Abstract
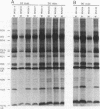
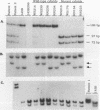
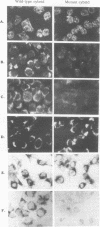
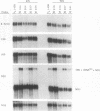
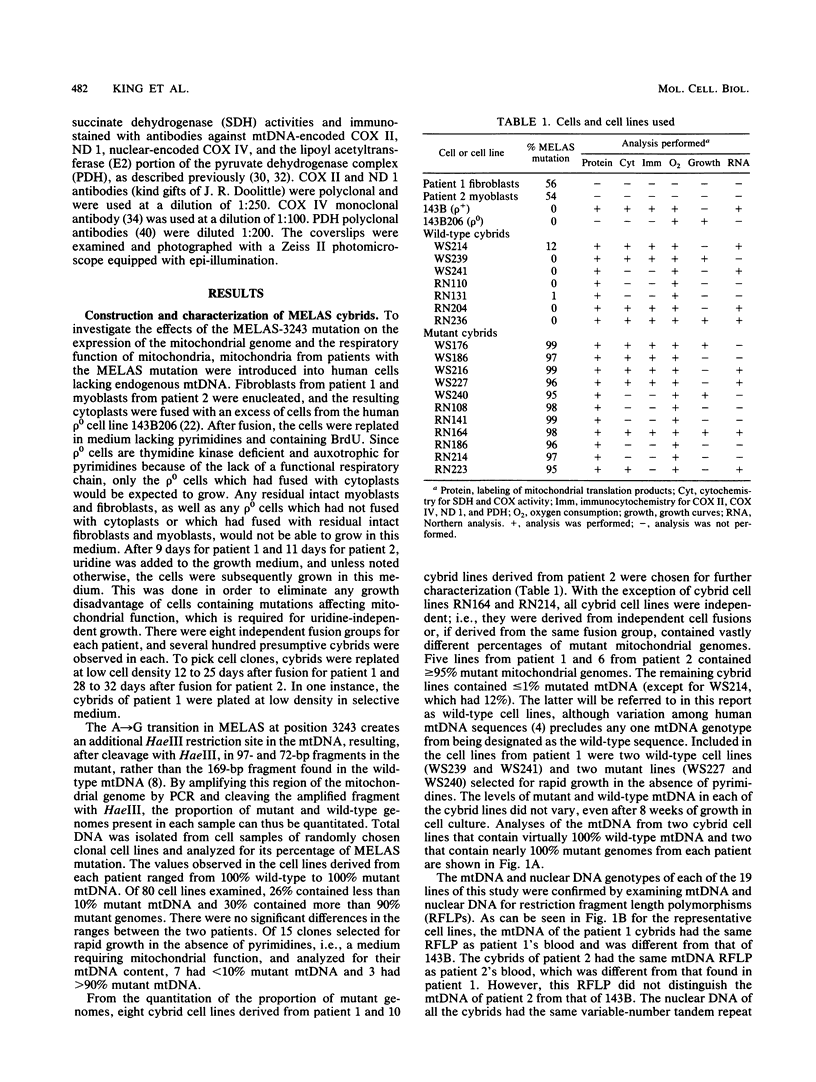
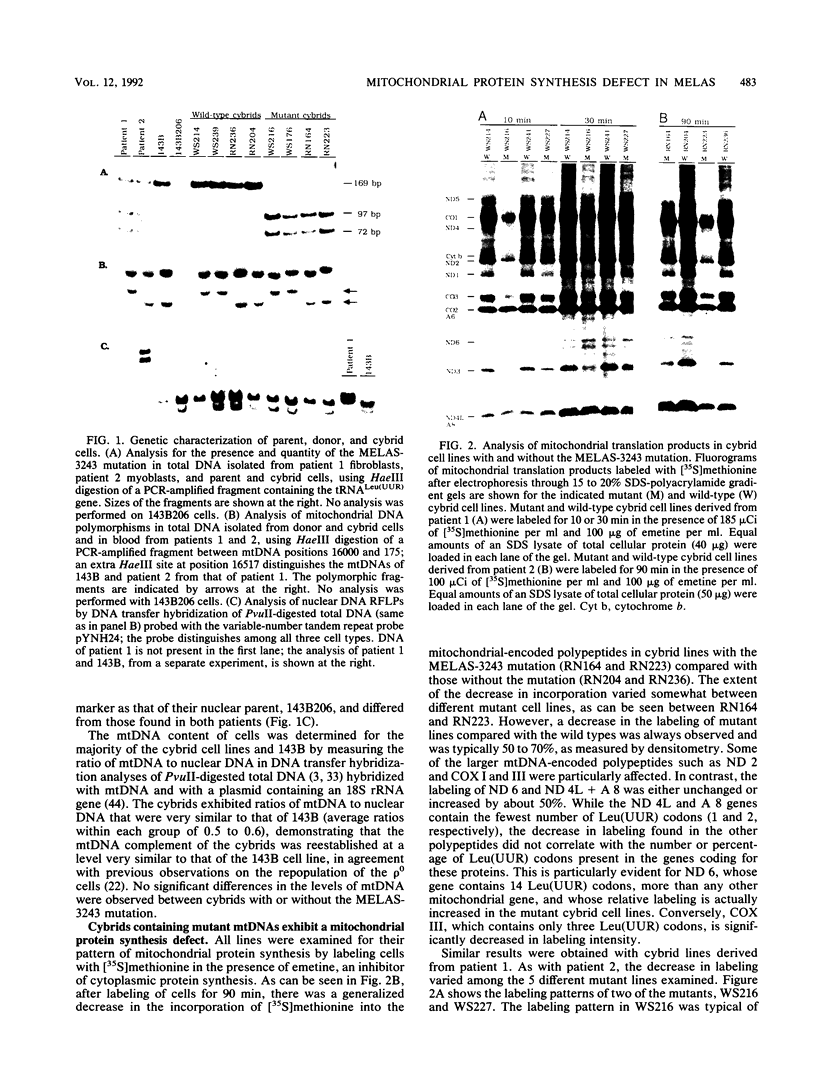
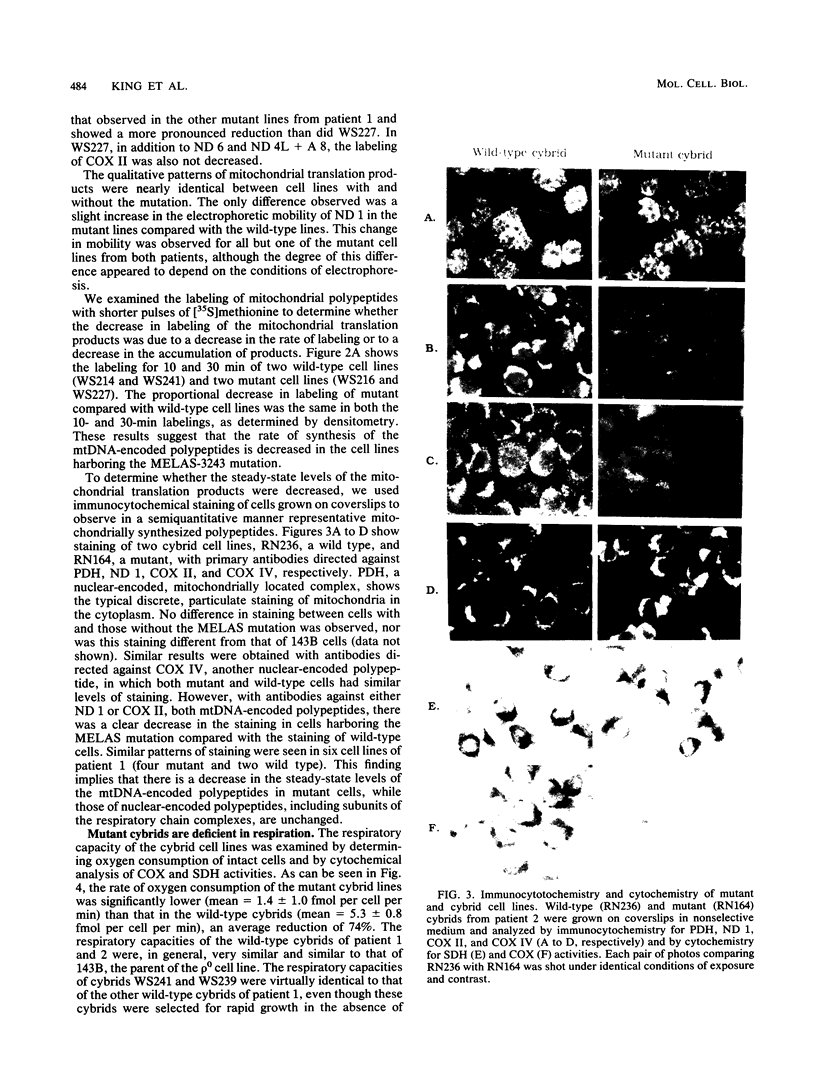
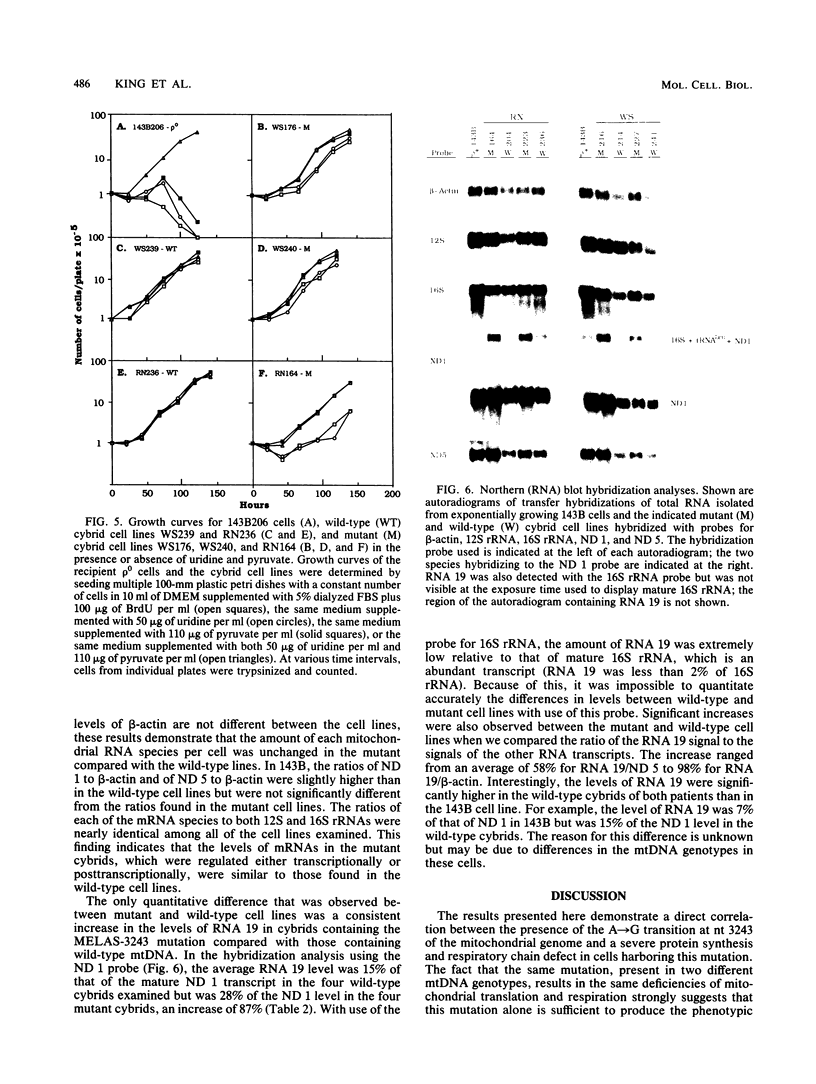
Cytoplasts from two unrelated patients with MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes) harboring an A----G transition at nucleotide position 3243 in the tRNA(Leu(UUR)) gene of the mitochondrial genome were fused with human cells lacking endogenous mitochondrial DNA (mtDNA) (rho 0 cells). Selected cybrid lines, containing less than 15 or greater than or equal to 95% mutated genomes, were examined for differences in genetic, biochemical, and morphological characteristics. Cybrids containing greater than or equal to 95% mutant mtDNA, but not those containing normal mtDNA, exhibited decreases in the rates of synthesis and in the steady-state levels of the mitochondrial translation products. In addition, NADH dehydrogenase subunit 1 (ND 1) exhibited a slightly altered mobility on polyacrylamide gel electrophoresis. The mutation also correlated with a severe respiratory chain deficiency. A small but consistent increase in the steady-state levels of an RNA transcript corresponding to 16S rRNA + tRNA(Leu(UUR)) + ND 1 genes was detected. However, there was no evidence of major errors in processing of the heavy-strand-encoded transcripts or of altered steady-state levels or ratios of mitochondrial rRNAs or mRNAs. These results provide evidence for a direct