individual. Multiple measurements in one individual have less variance and therefore more power to detect benefit or lack thereof. The concomitant use of ambulatory monitoring and subject interaction through cell phone and wireless technology increases patient involvement and is a convenient way to collect large amounts of data. The benefit of a carryover effect of a drug (or the disease-modifying effect) may be demonstrated in the exercise test or measurements taken following a crossover. “N-of-1” trials might be used to investigate add-on therapy to stable patients with PHVD already receiving background treatment. “N-of-1” trials have been performed in children with chronic arthritis, attention-deficit disorder and cystic fibrosis.[30–32] “N-of-1” trials remain untried in children with PHVD.
Pharmacokinetics
Pharmacokinetic modeling and physiologically based simulation afford other opportunities to reduce sample size and maximize the information obtained from children in clinical studies[6] (Fig. 2).
Figure 2.
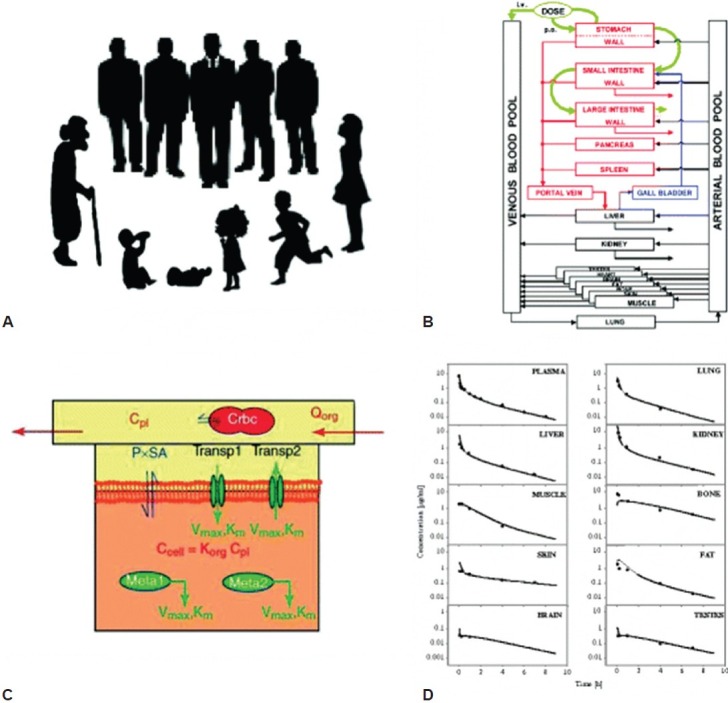
Illustration of the concept for building a physiologically based pharmacokinetic model modified according to Willmann et al. (2003). (A) Organisms, for example, human beings of different ages or populations, are the basis for the model. (B) The organism is divided into a number of compartments, each representing a single organ. To describe the distribution of compounds in the body, the organs are connected via their arteries and veins to the arterial and venous blood pool. Inter-compartmental mass transport occurs via organ-specific blood flow rates. The organs are mathematically connected. (C) Division of each organ into three sub-compartments representing the vascular (with blood cells), interstitial and cellular space. The interstitial space is assumed to be in direct contact with the plasma. The exchange of substances between the cellular and interstitial compartment can occur by permeation across theembranes via passive diffusion as well as active influx and efflux transport processes by saturable Michaelis-Menten (MM) kinetics (parameters: Vmax, Km). Metabolization of substances (Meta1, Meta2) occurs via active enzymes (MM kinetics). Finally, the model consists of a large number of coupled differential equations. (D) Output of the model: Concentration time curves for the substances. Shown are simulated and observed ciprofloxacin concentrations in various organs after intravenous application of ciprofloxacin 5 mg/kg to a rat. Originally from references[6,86] with permission.
In the pediatric population, growth and developmental changes can influence absorption, distribution, metabolism and elimination and lead to changes in pharmacokinetic measures and/or parameters. These changes in drug concentrations can lead to changes in the effectiveness and safety profile of a drug and thus require adjustment in dose within different age groups. Pharmacometric tools such as (1) population pharmacokinetic/pharmacodynamics (POP-PK/PD) and/or (2) physiology-based pharmacokinetic (PBPK) modeling can help to translate maximal information gathered in studies in adult patients, as well as for extrapolating data from older to younger children:
POP-PK/PD modeling techniques explore measurable physiological and pathophysiological sources of variability (e.g., bodyweight, age, glomerular filtration rate, co-administration of other drugs that can affect the concentration of the study drug). Such physiological and pathophysiological sources can influence the dose-drug concentration-effect relationship.
PBPK models use a priori information about the anatomical and physiological structure of the body, as well as certain physicochemical properties of the drug to predict the absorption, distribution, metabolism and excretion (ADME) characteristics and the resulting PK of the drug. This approach can help interpolate and extrapolate between different populations (e.g., adult and pediatric patients). The applicability of the data depends on disease similarity between adults and children, the availability of PK, PD, efficacy and safety information in adults, as well as a detailed understanding of the drug ADME.
Based on an understanding of these concepts, the following approaches are proposed for product approach and labeling in pediatric medicines and are supported by the Federal Drug Agency of the USA (FDA) and European Medicines Agency (EMA).[33]
The PK-only approach can be selected when the disease and the exposure-response relationship in the pediatric population are anticipated to be similar to that in adults. The PK study in pediatric patients is performed to identify doses resulting in a similar exposure as known to be effective and safe in adults
The PK/PD approach is considered suitable if the disease characteristics and the intervention/target PD parameters are expected to be similar between adult and pediatric patients, but the exposure-response relationship in the pediatric population is not sufficiently known. The goal of the study is to adequately characterize and compare the exposure-response relationship in pediatric compared to adult patients based on defined clinical measures (PD-measurements, symptoms, biomarkers, outcomes, etc.). The established exposure-response relationship in the pediatric population can then be used to select appropriate doses/dosing schedule for children
The PK and efficacy approach is required if the disease is exclusively occurring in the pediatric population and the disease process is unknown and/or different to that in adults and/or a predictive biomarker for efficacy is not available. In this case, studies investigating appropriate doses leading to a defined exposure-response relationship in the pediatric population need to be carried out in a first step, followed by demonstration of safety and efficacy in a second step.
