Background: Increased Fap1 expression in chronic myeloid leukemia (CML) is associated with Fas resistance.
Results: Fap1 also increases βcatenin activity in CML by inactivating Gsk3β, a serine/threonine kinase that effects inhibitory phosphorylation of βcatenin.
Conclusion: Fap1 influences cell proliferation and survival by regulating βcatenin protein stability.
Significance: Fap1 contributes to the pathogenesis of CML by inhibiting apoptosis and increasing βcatenin activity.
Keywords: βcatenin, Fas, Gene Regulation, Glycogen Synthase Kinase 3, Leukemia
Abstract
Increased βcatenin activity correlates with leukemia stem cell expansion and disease progression in chronic myeloid leukemia (CML). We found previously that expression of the CML-related Bcr-abl oncoprotein in myeloid progenitor cells increases expression of Fas-associated phosphatase 1 (Fap1). This resulted in Fap1-dependent resistance to Fas-induced apoptosis in these cells. Fap1 also interacts with the adenomatous polyposis coli (Apc) protein, but the functional significance of this interaction is unknown. Apc participates in a complex that includes glycogen synthase kinase β (Gsk3β) and βcatenin. Assembly of this complex results in phosphorylation of βcatenin by Gsk3β, which facilitates βcatenin ubiquitination and degradation by the proteasome. In this study, we found increased association of Fap1 with the Apc complex in Bcr-abl+ myeloid progenitor cells. We also found Fap1-dependent inactivation of Gsk3β and consequent stabilization of βcatenin in these cells. Consistent with this, Bcr-abl+ cells exhibited a Fap1-dependent increase in βcatenin activity. Our studies identified Fap1-dependent Gsk3β inactivation as a molecular mechanism for increased βcatenin activity in CML.
Introduction
Fap1 is a ubiquitously expressed protein tyrosine phosphatase (PTP)2 that interacts with the C terminus of Fas (1, 2). This interaction results in Fas dephosphorylation and inhibition of Fas-induced apoptosis. The interaction of Fap1 with Fas is blocked by a tripeptide representing the Fas-C terminus (the Serine-Leucine-Valine peptide), and in vitro treatment of Fas-resistant cells with this peptide increases sensitivity to Fas-induced apoptosis (2–4). Fap1 also interacts with other proteins, including RapGEF6 and adenomatous polyposis coli (Apc) (5, 6). Fap1 interacts with the C termini of these partners through the same domain that mediates Fas interaction. The functional significance of these Fap1 interactions is unknown, and Fas is the only known substrate for Fap1-PTP activity.
Chronic myeloid leukemia (CML) is characterized by translocations involving chromosomes 9 and 22 that produce the Bcr-abl oncoprotein. Previous investigations characterized the CML leukemia stem cell (LSC) as a granulocyte-monocyte progenitor (GMP) with increased βcatenin activity (7, 8). These investigators found an association between increasing βcatenin activity in the bone marrow and disease progression in human CML (7).
The human CML LSC is also characterized by decreased expression of the interferon consensus sequence binding protein (Icsbp, also referred to as interferon regulatory factor 8) (9)3. In murine bone marrow, transduction with a Bcr-abl expression vector decreases Icsbp expression in vitro and in vivo (11–13). Re-expression of Icsbp in Bcr-abl+ murine bone marrow decreases myeloproliferation and delays blast crisis in vivo (12). Mice with IRF8 gene disruption phenocopy some aspects of CML, suggesting that Icsbp regulates target genes that are relevant to proliferation and survival of CML LSCs (14, 15).
Consistent with this hypothesis, we found that Icsbp represses PTPN13, the gene encoding Fap1. Search of publically available databases determined that Fap1 expression is increased in human CML3. We found previously that Bcr-abl-expressing murine myeloid progenitor cells exhibited Icsbp- and Fap1-dependent Fas resistance (3, 4). We also found that treatment of these cells with SLV peptide partly reversed Fas resistance (4).
In this study, we consider the possibility that Fap1 also contributes to the pathogenesis of CML by interacting with Apc. Apc assembles a multiprotein complex with Axin, Gsk3β, and βcatenin. Serine/threonine phosphorylation of βcatenin by Gsk3β facilitates βcatenin ubiquitination and degradation by the proteasome (16, 17). This provides a potential mechanism for Fap1 to influence βcatenin.
