Abstract
The growth and differentiation of the central nervous system are closely related to the presence of iodine and thyroid hormones. It has been hypothesized that neurobehavioral disabilities of childhood, such as attention deficit hyperactivity disorder (ADHD), learning disorders, and autism can be attributed to fetal thyroidal endocrine disruption in utero. To determine whether there is an association between neonatal thyroid status and a subsequent diagnosis of a neurobehavioral disability, neonatal thyroxine (T4) levels have been used as the indicator of the presence of intrauterine thyroidal dysfunction. Neonatal T4 levels were obtained from the neonatal hypothyroidism screening program. All cases were diagnosed at medical school diagnostic clinics, the diagnostic categories being ADHD, autism spectrum disorder, behavioral disorder, cognitive disorder, developmental delay, emotional disorder, learning disability, and speech/language disorder. Conditional logistic regression analysis was performed for each clinical condition. Odds ratios for the conditions ranged from 0.92 to 1.13 with p values ranging between 0.19 and 0.84. No significant differences were detected between neonatal T4 values of the cases and the controls for any of the neurobehavioral conditions. All neonatal T4 values were within normal ranges. The data provide no evidence to suggest that intrauterine thyroid status as reflected by the neonatal T4 values had an impact on the neurologic disorders diagnosed in childhood.
Introduction
Insufficient Levels of Thyroid Hormones, mainly free thyroxine (FT4), during fetal brain development lead to deficiencies in differentiation and maturation of the central nervous system, later resulting in poor motor skills and deficient intellectual and behavioral development (1). The human fetal thyroid begins to produce thyroid hormones by approximately week 10 of gestation. The fetus is dependent on maternal thyroid hormones during those first weeks of gestation and at least the first part of the second trimester of pregnancy (2, 3). During the later gestational period the fetal thyroid has the capacity to make sufficient thyroxine (T4) on its own (1). It has been suggested that fetal neurologic damage is inversely related to maternal serum T4 levels in the first and second trimesters. Triiodothyronine (T3) is the main form of thyroid hormone that regulates fetal brain development by its binding to brain nuclear receptors. Maternal T4 and T3 that cross the placenta are converted to T3 at the fetal brain tissue level (4).
Iodine plays a central role in thyroid physiology, being both a major constituent of thyroid hormones and a regulator of thyroid gland function. Iodine deficiency has been shown to result in decreased serum T4 levels and in increased thyroid stimulating hormone (thyrotropin [TSH]) levels (5). In mildly iodine-deficient areas (median urinary iodine [UI], 50–99 µg/L) and in iodine-sufficient areas where maternal T4 concentrations are in the low-normal range, mild and subclinical neuropsychomotor deficits have been observed in neonates (6). Congenital hypothyroidism can cause motor and cognitive development abnormality in children (7), such motor and cognitive development abnormalities have occurred when blood T4 levels failed to reach a threshold level of 43 nmol/L at the neonatal screening after birth (8, 9). It is assumed that when T4 levels fall below such a threshold at a critical time of development and for an extended length of time there will be a neurobehavioral effect.
Newborns with congenital hypothyroidism who are identified by screening programs and treated promptly by T4 supplementation usually have IQs in the normal range at 5 to 7 years of age, as well as normal growth and development (1). Even children born without a thyroid have normal intellect if the absence is detected early enough after birth and thyroid hormone replacement is initiated early. Autism may also be a disease of early fetal brain development (approximately day 20–24 of gestation) (10). We examined the hypothesis that intrauterine thyroid dysfunction may be an early factor in the development of neurobehavioral disorders by testing whether T4 levels of newborns who were subsequently diagnosed with neurobehavioral disorders differed from T4 levels of the general community of newborns.
Methods
Study design
This matched case-control study was designed to determine whether children who were subsequently diagnosed with specific neurobehavioral disorders had abnormal T4 levels as newborns. The methodology has previously been described (11). Cases diagnosed at the pediatric neurodevelopmental diagnostic clinics of the three medical schools in Washington, D.C., served as potential cases. The data from the Washington, D.C. neonatal screening program were reviewed to identify the records of cases that had been born in Washington, D.C., and to record their neonatal T4 values. The controls were those newborns born and screened on the same day as the case and at the same hospital. The neonatal T4 values of the controls were identified and compared to those of the cases.