POP-PK/PD and PBPK modeling are first used to plan a pediatric study, especially in the case of a “first in pediatric study,” including study design, recommended doses to be tested and informed selection of times for sparse blood sampling for PK, biomarkers, etc., During interim analysis and/or after completion finalization of a pediatric study, existing POP-PK/PD and/or PBPK models can be refined or established de novo. POP-PK/PD modeling is usually used to evaluate key pharmacokinetic measures such as area under the curve, peak serum concentration, clearance, volume of distribution, half-life, etc., including their intra- and inter-subject variability. PD endpoints can be used to establish some understanding of concentration-response relationships for both efficacy and toxicity. In contrast, the application of “refined” PBPK models by using information gathered in the pediatric study is more focused on the prediction of the influence of the effect of intrinsic (organ dysfunction, genetics, etc.) and extrinsic (e.g., drug-drug interactions) factors on drug exposure not yet studied in detail.
Unfortunately, the number of scientists with dedicated PBPK know-how and practical experience in drug development is limited. Standard approaches and with full transparency for review by regulatory agencies, are not established routinely. Therefore, the use of PBPK was favored by a recent FDA advisory committee, but as a recommendation, not a requirement.
In summary, pharmacometric modeling techniques such as POP-PK/PD and PBPK help to efficiently and quantitatively translate information from adult to pediatric patients, especially when target disease characteristics are similar between the populations, as is the case for some types of PHVD. The use of POP-PK/PD and PBPK in the planning and evaluation of pediatric studies may prevent duplication of studies, reduce the burden of unnecessary blood sampling and testing of ineffective or toxic doses. This technique has been used to model dosing with sildenafil in children.[6] The FDA recommends clinical trial simulation as a routine approach to assess the appropriateness of trial designs in pediatric drug development.[34]
COMPOUNDING AND DOSING OF TARGETED MEDICATIONS
The formulation and dosing of drugs require more thought and a different emphasis in children.[35] Children vary in age and size, and so dose ranges will likely be broader. Tablets need to be scored or specifically made for children so that intermediate doses can be given easily and changed with growth without the risk of dosing errors. Many children find large tablets difficult to swallow and the 3-mm-diameter “minipill” increases the percentage of doses swallowed.[36] In addition, specific amounts of drug in tablet form likely will produce a larger range in drug levels per weight; dose ranging studies need to take into account the safety and efficacy of this greater variability of drug concentration and exposure.
There are a number of imaginative but underutilized solutions to overcome the reluctance of children to take medications, improve the dosing, decrease parental anxiety and avoid the parental quandary of the partial spit-out dose. These include single-use prepackaged spoons, dosed pacifiers and calibrated droppers. The calibrated drinking straw, which delivers a prespecified dose, universally appeals to children and can improve compliance.[37]
The availability of stable liquid formulations provides a more accurate method of titrating dose to weight for less variability of drug concentrations and is helpful in treating neonates and smaller children. A stable formulation decreases the requirement of parents to make frequent visits to the pharmacy and spend time on filling of prescriptions. Compounding of the formulations is important. Children may be more accepting of pleasantly flavored medicine. On the other hand, unpalatable or bitter-tasting compounds will be refused. The regular administration of medicine to an unwilling infant or child is a huge source of parental stress.
Interactions of medicines with common drinks and foods of childhood and feeding formulas of babies may be a source of unexpected changes in metabolism.[5] Furthermore, the excipients (e.g., solvents, vehicles, emulsifiers and preservatives) used in formulating drugs for neonates, particularly premature infants, may expose them to concentrations of toxic substances such as ethanol and propylene glycol that have lower safety thresholds in children than adults.[38,39]
A detailed concept paper on the formulations of choice for the pediatric population has been adopted by the EMA and is available on their website http://www.emea.europa.eu/ema.
EVALUATING CLINICAL STATUS AND DISEASE PROGRESSION/IMPROVEMENT IN CHILDREN WITH PVD
Standard measures of assessment
The World Health Organization (WHO) functional classification (an adaptation of the New York Heart Association heart failure functional classification) for pulmonary hypertension is unsuitable for use in neonates, infants and young children and misses important information about symptom severity.[40] The Panama Functional Classification, developed by the pediatric taskforce of the Pulmonary Vascular Research Institute, is designed specifically for children. The Panama Functional Classification divides functional class into four groups equivalent to the WHO Classes I, II, III and IV[40] (Tables 2–7). However, between birth and age 16 years, there are five age distributions with developmentally appropriate criteria for assessing symptoms. For younger children, for example, criteria are included based on feeding and the need for supplementing feeds; in older children, school days lost due to illness are assessed. The Panama Functional Classification assesses symptoms and performance and therefore would most likely be acceptable to drug agencies without validation or comparison to the WHO I-IV pulmonary hypertension functional classification. However, the use of both systems would be beneficial for older children, particularly when they transition from care by a pediatrician to an adult medicine specialist.
Table 2.
Pediatric Functional Classification for children aged 0 – 0.5 years

Table 7.
What to expect of children in Functional Class I

Table 3.
Pediatric Functional Classification for children aged 0.5 – 1 years
Table 4.
Pediatric Functional Classification for children aged 1 – 2 years
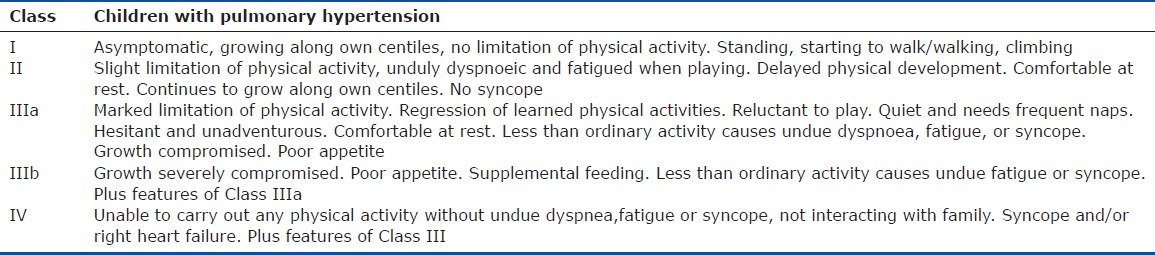
Table 5.