relationship between the tRNALeu(UUR) mutation and the pathogenesis of this mitochondrial disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Merkel C., Gelfand R., Attardi G. Fractionation of mitochondrial RNA from HeLa cells by high-resolution electrophoresis under strongly denaturing conditions. J Mol Biol. 1978 Jan 5;118(1):1–25. doi: 10.1016/0022-2836(78)90241-3. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Arnaudo E., Dalakas M., Shanske S., Moraes C. T., DiMauro S., Schon E. A. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991 Mar 2;337(8740):508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. In vitro transcription of human mitochondrial DNA: accurate termination requires a region of DNA sequence that can function bidirectionally. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6277–6281. doi: 10.1073/pnas.83.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gelfand R., Attardi G. Synthesis and turnover of mitochondrial ribonucleic acid in HeLa cells: the mature ribosomal and messenger ribonucleic acid species are metabolically unstable. Mol Cell Biol. 1981 Jun;1(6):497–511. doi: 10.1128/mcb.1.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Stanbridge E. J. Cloned mycoplasma ribosomal RNA genes for the detection of mycoplasma contamination in tissue cultures. Science. 1984 Dec 7;226(4679):1211–1213. doi: 10.1126/science.6505688. [DOI] [PubMed] [Google Scholar]
- Hammans S. R., Sweeney M. G., Brockington M., Morgan-Hughes J. A., Harding A. E. Mitochondrial encephalopathies: molecular genetic diagnosis from blood samples. Lancet. 1991 Jun 1;337(8753):1311–1313. doi: 10.1016/0140-6736(91)92981-7. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Parisi M. A., Bennett J. L., Clayton D. A. Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1991 May 16;351(6323):236–239. doi: 10.1038/351236a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D. A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991 May;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Klug A., Robertus J. D., Ladner J. E., Brown R. S., Finch J. T. Conservation of the molecular structure of yeast phenylalanine transfer RNA in two crystal forms. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3711–3715. doi: 10.1073/pnas.71.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Koga Y., Nonaka I., Kobayashi M., Tojyo M., Nihei K. Findings in muscle in complex I (NADH coenzyme Q reductase) deficiency. Ann Neurol. 1988 Dec;24(6):749–756. doi: 10.1002/ana.410240609. [DOI] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Ishii S., DiMauro S., Shay J. W. Cytochrome c oxidase deficiency in Leigh's syndrome: genetic evidence for a nuclear DNA-encoded mutation. Neurology. 1989 May;39(5):697–702. doi: 10.1212/wnl.39.5.697. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Schon E. A., DiMauro S., Miranda A. F. Heteroplasmy of mitochondrial genomes in clonal cultures from patients with Kearns-Sayre syndrome. Biochem Biophys Res Commun. 1989 Apr 28;160(2):765–771. doi: 10.1016/0006-291x(89)92499-6. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Shanske S., Tritschler H. J., Aprille J. R., Andreetta F., Bonilla E., Schon E. A., DiMauro S. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Miranda A. F. A monoclonal antibody against cytochrome c oxidase distinguishes cardiac and skeletal muscle mitochondria. Exp Cell Res. 1987 Jan;168(1):44–52. doi: 10.1016/0014-4827(87)90414-9. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Gillilan S., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pYNH24 on chromosome 2 (D2S44). Nucleic Acids Res. 1987 Dec 10;15(23):10073–10073. doi: 10.1093/nar/15.23.10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov H. M., Benson S., Robinson J. J., Britten R. J., Wilt F., Davidson E. H. A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. II. Structure of the gene and derived sequence of the protein. Dev Biol. 1987 Apr;120(2):507–519. doi: 10.1016/0012-1606(87)90254-5. [DOI] [PubMed] [Google Scholar]
- Sudoyo H., Marzuki S., Trounce I., Byrne E. Antimitochondrial autoantibodies of primary biliary cirrhosis as a novel probe in the study of 2-oxo acid dehydrogenases in patients with mitochondrial myopathies. J Neurol Sci. 1990 Sep;98(2-3):185–193. doi: 10.1016/0022-510x(90)90259-p. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Ohbayashi T., Ikebe S., Sano T., Ichiki T., Kobayashi M., Wada Y., Ozawa T. Mitochondrial DNA mutations in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Biophys Res Commun. 1991 Jan 31;174(2):861–868. doi: 10.1016/0006-291x(91)91497-z. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Antozzi C., Rimoldi M., Morandi L., Villani F., Tiranti V., DiDonato S. Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 1991 Jul 20;338(8760):143–147. doi: 10.1016/0140-6736(91)90136-d. [DOI] [PubMed] [Google Scholar]