The pediatric neurodevelopmental diagnostic clinics of the three medical schools in Washington, D.C., were recruited for participation in this study. The institutional review board of each medical school approved the protocol, as did the director of the D.C. Department of Health. Each clinic prepared a list of children diagnosed there as having a neurobehavioral disorder along with their date of birth. These lists were submitted to the Neonatal Screening Laboratory at Howard University Hospital that had conducted the screening for the Division of Maternal Child Health, D.C. Department of Health, Washington, D.C. Laboratory records were searched to identify the screening results for each case. Neonatal T4 level measurements were retained in this laboratory as numeric date with actual values, in contrast to laboratories elsewhere that retained only the categorical information of normal or abnormal.
Matched controls for each case were all children who were born in the same Washington, D.C., hospital as the case and had their neonatal screening blood sample taken on the same day as the case. Neonatal T4 level as well as the date of sampling, date of birth, race, and gender of each case and all of its controls were recorded as a set with study numbers replacing personal identifiers. Cases or controls who were born prematurely were noted and excluded from the analysis. Because all the children who met the matching criteria were retained as controls, the number of matched controls per case varied. The data from the three centers were pooled for analysis.
Subject recruitment, screening, and diagnosis
Diagnostic clinics of the three medical schools in Washington, D.C. (Howard University, George Washington University Childrens’ National Medical Center, and Georgetown University) diagnosed the cases independent of this analysis. Assessments involved a full psychiatric interview with each child and each parent, and supplementary parental, teacher, and child interview materials and questionnaires. The children included in this study were diagnosed by an interdisciplinary team. Diagnostic teams consisted, at a minimum, of a psychologist and a developmental pediatrician, with additional team members, such as a physical therapist, occupational therapist, special educator, and speech and language specialist, added based on the specific needs of each individual child. All children received a neuropsychologic assessment designed to be age appropriate and to address the presenting symptoms of the child. This study was designed to maintain confidentiality with the specific diagnoses of individual cases maintained by the child’s clinic. Informed consents were not required as this study was limited to the analysis of currently existing datasets and did not involve patient contact or follow-up.
Diagnosis of cases was performed according to usual clinical standards. All case children met the criteria of the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV. While the test procedures varied among institutions, the domains measured included abstract and concept formation, complex problem solving, cognitive speed, verbal learning, long- and short-term memory, attention and alertness, visuospatial relationships, sensory function, and motor function. Age and presenting questions influenced the choice of evaluation tools in most instances.
Laboratory methods
Newborn thyroxine laboratory measurements
Collection of samples
All newborn blood specimens were obtained by the heel-stick method, within 48–72 hours of birth. The blood specimens were collected at the nursery on a special S & S #903 filter paper. The specimens were allowed to dry at room temperature prior to transport of the dry blood specimen (DBS) to the laboratory.
T4
Newborn T4 was measured using an immunoassay for T4 ICN neonatal (125I) T4 solid phase radioimmunoassay system (ICN Biomedicals, Irvine, CA) (12) which had a reference (normal) range of 7 µg/dL to 25 µg/dL. Values lower than 7 µg/dL were considered abnormal. The abnormal specimens were then assayed for TSH.
TSH
TSH was measured with ICN ImmunoChem™ Neonatal hTSH IRMA kit (ICN Biomedicals) (13). The same procedure and equipment were used to measure the samples of all of the cases and all of the controls. The TSH assays were performed on a one-quarter inch disc of the DBS using ICN ImmunoChem Neonatal hTSH IRMA kit (13).