Paediatric Functional Classification for children aged 2 – 5 years
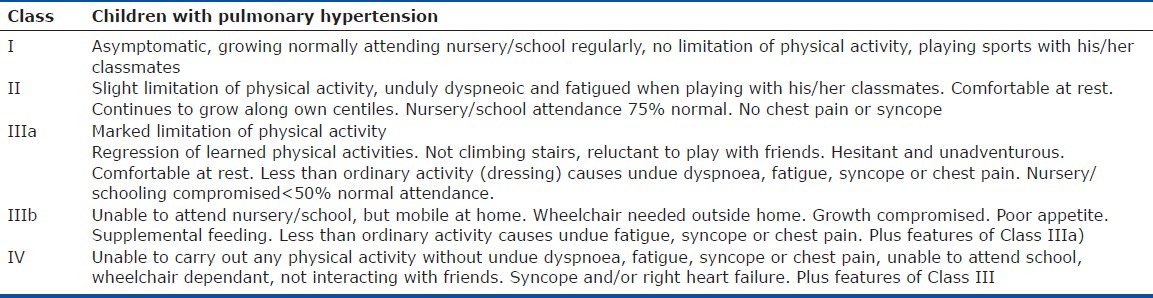
Table 6.
Pediatric Functional Classification for children aged 5 – 16 years

Growth
Appropriate height and weight for age and appropriate incremental gains are general predictors of health in a child. Recent observations from the UK Network would suggest that most children with idiopathic or isolated PHVD are below the 5th percentile for height and weight and that, with current therapy, most children do not exhibit catch-up growth.[1] This may not hold true for all populations and may be influenced by prevailing nutrition and childhood obesity.[24] Although height and weight may not be discriminatory enough as endpoints for drug trials, it should be noted that failure to gain weight or grow taller is a sign of illness. Thus, longitudinal changes in height and weight should be included in the assessment of childhood PHVD.
Exercise Capacity
Distance walked in six minutes. Children as young as four years of age may be able to undertake a measured test of the distance walked in six minutes (6MWD). However, it is not until six to eight years of age when one may expect a reliable performance. Even then, children under 10 years of age may be distracted easily, which makes interpretation of the test more difficult. Normal values and percentiles for the 6MWD in children aged 4-17 years have been published.[41–44] Studies are underway in India to establish normal values for children, as there may be some variability in the distance walked in different countries and cultures. However, comparisons of 6MWD results made before and after intervention in the same child should reduce the importance of these differences. Without normal values for children in different countries and cultures, it may not be possible to express the 6MWD as percent-predicted 6MWD. The utility of the 6MWD appears less useful if the child can walk more than 300 m; if this is the case, cardiopulmonary exercise testing (CPET) is more useful. This is similar to the “ceiling effect” observed in adult PAH trials. Nevertheless, it is clear that this is an effort-dependent test with an inherent variability even on successive tests that is inversely proportional to the child's level of maturity. We note that the EMA, after extensive discussion, suggests that the distance walked in six minutes may be used as a primary endpoint in children > 12 years old.
Ambulatory physiological monitoring. Innovations in ambulatory physiological monitoring offer the potential to collect and evaluate physiological data in response to individual specific activity at home for clinical studies. For example, inexpensive “bracelets” that can transmit vital signs to cell phone technology provide the possibility of monitoring continuous heart rate, ventilation and activity monitoring, as well as the response to spontaneous or structured exercise. The 6MWD could, for instance, be performed before and after therapy, more often and at home and without the vagaries imposed by a child's visit to the hospital. The challenges of structured exercise tests in children with physical and neurodevelopmental disabilities may be overcome by evaluation of the effect of therapy on their activity at home. Pedometers or accelerometers seem suited ideally to pediatrics to measure changes in physical activity in response to therapy. Heart rate, pedometer and accelerometer recordings correlated with oxygen consumption and predicted energy expenditure in children.[45]
Cardiopulmonary exercise testing. CPET, especially with measurement of maximal oxygen consumption (VO2 max), ventilatory efficiency (VE/VCO2) and end-tidal CO2, is only feasible in children if they are both tall enough to use the equipment and developmentally able to follow protocols reliably. Many centers suggest a minimum age of seven years before attempting CPET. However, the age at which, for instance, a bicycle test could be performed would be lower if the equipment was modified appropriately to accommodate children (e.g., adjustable saddle to pedal lengths, smaller mouthpieces, etc.). Paradoxically, although more children than adults ride a bicycle, clinical bicycle exercise equipment is unsuitable for most children to use. Treadmill testing is an alternative and with training has been performed in children as young as seven years old. Ventilatory efficiency (VE/VCO2 slope) should be independent of cooperation and may prove useful in the cardiopulmonary assessment,[46] particularly RV to pulmonary artery coupling, of children with PHVD. Similarly, end-tidal CO2 measurements during exercise may prove useful.
Pulmonary vascular hemodynamics. Deterioration or improvement in hemodynamics is most commonly used in clinical practice to assess disease severity including response to therapeutic interventions, especially in children too young to complete formal testing of exercise capacity or tolerance in most, but not all, countries.