Molecular mechanisms that increase βcatenin activity in CML are not well defined. Neither increased CTNNB1 transcription nor Wnt signaling are found consistently in this disease (18). However, we determined previously that Icsbp influences βcatenin activity by regulating the GAS2 gene (13). Growth arrest-specific 2 (Gas2) is a calpain inhibitor, and βcatenin is a calpain substrate (19). We found that increased Gas2 expression in Bcr-abl+ myeloid progenitor cells contributed to stability and activity of βcatenin (13).
In this study, we investigate the hypothesis that Fap1 also stabilizes βcatenin in Bcr-abl+ cells by interfering with the Apc-Gsk3β-βcatenin complex. Fap1 might exert this effect by preventing assembly of the complex or by inactivating (dephosphorylating) a component protein. If this hypothesis is correct, therapeutic targeting of Fap1 might increase Fas sensitivity and decrease βcatenin activity, impacting disease progression in CML.
EXPERIMENTAL PROCEDURES
Plasmids and Protein Expression Vectors
The Icsbp cDNA was obtained from Dr. Ben Zion-Levi (Technion, Haifa, Israel). The full-length cDNA was generated by PCR and subcloned into the mammalian expression vector pcDNA (Stratagene, La Jolla, CA) as described (20). The Bcr-abl (p210) cDNA in the MiGR1 retroviral vector was obtained from Dr. Ravi Bhatia (City of Hope National Medical Center, Duarte, CA) and was subcloned into the pcDNA expression vector. The cDNA for Gsk3β was obtained from and subcloned into the pcDNAamp expression vector. A form of Gsk3β with mutation of Tyr-216 to Asp was generated by PCR-based mutagenesis as described (21).
Plasmids and Reporter Constructs
The TopFlash and FopFlash reporter constructs were purchased from Millipore (Billerica, MA). TopFlash contains three copies of a consensus binding site for βcatenin/Tcf-Lef linked to a minimal promoter and a luciferase reporter. FopFlash is a similar construct but with a mutation that abolishes βcatenin/Tcf-Lef binding.
Myeloid Cell Line Culture
The human myelomonocytic leukemia cell line U937 (22) was obtained from Andrew Kraft (Hollings Cancer Center, Medical University of South Carolina, Charleston, SC). Cells were maintained as described (21).
Murine Bone Marrow Culture
Animal studies were performed according to a protocol approved by the Animal Care and Use Committees of Northwestern University and Jesse Brown Veterans Affairs Medical Center. Bone marrow mononuclear cells were obtained from the femurs of WT or Icsbp−/− C57/BL6 mice. Sca1+ cells were separated using the Miltenyi magnetic bead system according to the instructions of the manufacturer (Miltenyi Biotechnology, Auburn, CA).
Bipotential GMPs were cultured (at a concentration of 2 × 105 cells/ml) for 48 h in DMEM supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, 10 ng/ml murine granulocyte-monocyte colony stimulating factor (GM-CSF) (R&D Systems, Inc., Minneapolis, MN), and 10 ng/ml murine recombinant IL-3 (R&D Systems, Inc.). Cells were maintained in this medium for 48 h, and CD34+ cells were separated using the Miltenyi magnetic bead system, as above, for extraction of RNA or proteins.
Quantitative Real-time PCR for mRNA Expression
RNA was isolated using the TRIzol reagent according to the instructions of the manufacturer (Invitrogen). RNA was tested by denaturing gel electrophoresis to determine the integrity of the 18 S and 28 S ribosomal bands. Primers were designed with the software from Applied Biosystems, and real-time PCR was performed using SYBR Green according to the “standard curve” method. Result were normalized to 18 S to control for RNA abundance in various samples.
Stable Transfectant Myeloid Cell Lines
U937 cells were transfected by electroporation with a p210 Bcr-abl expression vector or empty vector control (Bcr-abl/pcDNAamp or pcDNAamp) plus a vector with a neomycin phosphotransferase cassette (pSRα) (30 μg each). Stable pools of cells were selected in G418 (0.5 mg/ml).
Transient Transfections for Reporter Gene Assays
U937 cells (32 × 106/ml) were transfected with various combinations of vectors to express p210 Bcr-abl or control (50 μg), βcatenin or control (50 μg), Gsk3β or Y216D-Gsk3β, or control (50 μg). Cells were cotransfected with the TopFlash reporter vector or FopFlash control (70 μg) and a cytomegalo-virus/β-galactosidase reporter (to control for transfection efficiency). Transfectants were assayed for luciferase expression using a luciferase assay system according to the instructions of the manufacturer (Promega, Madison, WI). Assays for β-galactosidase expression were performed described previously (23, 24).