Reference ranges
The reference ranges for T4 were: 7–25 µg/dL (90–276 nmol/L), and values lower than 7 µg/dL (90 nmol/L) were considered abnormal. The reference ranges quoted were supplied by the kit manufacturers and were used throughout the study. The abnormal specimens are then assayed for TSH. The reference ranges for TSH 0–30 µIU/mL are the normal limits, and TSH values above 40 µIU/mL are suggestive of hypothyroidism. The reference values for T4 and TSH were derived from six controls: three controls from the specific manufacturer of these kits and three controls from the Center for Disease Control (CDC). Additionally, five controls from the CDC were assayed quarterly for the proficiency testing. All the participating laboratories performing the neonatal newborn screening internationally and in the United States assay these CDC proficiency-testing samples.
The reference values for T4 and TSH were derived from six standards: three standards from the specific manufacturer of the kits and three standards from the CDC. Additionally, five standards from CDC were assayed quarterly for the proficiency testing.
Statistical analysis
To explore the relation between neonatal blood thyroid hormone levels (T4) and subsequent diagnosis of each of the neurobehavioral disorders, a matched case-control design was used. In order to improve generalizability, multiple sets of matched controls were defined to compare with cases in terms of neonatal blood thyroid hormone levels. Matched controls were created as follows: (1) controls matched for age, gender, and race; (2) controls matched for age and gender; (3) controls matched for age and race; and (4) controls matched for gender and race. Distribution of cases and controls can be found in Table 1.
Table 1.
Distribution of Cases and Controls by Diagnosis
| Disorder | No. cases | No. controls |
|---|---|---|
| Attention deficit (ADHD) | 53 | 231 |
| Autism (ASD) | 6 | 23 |
| Behavioral disorder | 37 | 154 |
| Cognitive disorder | 22 | 95 |
| Developmental disorder | 9 | 36 |
| Emotional disorder | 7 | 27 |
| Learning disorder | 67 | 286 |
| Speech/language disorder | 26 | 96 |
| Total (1175) | 227 | 948 |
Conditional logistic regression analysis with unequal numbers of controls was performed because this is a matched case-control study with unequal numbers of controls (14). Controls/case ratio average was 4.2, the maximum, 9. Conditional logistic regression analyses were performed separately for different sets of controls.
To examine the relation between T4 and disease status, T4 was treated both as a categorical and as a continuous variable in the logistic regression analysis. Using T4 as a categorical variable, quantiles were calculated (the first, 25th percentile; the second, 50th percentile or median; and the third, 75th percentile) of T4. The values of T4 above the third quantile were used as a reference group. Thus, three dummy variables were then created and were put into the logistic regression analysis. Statistical tests were considered to be significant at an α level of 0.05 on a two-tailed test.
Results
The children included in this study were 5½ to 12 years of age at the time of diagnosis and had been diagnosed with neurobehavioral disorders at three independent medical school diagnostic clinics in Washington, D.C. (Table 1). The disorders included in this study include (1) ADHD, (2) autism spectrum disorder, (3) behavioral disorder, (4) cognitive disorder, (5) developmental delay, (6) emotional disorder, (7) learning disability, and (8) speech/language disorder.
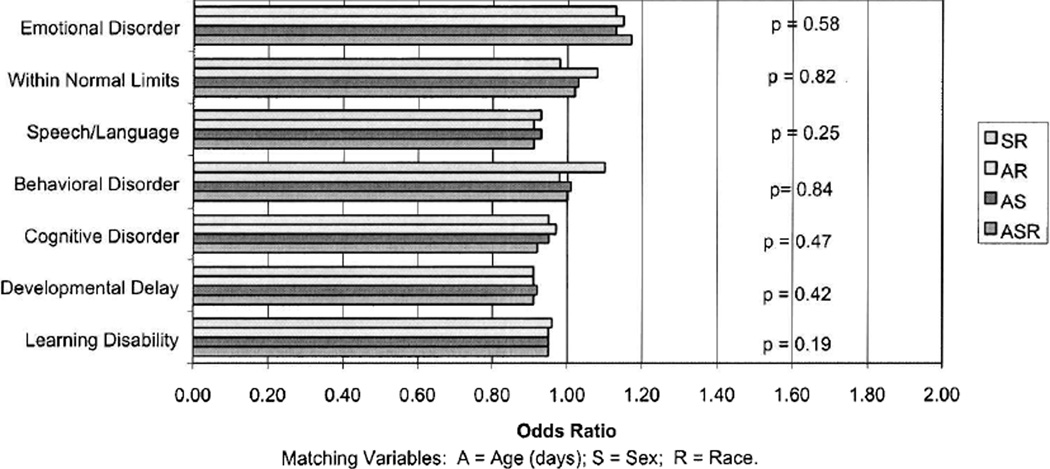
Matched controls were those newborns whose neonatal screening occurred on the same day and in the same hospital as the case, with one to five matched controls per case. Information on cases and controls included age at time of T4 sampling, race, and gender. Analysis took these variables into consideration. The odds ratios and p values by diagnosis are charted in Table 2, and more specifically, including matching by varying sets of demographic variables in Figure 1. All cases of neurobehavioral disorders seen here were found to have neonatal T4 levels within the normal range for newborns, as did the controls.