Echocardiography. Echocardiography offers useful information, but has the significant drawback that not all information is available in all patients with the exception of septal position. Septal position is used often clinically in infants and is useful in day-to-day clinical practice, but lacks the precision required in a clinical trial.[47,48]
Cardiac catheterization. Clinicians regard the use of serial changes in pulmonary artery pressure and pulmonary vascular resistance index (PVRI) as the most objectively reassuring evidence of the state of the disease. The PVRI reflects both the transpulmonary gradient and the cardiac index and offers additional information over and above the pulmonary artery pressure. We presume that the pathophysiological process that causes an increased pulmonary artery pressure and/or PVRI results in the symptoms and impaired exercise capacity that are hallmarks of the disease. An analysis of 13 clinical trials in adults with IPAH or heritable PAH suggested that, as a group, a change in PVRI between baseline and after drug or placebo administration showed a significant negative correlation with change in distance walked in six minutes, although for individual patients, correlation of PVRI and distance walked in six minutes was poor. Furthermore, change in PVRI showed a significant relationship in 12 out of 13 trials with the 95% confidence intervals overlapping among the majority of trials.[49] The disadvantage of PVRI as an endpoint is that the data must be obtained by invasive cardiac catheterization, which carries a higher risk in pediatric PHVD than in adult PAH.[50,51] Almost all children under the age of 15 years require either general anesthesia or conscious sedation to tolerate a cardiac catheterization. The drugs used for sedation, the effects on hemodynamics and ventilation and the response to these changes may confound the hemodynamic measurements obtained. The cardiac catheterization data need to be reviewed and interpreted carefully by an expert in caring for children with PHVD. However, it is generally agreed that in centers with experience in managing children with PHVD, cardiac catheterization provides extremely useful information with an acceptably low risk and high benefit. We need to define when and how often, a cardiac catheterization is the standard of care. There is consensus that the standard of care in most instances requires a cardiac catheterization to establish the diagnosis and severity before starting therapy, exceptions including PPHN (these patients generally only require short-term treatment) and very sick patients, at least until the latter are stabilized with therapy. All patients receiving long-term therapy should have had cardiac catheterizations at some time to establish the diagnosis and severity of PHVD and the response to treatment. Criteria for undertaking follow-up cardiac catheterization in children are yet to be established. Most would agree that cardiac catheterization is justified during follow-up after beginning or changing therapy. Cardiac catheterization is also justified if patients are failing therapy or have new symptoms such as syncope, but not at the expense of unduly delaying additional therapeutic interventions. There is value also in repeat catheterizations of children who have demonstrated acute pulmonary vascular reactivity, as this response may diminish with time.[52] The more difficult scenario is the child with IPAH who appears to be doing well even though the PVRI may be increasing and is not receiving maximum therapy. We need to recognize these children and increase therapy before they deteriorate clinically. The best method to detect disease deterioration, before clinically evident, is a cardiac catheterization. The availability of multiple drugs and the accumulating data that early treatment impacts disease progression[53] and that a goal-oriented approach[54] to treatment may improve outcome are strong arguments in favor of frequent surveillance of children by cardiac catheterization. Therefore, many children require recatheterization on clinical grounds, usually within a year of initiation of oral therapy. It is important that children with PHVD undergo invasive procedures only at experienced pediatric PH centers with pediatric anesthesiologists experienced in the care of children with PHVD. Careful observation postanesthesia is imperative to minimize complications during recovery. If these caveats are adhered to, then cardiac catheterization is invaluable in making the correct diagnosis and assessing response to therapeutic interventions with a very favorable risk-benefit profile. Nevertheless, it would seem prudent to link changes in PVRI with endpoints that indicate a meaningful symptomatic benefit to the child. At present, the only tool we have to assess PHVD in children too young to undertake formal assessment of exercise is the Panama Functional Classification.[40] Cardiac catheterization remains the only objective and universally applicable method to assess PHVD in all neonates, infants and children. Unfortunately, we lack a reliable, portable and noninvasive measure of pulmonary artery pressure akin to the systemic blood pressure cuff and we have no validated means of assessing PVRI noninvasively. This highlights the need for continued research in this area.
Patient quality of life and parental and family stress. Quality of life and occurrence of depression are important aspects of all chronic illnesses. Complex time-consuming therapy, such as continuous parenteral (intravenous or subcutaneous) prostacyclin, may have beneficial or adverse effects on the quality of life of the patient and the patient's family. An important difference between adults and children is that children are dependent completely on an adult for their care and the impact of the disease and therapy will impact not only the patient, but also the caregiver, siblings and family unit. Parents of affected children with inherited PAH may be burdened by guilt associated with transmission of the gene, especially if the parent is an asymptomatic carrier. Mullen et al. (in press Chest, 2012) reported that children with PHVD scored significantly less than healthy children on the Pediatric Quality of Life Inventory.[55] Severe illness in a child affects the parents as 34% of parents met the criteria for a presumed psychiatric diagnosis and parents reported encountering stressful events more frequently than children with cancer. They reported also resorting to coping mechanisms more often than a sample of parents without children with PHVD, according to Mullen et al. (in press Chest, 2012).
The Pediatric Quality of Life Inventory is a tool that may be administered to parents to assess physical, emotional, social and academic functioning of their child.[56] Parental stress maybe assessed with the Pediatric Inventory for Parents and is a reliable and validated caregiver measure assessing parenting stress related to caring for a child with an illness.[57] Parental adjustment to their child's illness maybe assessed by the Brief Symptom Inventory (BSI) and is a well-accepted method of screening for psychological problems in adults.[58] Parental coping strategies may be quantified by the Ways of Coping Questionnaire (WCQ). WCQ is a reliable and validated instrument that measures the use of coping strategies.[59] Family function may be assessed with the Global Assessment of Relational Functioning Scale (GARF). GARF is a clinician-rated measure of the relational context in which patients live. This measure comprises a single score ranging from 0 to 100, analogous to the DSM-IV Global Assessment of Functioning Scale used to assess individual psychosocial functioning.[60]
The Multi-Attribute Health Status Classification (MAHSC) determines outcome in six health domains (sensation, mobility, emotion, cognition, self-care and pain) with excellent inter-rater agreement between parents, medical staff and investigators.[61] It is ideally suited to neonatal follow-up, but has been used following pediatric intensive care admission.[62] It may be used as a parent-report surveillance tool which would decrease the need for every child in a study to be re-evaluated by an expert and would focus recall only on those with abnormal parent reports. This would increase the cost-effectiveness of follow-up. All of these tests have yielded important information on the quality of life of children with PHVD and their families (Mullen et al. in press Chest, 2012).
ADDITIONAL MEASURES OF ASSESSMENT
Biomarkers
The use of biomarkers as surrogates for symptoms, functional capacity, or outcome is appealing and would address many of the drawbacks of comparing children of different ages. However, skepticism for this approach has yet to be completely allayed. Of all the biomarkers used, those which reflect RV function, such as brain natriuretic peptide (BNP or N Terminal pro BNP), may be the most promising. Considerable data on age-dependent differences and alterations in levels with different conditions are emerging.[63,64] Several investigators have concluded that BNP or N Terminal pro BNP levels have value in the assessment of PHVD in children. BNP correlates with WHO functional class, but has limited sensitivity to predict death or need for transplantation.[65] BNP and N Terminal pro BNP have been investigated in children with PHVD and unit for unit increases in the logarithmically transformed value are associated with increases in PVRI. N Terminal pro BNP correlates with exercise data better than BNP and showed less patient variability over time. In this study, no patient died or was transplanted; therefore, the relationship with adverse events could not be demonstrated.[66] BNP may also decrease with successful iNO therapy in PPHN and predict the occurrence of rebound pulmonary hypertension on withdrawal of iNO.[67] The consensus is that BNP and N Terminal pro BNP have value especially in describing cohorts of patients with PHVD, but it remains uncertain if they are sufficiently sensitive to predict individual outcomes or response to therapy. In addition, children may have extremely elevated PVRI without right heart failure or increased BNP and thus both the 6-Minute Walk Test and BNP could be insensitive tests in these children. Plasma proteomics and circulating progenitor cells may have the potential as biomarkers in childhood PHVD, but are largely unexplored.[68–71]
RV-pulmonary artery coupling
The need for comprehensive functional assessment of RV-pulmonary artery coupling was reinforced at the recent NIH-sponsored workshop, “Improving Outcomes for Pulmonary Vascular Disease” held on August 11-12, 2011, which was convened to identify critical areas for future research. The need for better measures of RV-specific functional performance was identified. Further, there was clear recognition of the differences between the adult (fully developed) and pediatric (developing) pulmonary circulations. It is increasingly evident that the pediatric RV-pulmonary vasculature axis under hypertensive load represents an extraordinarily unique system, not only for its intrinsic importance to clinical outcomes but also for its biologically and mechanically adaptive capabilities, many of which are only now beginning to be understood. Recent clinical work has highlighted the importance of pulmonary vascular stiffness in the progression of PH;[72–74] the inclusion of impedance and pulmonary vascular stiffness in outcomes analysis may be better than PVRI alone.[75–77]
Several other investigators, working primarily on adult patients with PH, but also in children,[77] have established also that the stiffness of lung elastic pulmonary arteries increases in several forms of pulmonary hypertension and that this change affects disease course.[74,78,79] Increased pulmonary vascular stiffness has been shown to stimulate production of vasoconstrictive and proliferative factors from distal pulmonary vascular cells.[80,81]
ENDPOINTS IN CLINICAL TRIALS
The optimal selection of efficacy endpoints in adult trials of PHVD is the subject of much discussion and debate and the same arguments apply to pediatric trials where selection of endpoints is even more difficult. Functional class should be a more reliable endpoint now, using the age-specific Panama classification of functional class, but determination of exercise capacity is still problematic in the young child and assessments of quality of life are second-hand, depending primarily on the parental report. Echocardiographic techniques carry the same caveats as in adult trials and can be less reliable in the very young or uncooperative child. Cardiac catheterization, although providing the most robust and objective data, carries a risk, however minimal and repeat catheterization to study drug effect unfortunately may not appear acceptable to either parent or ethics committee.
Selection of endpoints may be influenced also by the experience and training of trial sites. CPET in children, for example, can only be carried out reliably in experienced pediatric centers. Trials which include sites in developing countries can be complicated by issues of informed consent, reliable dosing, follow-up and the technical facilities of the laboratories involved.
Discussion about the pros and cons of single versus composite endpoints applies to children as well as adults. Time to clinical worsening has become a useful endpoint in adult trials, but events signifying clinical worsening are low in children in the short term. Trial times lasting for a minimum of one year would be required if these criteria were to be used. Whether novel composite scores for children incorporating pediatric specific criteria could be developed and utilized for time to clinical worsening or improvement in children in long-term studies is worth exploring. Clear, unambiguous events such as death or lung transplantation are uncommon events in short-term drug trials in children, but alternatives, such as need for hospitalization, may be soft endpoints and open to wide interpretation.
The need for long-term monitoring
The detection of unsuspected side effects should be considered when planning any trial in children, particularly those related to neurological and endocrine development.
Neurodevelopment
Neurodevelopment testing may be initiated in the neonatal period and incorporates standardized interpretations of results for all developmental stages through childhood, depending on the specific test administered. Outcomes are scored by cognitive, motor and sensory development. Extensive neuropsychological and cognitive testing can be performed as early as three to four years of age with good prediction of late outcome. These tests take two to three hours and can be applied from three to 17 years of age. Neurodevelopmental testing in the Alberta high-risk therapies cohort group is administered at six months (neurological examination with Alberta Infant Motor Scale (AIMS) and hearing check), 18 months (Bayley scales of infant development, hearing and cognitive testing), three to four years and eight years.[82] The financial burden of this amount of high-risk follow-up would vary by country and individual center. In Alberta, each evaluation costs between $600 and $1,300. In young children, the Bayley scales of infant development have been used, but recently questions have been raised concerning their prediction of ultimate abilities. These scores are considered by some to be too dependent on socioeconomic background.
Endocrine status
Long-term monitoring will be required to determine whether current and future PHVD-targeted drugs affect the patient's hormonal status, fertility and/or fetal development. The development of secondary sexual characteristics in a staged fashion is an important marker of normal childhood development. Tanner staging of males and females is an accepted standard of evaluation in both clinical practice and clinical research. Pubertal timing is determined by maternal and paternal height and peak growth velocity, as well as linear growth. Laboratory studies can predict central puberty by LH/FSH ratios; additionally, ultrasensitive assays are available that begin to increase in children as young as three to four years of age and offer the possibility of detecting an abnormal hormone status before the onset of puberty. Inhibin B is a marker of Sertoli cell function and correlates with sperm counts in adults; it may be pertinent to undertake follow up studies of children exposed to ERA's as they are associated with reduced sperm counts, reversible on drug discontinuation, in 4% of studied subjects (Tracleer product Monograph). Anti-Mullerian hormone may be used in the same way to assess female development. Whether current or future PH-targeted drugs affect fertility may have to wait until the exposed children reproduce. Teratogenicity can be assessed only by follow-up, which includes offspring evaluation. Devastating effects of drugs given to mothers on their offspring are well documented. Pregnancy is contraindicated generally in women with PHVD and in those taking ERAs.
We recommend that future drug trials in children should be for longer periods (three to five years) with at least long-term extensions of different and blinded doses. We acknowledge the economic burden of long trials, but pharmacovigilance to adult life is the ideal.