Table 2.
ODDS Ratios and p Values by Diagnosis
| Disorder/disability | Odds ratio | p value |
|---|---|---|
| Attention deficit (ADHD) | 0.99 | 0.81 |
| Autism (ASD) | 1.08 | 0.73 |
| Behavioral disorder | 1.01 | 0.84 |
| Cognitive disorder | 0.95 | 0.47 |
| Developmental delay | 0.92 | 0.42 |
| Emotional disorder | 1.13 | 0.58 |
| Learning disorder | 0.95 | 0.19 |
| Speech/language disability | 0.93 | 0.25 |
| Total (n = 1175) |
FIG. 1.
Odds ratio for neurobehavioral disorders of children by neonatal thyroxine (T4) as a continuous variable and matched on varying set of demographic variables.
The actual T4 values for the cases were (Table 3): mean T4 was 13.95 µg/dL and standard deviation (SD) (3.94 µg/dL). The T4 concentration range was 2.6–25.3 µg/dL; the quantiles: 11.2, 14.0, 16.5. For the controls, the mean T4 (SD) was 14.47 µg/dL (3.38 µg/dL), and the T4 concentration range, 2.4–25.1 µg/dL; the quantiles: 12.3, 14.5, 16.7.
Table 3.
T4 Concentrations, Means, Ranges, and Quantiles for Cases and Controls
| T4 concentrations (µg/dL) |
Cases (SD) (µg/dL |
Controls (SD) µg/dL) |
|---|---|---|
| Mean | 13.95 (3.94) | 14.47 (3.38) |
| Range | 2.6–25.3 | 2.4–25.1 |
| Quantiles | 11.2, 14.0, 16.5 | 12.3, 14.5, 16.7 |
T4, thyroxine; SD, standard deviation.
As illustrated in Figure 1, no differences were found between the odds ratio of the different neurobehavioral dysfunctions and none were found to have any statistical significance.
Table 2 lists the different disorders, their odds ratio, and p values. The odds ratios range from 0.92 to 1.13. As noted in Table 2, the odds ratio and p values by diagnosis show no significant differences for any of the disorders or disabilities.
These results illustrate that although the children who served as cases in our study had neurobehavioral disorders that manifested during childhood, their levels of T4 as neonates (as measured by the neonatal screening program) were within the normal pediatric T4 reference range (11). There is no evidence to suggest that neonatal T4 level had a prominent impact and was the cause of later manifestation of neurobehavioral disorders of interest in this study. All analytic sets found no difference in the neonatal T4 levels for all the neurobehavioral disorders for the cases as well as for their controls, whether T4 was analyzed as a categorical or continuous variable.
Discussion
It is well established that thyroid hormones play a critical role in brain development (15–18). Both maternal and neonatal thyroid glands play an important role in normal neuropsychointellectual development. Lesser degrees of thyroid dysfunction do not cause frank mental retardation, but can result in more subtle yet measurable neurocognitive and psychomotor deficits, as observed in some children with early-treated congenital hypothyroidism (7, 19–23). There will only be an adverse effect if these factors cause FT4 insufficiency (hypothyroxinemia) during development fetus. Consistent with these observations, pregnant women with untreated hypothyroidism (24, 25), women in geographical areas of moderate iodine deficiency (26), women with first trimester but low FT4 (27), and women with antithyroid peroxidase anti-bodies (28) may give birth to children who subsequently demonstrate mild but measurable neurocognitive and psychomotor deficits in early childhood. Any combination of these factors, which alone may not be detectable, may lead to a synergistic effect that may result in a disruption leading to neurobehavioral disorders.