CONCLUSIONS
Drug trials in neonates and children with PHVD pose unique but not insurmountable challenges. The recent development of the Panama Classifications of PHVD in Childhood facilitates a more precise definition of disease phenotype. The Panama Classification of Functional Class provides a robust, age-related means of assessing functional status from age 0 to 18 years, which will be important in all future clinical trials, especially if children too young to perform exercise testing are included. The Panama Classification of Functional Class is symptom based and will likely be acceptable to drug regulatory agencies as a more precise tool for evaluating children than the current WHO functional class designed for use in adults. Precise phenotyping and genotyping of PHVD that reflects the heterogeneity of childhood disease should improve our ability to detect treatment and outcome differences. However, such stratification may be limited by the numbers of children that can be enrolled, unless efforts are made to (1) increase the availability of trial results to a wider group of investigators as in the Starchild project,[83] (2) extend the judicious use of PK and PBPK modeling and simulations, and (3) trials can be designed to optimize and analyze data using novel statistical techniques obtained from small recruited populations.
The selection of clinically meaningful endpoints is difficult. Determination of PVRI by cardiac catheterization may be the best objective measure of disease status and response to therapy that can be used in all children entered into a trial, especially if children under six to eight years of age are included. However, the risks, albeit low in experienced centers and the timing of repeat cardiac catheterizations will need careful consideration. Provision of robust data for a clinical trial usually requires a baseline cardiac catheterization and a follow-up cardiac catheterization to assess response after new or additional treatments. We emphasize that changes in PVRI and/or other hemodynamic parameters such as those which reflect RV to pulmonary artery coupling should be linked to meaningful measures that show symptomatic benefit to the child. The EMA and FDA remain skeptical about the use of hemodynamic endpoints alone, primarily due to safety concerns; yet, objective data are required and all children cannot undertake exercise testing in the traditional manner. Morbidity/mortality endpoints are not considered feasible (event driven, high sample size, long study duration) in the short term. In these circumstances, composite outcome scores, which include functional class, exercise data, biomarkers and hemodynamics, may be the most appropriate primary or secondary endpoints in pediatric trials. Novel uses of physiological monitoring and accelerometers may be more suited to children, but are untried as of yet in children with PHVD.
Adverse event profiling of new drug clinical trials in children would include a minimum long-term assessment of neurodevelopment outcome (sensory, cognitive and motor) and peripubertal endocrine evaluation. Paradoxically, children with milder PHVD are likely to be treated longer with newer oral drugs and will, therefore, be most at risk for the development of late-onset adverse events. Repeated questionnaires with specific evaluation of reported abnormalities may be the most cost-effective and universally applicable method available at present. It is important to consider the parents and family unit when assessing the impact of new medications in children.
Age-appropriate compounds (small and/or easily scored tablets, dose variety and liquid formulations) may increase compliance in the trial, as well as reduce parental stress and improve the child's quality of life.
The lessons learned from the pediatric monotherapy sildenafil trial in treatment-naïve children,[9] the use of growth hormone,[20,84] and furosemide ototoxicity[19] make very strong cases for drug trials of at least three to five years to establish both long- and short-term efficacy and particularly to evaluate long- and short-term adverse effects in developing children. Further, outcomes may differ with combination therapy. Long-term continuation of blinded dose ranging after completion of the short-term trials is extremely important in adverse effect surveillance, particularly those that may affect adversely developmental, neurocognitive and endocrine systems.
Carrying out drug trials in children with PHVD is difficult, but the observations made in this paper suggest how we might advance solutions to the problem and demonstrate that successful pediatric clinical trials are feasible. The search for robust evidence to guide therapy of children and neonates with PHVD is a crucial and necessary goal.
ACKNOWLEDGMENTS
The authors are grateful for the ideas that arose out of discussions with Dr. Mark Palmert, Hospital for Sick Children, Toronto, Canada and Dr. Tom MacDonald, Ninewells Hospital and Medical School, Dundee, Scotland.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: A national cohort study. Heart (British Cardiac Society) 2010;96:1401–6. doi: 10.1136/hrt.2009.182378. [DOI] [PubMed] [Google Scholar]
- 2.van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, et al. Pediatric Pulmonary Hypertension in the Netherlands.Epidemiology and Characterization During the Period 1991 to 2005. Circulation. 2011;124:1755–64. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 3.Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–6. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 4.Reid LM. Lung growth in health and disease. Br J Dis Chest. 1984;78:113–34. [PubMed] [Google Scholar]
- 5.de Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol. 2011;7:935–48. doi: 10.1517/17425255.2011.577739. [DOI] [PubMed] [Google Scholar]
- 6.Laer S, Meibohm B. Study design and simulation approach. Handb Exp Pharmacol. 2011;205:125–48. doi: 10.1007/978-3-642-20195-0_6. [DOI] [PubMed] [Google Scholar]
- 7.Weiss CF, Glazko AJ, Weston JK. Chloramphenicol in the newborn infant. A physiologic explanation of its toxicity when given in excessive doses. N Engl J Med. 1960;262:787–94. doi: 10.1056/NEJM196004212621601. [DOI] [PubMed] [Google Scholar]
- 8.Takatsuki S, Rosenzweig EB, Zuckerman W, Brady D, Calderbank M, Ivy DD. Clinical safety, pharmacokinetics and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol. 2013;48:27–34. doi: 10.1002/ppul.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 10.Rose K, Della Pasqua O. Development of paediatric medicines: Concepts and principles. Handb Exp Pharmacol. 2011;205:111–24. doi: 10.1007/978-3-642-20195-0_5. [DOI] [PubMed] [Google Scholar]
- 11.Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 12.de Vries MC, Wit JM, Engberts DP, Kaspers GJ, van Leeuwen E. Pediatric oncologists’ attitudes towards involving adolescents in decision-making concerning research participation. Pediatr Blood Cancer. 2010;55:123–8. doi: 10.1002/pbc.22510. [DOI] [PubMed] [Google Scholar]
- 13.UNICEF. State of the world's children main report 2011. 2011 [Google Scholar]
- 14.Nor Aripin KN, Sammons HM, Choonara I. Published pediatric randomized drug trials in developing countries, 1996-2002. Paediatr Drugs. 2010;12:99–103. doi: 10.2165/11316260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.McRorie TI, Lynn AM, Nespeca MK, Opheim KE, Slattery JT. The maturation of morphine clearance and metabolism. Am J Dis Child (1960) 1992;146:972–6. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 17.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PJ, Merrill RJ, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 18.Robertson CM, Tyebkhan JM, Peliowski A, Etches PC, Cheung PY. Ototoxic drugs and sensorineural hearing loss following severe neonatal respiratory failure. Acta Paediatr. 2006;95:214–23. doi: 10.1080/08035250500294098. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CM, Alton GY, Bork KT, Joffe AR, Tawfik GC, Sauve RS, et al. Bilateral sensory permanent hearing loss after palliative hypoplastic left heart syndrome operation. Ann Thorac Surg. 2012;93:1248–53. doi: 10.1016/j.athoracsur.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: Preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97:416–25. doi: 10.1210/jc.2011-1995. [DOI] [PubMed] [Google Scholar]
- 21.Laron Z. Growth hormone therapy: Emerging dilemmas. Pediatr Endocrinol Rev. 2011;8:364–73. [PubMed] [Google Scholar]
- 22.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1:286–98. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart (British Cardiac Society) 2009;95:312–7. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 24.Barst RJ, McGoon DC, Elliot CG, Foreman AJ, Miller DP, Ivy DD. Survival in Childhood Pulmonary Arterial Hypertension Insights From the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management. Circulation. 2012;125:113–22. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 25.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: A registry study. Lancet. 2012;379:537–46. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraisse A, Jais X, Schleich JM, di Filippo S, Maragnes P, Beghetti M, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis. 2010;103:66–74. doi: 10.1016/j.acvd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Fasnacht MS, Tolsa JF, Beghetti M. The Swiss registry for pulmonary arterial hypertension: The paediatric experience. Swiss Med Wkly. 2007;137:510–3. doi: 10.4414/smw.2007.11895. [DOI] [PubMed] [Google Scholar]
- 28.Offringa M, van der Lee H. Small sample approach and statistical and epidemiological aspects. Handb Exp Pharmacol. 2011;205:181–202. doi: 10.1007/978-3-642-20195-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8:161–73. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber AM, Tomlinson GA, Koren G, Feldman BM. Amitriptyline to relieve pain in juvenile idiopathic arthritis: A pilot study using Bayesian metaanalysis of multiple N-of-1 clinical trials. J Rheumatol. 2007;34:1125–32. [PubMed] [Google Scholar]
- 31.Nikles CJ, Mitchell GK, Del Mar CB, Clavarino A, McNairn N. An n-of-1 trial service in clinical practice: testing the effectiveness of stimulants for attention-deficit/hyperactivity disorder. Pediatr. 2006;117:2040–6. doi: 10.1542/peds.2005-1328. [DOI] [PubMed] [Google Scholar]
- 32.Suri R, Metcalfe C, Wallis C, Bush A. Predicting response to rhDNase and hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol. 2004;37:305–10. doi: 10.1002/ppul.10442. [DOI] [PubMed] [Google Scholar]
- 33. EMA Guideline General Considerations for Pediatric Pharmacokinetic Studies for Drugs and Biological Products, EMA guideline. [Google Scholar]
- 34.Gobburu JV. How to double success rate of pediatric trials? US Food and Drug Administration, Office of Clinical Pharmacology, Silver Spring, MD. 2010 [Google Scholar]
- 35.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59:674–6. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson SA, Tuleu C, Wong IC, Keady S, Pitt KG, Sutcliffe AG. Minitablets: New modality to deliver medicines to preschool-aged children. Pediatr. 2009;123:e235–8. doi: 10.1542/peds.2008-2059. [DOI] [PubMed] [Google Scholar]
- 37.Breitkreutz J. In: World Pharma. Copenhagen. 2010. Novel drug formulations for children. Available from: http://www.worldpharma2010.org/powerpoints.php . [Google Scholar]
- 38.Whittaker A, Currie AE, Turner MA, Field DJ, Mulla H, Pandya HC. Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F236–40. doi: 10.1136/adc.2008.146035. [DOI] [PubMed] [Google Scholar]
- 39.Glasgow AM, Boeckx RL, Miller MK, MacDonald MG, August GP, Goodman SI. Hyperosmolality in small infants due to propylene glycol. Pediatr. 1983;72:353–5. [PubMed] [Google Scholar]
- 40.Lammers AE, Adatia I, Cerro MJ, Diaz G, Freudenthal AH, Freudenthal F, et al. Functional classification of pulmonary hypertension in children: Report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ. 2011;1:280–5. doi: 10.4103/2045-8932.83445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–9. doi: 10.1016/j.jpeds.2006.12.052. 9 e1-2. [DOI] [PubMed] [Google Scholar]
- 42.Lammers AE, Hislop AA, Flynn Y, Haworth SG. The 6-minute walk test: Normal values for children of 4-11 years of age. Arch Dis Child. 2008;93:464–8. doi: 10.1136/adc.2007.123653. [DOI] [PubMed] [Google Scholar]
- 43.Li AM, Yin J, Au JT, So HK, Tsang T, Wong E, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med. 2007;176:174–80. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- 44.Nixon PA, Joswiak ML, Fricker FJ. A six-minute walk for assessing exercise tolerance in severely ill children. J Pediatr. 1996;129:362–6. doi: 10.1016/s0022-3476(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 45.Eston RG, Rowlands AV, Ingledew DK. Validity of heart rate, pedometry and accelerometry for predicting the energy cost of children's activities. J Appl Physiol. 1998;84:362–71. doi: 10.1152/jappl.1998.84.1.362. [DOI] [PubMed] [Google Scholar]
- 46.Schwaiblmair M, Faul C, von Scheidt W, Berghaus T. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm Med. 2012;12:23. doi: 10.1186/1471-2466-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–84. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humpl T, Reyes JT, Erickson S, Armano R, Holtby H, Adatia I. Sildenafil therapy for neonatal and childhood pulmonary hypertensive vascular disease. Cardiol Young. 2011;21:187–93. doi: 10.1017/S1047951110001745. [DOI] [PubMed] [Google Scholar]
- 49.Briefing information for cardiovascular and renal drugs advisory committee meeting Use of ĢPVRI for dosing recommendations of adult-approved drugs in pediatric PAH patients. In: Research USFDA Advisory Committee meeting 2010 July 29th [Google Scholar]
- 50.Taylor CJ, Derrick G, McEwan A, Haworth SG, Sury MR. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98:657–61. doi: 10.1093/bja/aem059. [DOI] [PubMed] [Google Scholar]