The fundamental causes of many of the neurobehavioral disorders are not known; however, many researchers agree that these may be a manifestation of brain disorders with a biologic basis (29, 30). Genetic and environmental elements are believed to be important contributors to the etiology of some neurobehavioral disorders (31, 32). In light of observations that intrauterine thyroid dysfunction can lead to neurobehavioral disturbances (33–37), the fact that premature birth accompanied by transient hypothyroidism increases the risk of neurologic development ADHD (38), and the comorbidity of ADHD with learning disabilities (30) we studied the relation between newborn T4 levels and neurobehavioral disorders. Although free triiodothyronine (FT3) is the physiologically active hormone that binds to the nuclear receptors (and is estimated as 0.2%–0.4% of the total T3) in this study T4 served as a surrogate for the active form of the hormone. It has been found that motor and cognitive impairment was correlated with the degree of maternal hypothyroxinemia, and not with circulating T3 or TSH levels. The concentrations of all T4 and T3 hormones tend to change together, although the ultimate regulation of thyroid hormone metabolic activity is derived by the relative rates of formation of T3 and reverse triiodothyrone (rT3) from T4.
Fetal total T4 and FT4 production do not clearly increase until week 20–24, and fetal serum FT3 is undetectable until gestational week 32 (1). Although the fetal thyroid can synthesize its own T4maternal T4 may still contribute significantly to fetal needs, at levels 20%–50% of normal values until term (39). We did not assess maternal thyroid function during pregnancy although the study’s underlying hypothesis was that intrauterine thyroid dysfunction would be a contributing factor for subsequent neuropsychiatric disorders. Because neonatal T4 levels reflect primarily T4 levels produced by the neonate’s thyroid gland on the second or third day of life, differences in T4 levels between cases and controls would have suggested a pattern continuous with that of fetal T4 levels during gestation. However, all analytic sets found no difference in the neonatal T4 concentrations for all the neurobehavioral disorders for the cases as well as for their controls, whether T4 was analyzed as a categorical or as a continuous variable. It is likely that neurobehavioral deficits result from complex processes and result from exposure to multiple factors in the internal and external environment. Because T4 concentrations of the cases and of the controls in our study were all within normal range (Table 3), although we have no record of the maternal T4 concentrations at weeks 0–12 of gestation, it is highly unlikely that it is the intrauterine T4 concentrations of the mothers that are responsible for the resulting neurobehavioral disorders in their offspring.
The number of cases of autism in this study (6 cases, 11 controls) is only sufficient to yield an indication rather than a strong finding that neonatal T4 concentrations cannot predict autism. Similarly, in the other groups, the numbers are only suggestive of no relation between the disorder and newborn T4 concentrations. Each of the cases in this study had normal neonatal T4 concentrations that did not differ from the neonatal T4 levels of their matched controls, demonstrating no relation between neonatal T4 levels at birth and the development of neurobehavioral disorders later on in childhood. Studies similar in methodology to this study might be more conclusive.
Acknowledgments
The study was supported in part by the Perchlorate Study Group. The authors wish to thank Dr. M. Stein, Children’s National Medical Center, George Washington University School of Medicine, Washington, D.C.; and Dr. P. Magrab Georgetown University School of Medicine, Washington, D.C., for assistance with neurobehavioral patient diagnosis and Dr. K.N. Nandedkar and Mr. K.M. Japal, Howard University College of Medicine for the laboratory testing.
Footnotes
Presented in part at the 73rd Annual Meeting of the American Thyroid Association in Washington D.C., November 2001.