- 51.Carmosino MJ, Friesen RH, Doran A, Ivy DD. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–7. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 53.Galiè N, Rubin Lj, Hoeper M, Jansa P, Al-Hiti H, Meyer G, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trial. Lancet. 2008;371:2093–100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 54.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26:858–63. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 55.Mullen MP, Andrus J, Labella MH, Forbes P, Rao S, Harris JE, et al. Quality of life and parental functioning in pediatric pulmonary hypertension. Chest. 2012 doi: 10.1378/chest.13-0636. [DOI] [PubMed] [Google Scholar]
- 56.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Streisand R, Braniecki S, Tercyak KP, Kazak AE. Childhood illness-related parenting stress: The pediatric inventory for parents. J Pediatr Psychol. 2001;26:155–62. doi: 10.1093/jpepsy/26.3.155. [DOI] [PubMed] [Google Scholar]
- 58.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 59.Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status and psychological symptoms. J Pers Soc Psychol. 1986;50:571–9. doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- 60.Association AP. Diagnostic and Statistical Manual of Mental Disorders (Text Revision) 4th ed. USA: American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- 61.Gemke RJ, Bonsel GJ. Reliability and validity of a comprehensive health status measure in a heterogeneous population of children admitted to intensive care. J Clin Epidemiol. 1996;49:327–33. doi: 10.1016/0895-4356(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 62.Robertson CM, Watt JM, Joffe AR, Murphy DB, Nagy JM, McLean DE, et al. Childhood morbidity after severe traumatic brain injury: Increased detection with the Multiattribute Health Status Classification. Pediatr Crit Care Med. 2001;2:145–50. doi: 10.1097/00130478-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Shih CY, Sapru A, Oishi P, Azakie A, Karl TR, Harmon C, et al. Alterations in plasma B-type natriuretic peptide levels after repair of congenital heart defects: A potential perioperative marker. J Thorac Cardiovasc Surg. 2006;131:632–8. doi: 10.1016/j.jtcvs.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 64.Chikovani O, Hsu JH, Keller R, Karl TR, Azakie A, Adatia I, et al. B-type natriuretic peptide levels predict outcomes for children on extracorporeal life support after cardiac surgery. J Thorac Cardiovasc Surg. 2007;134:1179–87. doi: 10.1016/j.jtcvs.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Lammers AE, Hislop AA, Haworth SG. Prognostic value of B-type natriuretic peptide in children with pulmonary hypertension. Int J Cardiol. 2009;135:21–6. doi: 10.1016/j.ijcard.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Takatsuki S, Wagner BD, Ivy DD. B-type Natriuretic Peptide and Amino-terminal Pro-B-type Natriuretic Peptide in Pediatric Patients with Pulmonary Arterial Hypertension. Congenit Heart Dis. 2012;7:259–67. doi: 10.1111/j.1747-0803.2011.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vijlbrief DC, Benders MJ, Kemperman H, van Bel F, de Vries WB. B-type natriuretic peptide and rebound during treatment for persistent pulmonary hypertension. J Pediatr. 2012;160:111–5 e1. doi: 10.1016/j.jpeds.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Yeager ME, Colvin KL, Everett AD, Stenmark KR, Ivy DD. Plasma proteomics of differential outcome to long-term therapy in children with idiopathic pulmonary arterial hypertension. Proteomics Clin Appl. 2012;6:257–67. doi: 10.1002/prca.201100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, et al. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J. 2012;39:104–11. doi: 10.1183/09031936.00072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levy M, Maurey C, Celermajer DS, Vouhe PR, Danel C, Bonnet D, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007;49:803–10. doi: 10.1016/j.jacc.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 71.Smadja DM, Gaussem P, Mauge L, Israel-Biet D, Dignat-George F, Peyrard S, et al. Circulating endothelial cells: A new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009;119:374–81. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 72.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–12. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 73.Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–74. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 75.Hunter KS, Feinstein JA, Ivy DD, Shandas R. Computational Simulation of the Pulmonary Arteries and its Role in the Study of Pediatric Pulmonary Hypertension. Prog Pediatr Cardiol. 2010;30:63–9. doi: 10.1016/j.ppedcard.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–74. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sajan I, Manlhiot C, Reyes J, McCrindle BW, Humpl T, Friedberg MK. Pulmonary arterial capacitance in children with idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with congenital heart disease: relation to pulmonary vascular resistance, exercise capacity and survival. Am Heart J. 2011;162:562–8. doi: 10.1016/j.ahj.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Hunter KS, Lammers SR, Shandas R. In: Comprehensive Physiology. 2011. Pulmonary Vascular Stiffness: Measurement, Modeling and Implications in Normal and Hypertensive Pulmonary Circulations; pp. 1413–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodes-Cabau J, Domingo E, Roman A, Majo J, Lara B, Padilla F, et al. Intravascular ultrasound of the elastic pulmonary arteries: a new approach for the evaluation of primary pulmonary hypertension. Heart (British Cardiac Society) 2003;89:311–5. doi: 10.1136/heart.89.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Engg. 2009;37:1082–92. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li M, Stenmark KR, Shandas R, Tan W. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J Vasc Res. 2009;46:561–71. doi: 10.1159/000226224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson CM, Sauve RS, Joffe AR, Alton GY, Moddemann DM, Blakley PM, et al. The registry and follow-up of complex pediatric therapies program of Western Canada: A mechanism for service, audit and research after life-saving therapies for young children. Cardiol Res Pract. 2011;2011:1–11. doi: 10.4061/2011/965740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klassen TP, Hartling L, Hamm M, van der Lee JH, Ursum J, Offringa M. StaR Child Health: An initiative for RCTs in children. Lancet. 2009;374:1310–2. doi: 10.1016/S0140-6736(09)61803-1. [DOI] [PubMed] [Google Scholar]
- 84.Savendahl L, Maes M, Albertsson-Wikland K, Borgstrom B, Carel JC, Henrard S, et al. Long-term mortality and causes of death in isolated GHD, ISS and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands and Sweden: Preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. 2012;97:E213–7. doi: 10.1210/jc.2011-2882. [DOI] [PubMed] [Google Scholar]
- 85.del Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1:286–98. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.William S, Lippert J, Sevestre M, Solodenko J, Fois F, Schmitt W. PK-Sim: A physiologically based pharmacokinetic ‘whole-body’ model. Biosilico. 2003;1:121–4. [Google Scholar]