References
- 1.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 2.Glinoer D. Potential repercussions for the progeny of maternal hypothyroxinemia during pregnancy. Thyroid. 2000;10:59–62. doi: 10.1089/thy.2000.10.59. [DOI] [PubMed] [Google Scholar]
- 3.Glinoer D. Potential consequences of maternal hypothyroidism on the offspring: Evidence and implications. Horm Res. 2001;55:109–114. doi: 10.1159/000049981. [DOI] [PubMed] [Google Scholar]
- 4.Bernal J. Iodine and brain development. Biofactors. 1999;10:271–276. doi: 10.1002/biof.5520100227. [DOI] [PubMed] [Google Scholar]
- 5.Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–128. doi: 10.1089/thy.1994.4.107. [DOI] [PubMed] [Google Scholar]
- 6.Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M, Grun JP, Kinthaert J, Lejeune B. A randomized trial for the treatment of mild iodine deficiency during pregnancy: Maternal and neonatal effects. J Clin Endocrinol Metab. 1995;80:258–269. doi: 10.1210/jcem.80.1.7829623. [DOI] [PubMed] [Google Scholar]
- 7.Rovet JF, Ehrlich R. Psychoeducational outcome in children with early-treated congenital hypothyroidism. Pediatrics. 2000;105(3 Pt 1):515–522. doi: 10.1542/peds.105.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Kooistra L, Laane C, Vulsma T, Schellekens JM, van der Meere JJ, Kalverboer AF. Motor and cognitive development in children with congenital hypothyroidism: A longterm evaluation of the effects of neonatal treatment. J Pediatr. 1994;124:903–909. doi: 10.1016/s0022-3476(05)83178-6. [DOI] [PubMed] [Google Scholar]
- 9.Tillotson SL, Fuggle PW, Smith I, Ades AE, Grant DB. Relation between biochemical severity and intelligence in early treated congenital hypothyroidism: A threshold effect. BMJ. 1994;309:440–445. doi: 10.1136/bmj.309.6952.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London EA. The environment as an etiologic factor in autism: A new direction for research. Environ Health Perspect. 2000;108(Suppl 3):401–404. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soldin OP, Nandedkar AK, Japal KM, Stein M, Mosee S, Magrab P, Lai S, Lamm SH. Newborn thyroxine levels and childhood ADHD. Clin Biochem. 2002;35:131–136. doi: 10.1016/s0009-9120(02)00284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dussault JH, Laberge C. Thyroxine (T4) determination by radioimmunological method in dried blood eluate: New diagnostic method of neonatal hypothyroidism? Union Med Can. 1973;102:2062–2064. [PubMed] [Google Scholar]
- 13.Gruters A. Congenital hypothyroidism. Pediatr Ann. 1992;21:15, 18–21, 24–28. doi: 10.3928/0090-4481-19920101-06. [DOI] [PubMed] [Google Scholar]
- 14.Breslow NE, Day NE. The analysis of case-control studies. Lyon, France: IARC Scientific Publications No. 32; 1987. Statistical methods in cancer research. [PubMed] [Google Scholar]
- 15.Anderson GW. Thyroid hormones and the brain. Front Neuroendocrinol. 2001;22:1–17. doi: 10.1006/frne.2000.0208. [DOI] [PubMed] [Google Scholar]
- 16.Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development—Current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- 17.Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000;11:123–128. doi: 10.1016/s1043-2760(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 18.Thompson CC, Potter GB. Thyroid hormone action in neural development. Cereb Cortex. 2000;10:939–945. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- 19.Delange F. Iodine deficiency as a cause of brain damage. Postgrad Med J. 2001;77:217–220. doi: 10.1136/pmj.77.906.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen M, Santavuori P, Hirvonen E, Perheentupa J. Multivariate analysis of psychomotor development in congenital hypothyroidism. Acta Paediatr Scand. 1989;78:405–411. doi: 10.1111/j.1651-2227.1989.tb11100.x. [DOI] [PubMed] [Google Scholar]
- 21.Rovet JF, Westbrook DL, Ehrlich RM. Neonatal thyroid deficiency: early temperamental and cognitive characteristics. J Am Acad Child Psychiatry. 1984;23:10–22. doi: 10.1097/00004583-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rovet JF, Ehrkich RM, Sobara DL. Neurodevelopment in infants and preschool children with congenital hypothyroidism: Etiological and treatment factors affecting outcome. J Pediatr Psychol. 1992;17:187–213. doi: 10.1093/jpepsy/17.2.187. [DOI] [PubMed] [Google Scholar]
- 23.Rovet J. The Effects of PTU Exposure During Pregnancy on Infant Cognitive Development. The American Thyroid Association Annual Meeting. 1999 [Google Scholar]
- 24.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 25.Man EB, Brown JF, Serunian SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci. 1991;21:227–239. [PubMed] [Google Scholar]
- 26.Glinoer D, Delange F. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid. 2000;10:871–887. doi: 10.1089/thy.2000.10.871. [DOI] [PubMed] [Google Scholar]
- 27.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drewhager NA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 28.Pop VJ, de Vries E, van Baar AL, Waelkens JJ, de Rooy HA, Horsten M, Donkers MM, Komproe IH, van Som MM, Vader HL. Maternal thyroid peroxidase antibodies during pregnancy: A marker of impaired child development? J Clin Endocrinol Metab. 1995;80:3561–3566. doi: 10.1210/jcem.80.12.8530599. [DOI] [PubMed] [Google Scholar]
- 29.Weiss RE, Stein MA, Trommer B, Refetoff S. Attentiondeficit hyperactivity disorder and thyroid function. J Pediatr. 1993;123:539–545. doi: 10.1016/s0022-3476(05)80947-3. [DOI] [PubMed] [Google Scholar]
- 30.Schweitzer JB, Cummins TK, Kant CA. Attentiondeficit/ hyperactivity disorder. Med Clin North Am. 2001;85:757–777. doi: 10.1016/s0025-7125(05)70339-4. [DOI] [PubMed] [Google Scholar]
- 31.Faraone SV, Doyle AE. Genetic influences on attention deficit hyperactivity disorder. Curr Psychiatry Rep. 2000;2:143–146. doi: 10.1007/s11920-000-0059-6. [DOI] [PubMed] [Google Scholar]
- 32.Lou HC. Etiology and pathogenesis of attention-deficit hyperactivity disorder (ADHD): Significance of prematurity and perinatal hypoxic-haemodynamic encephalopathy. Acta Paediatr. 1996;85:1266–1271. doi: 10.1111/j.1651-2227.1996.tb13909.x. [DOI] [PubMed] [Google Scholar]
- 33.Mirabella G, Feig D, Astzalos E, Perlman K, Rovet JF. The effect of abnormal intrauterine thyroid hormone economies on infant cognitive abilities. J Pediatr Endocrinol Metab. 2000;13:191–194. doi: 10.1515/jpem.2000.13.2.191. [DOI] [PubMed] [Google Scholar]
- 34.Rovet J, Alvarez M. Thyroid hormone and attention in school-age children with congenital hypothyroidism. J Child Psychol Psychiatry. 1996;37:579–585. doi: 10.1111/j.1469-7610.1996.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 35.Rovet J, Alvarez M. Thyroid hormone and attention in congenital hypothyroidism. J Pediatr Endocrinol Metab. 1996;9:63–66. doi: 10.1515/jpem.1996.9.1.63. [DOI] [PubMed] [Google Scholar]
- 36.Hauser P, Soler R, Brucker-Davis F, Weintraub BD. Thyroid hormones correlate with symptoms of hyperactivity but not inattention in attention deficit hyperactivity disorder. Psychoneuroendocrinology. 1997;22:107–114. doi: 10.1016/s0306-4530(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 37.Ishaik G, Asztalos E, Perlman K, Newton S, Frisk V, Rovet J. Hypothyroxinemia of prematurity and infant neurodevelopment: A pilot study. J Dev Behav Pediatr. 2000;21:172–179. [PubMed] [Google Scholar]
- 38.Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med. 1996;334:821–827. doi: 10.1056/NEJM199603283341303. [DOI] [PubMed] [Google Scholar]
- 39.Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321:13–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]