Western Blot Analyses of Lysate Proteins
U937 or murine bone marrow cells were lysed by boiling in 2× SDS sample buffer. Lysate proteins (50 μg) were separated by SDS-PAGE (8% acrylamide) and transferred to nitrocellulose according to standard techniques. Western blot analyses were serially probed with antibodies as described (3, 4). Each experiment was repeated at least three times with different batches of lysate proteins. A representative blot is shown. The band intensity on the blots was determined using UN-SCAN-IT software, and results were normalized for the band intensity of the loading control.
Immunoprecipitation and Western Blot Analyses
Lysate proteins from U937 or murine bone marrow cells were immunoprecipitated under denaturing conditions with antibody to Apc (Santa Cruz Biotechnology, Santa Cruz, CA). Precipitated proteins were collected with Staph protein A-Sepharose, separated by SDS-PAGE, and transferred to nitrocellulose as above. Western blot analyses were probed with antibodies as described. Each experiment was repeated at least three times with different batches of lysate proteins. A representative blot is shown.
In Vitro Protein Translation and PTP Assays
In vitro-translated, 35S-labeled Gsk3β was generated in rabbit reticulocyte lysate as described previously (21). For PTP assays, in vitro-translated protein was incubated for 30 min at 30° with recombinant Fap1 or control buffer. Proteins were diluted in radioimmune precipitation assay buffer, precipitated with antibody to phospho-Tyr-216-Gsk3β, and then immunoprecipitates were collected with staphlococcus protein A-Sepharose. Proteins were separated by SDS-PAGE and identified by autoradiography of immunoprecipitates. 1/10 input protein is included as a loading control and to demonstrate that Fap1 does not degrade Gsk3β.
Cell Proliferation Assays
Murine bone marrow cells were transduced with a retroviral vector to Bcr-abl or empty MiGR1 vector control and cultured in GM-CSF (10 ng/ml), IL3 (5 ng/ml), or stem cell factor (100 ng/ml); deprived of cytokines for 24 h (cultured in DMEM supplemented with 10% FCS only); and stimulated for 24 h with a dose titration of GM-CSF (0.02 to 20 ng/ml + 5 ng/ml of IL3). Cells were treated with SLV Fap1-blocking peptide or VLS control peptide, and cell proliferation was determined by incorporation of 3H-thymidine as described (13).
Confocal Fluorescent Microscopy
Cells were fixed with 4% w/v paraformaldehyde, permeabilized with 100% methanol at −20 °C for 10 min, and incubated with primary antibody overnight at 4 °C. Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc.) was added for 2 h at room temperature. Slides were mounted with ProLong Gold anti-fade reagent with DAPI (Invitrogen). Fluorescence signals were acquired using a Zeiss LSM 510 laser-scanning confocal microscope.
Statistical Analysis
Statistical significance was determined by Student's t test and analysis of variance methods using the SigmaPlot and SigmaStat software.
RESULTS
Blocking the Interaction between Fap1 and Apc Increases Gsk3β Activity and Decreases βCatenin
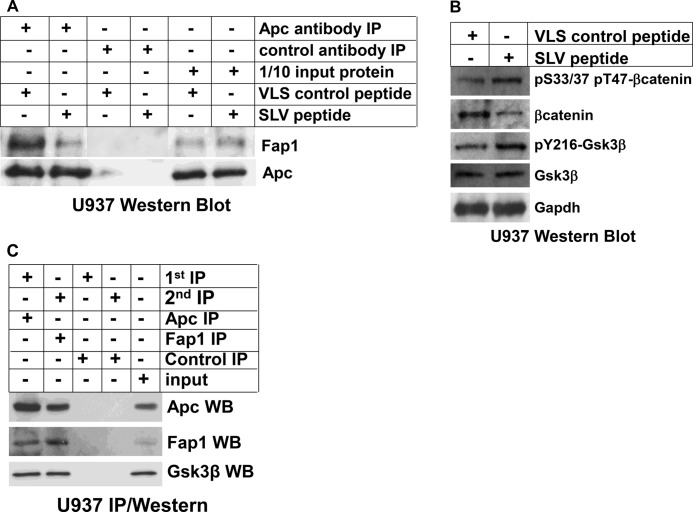
We found previously that treatment of myeloid leukemia cell lines with SLV peptide resulted in decreased Fap1/Fas interaction, increased Fas-tyrosine phosphorylation, and sensitivity to Fas-induced apoptosis (effects not observed with VLS control peptide) (3, 4). In this studies, we tested the ability of SLV peptide to decrease interaction of Fap1 with Apc. For these experiments, U937 myeloid leukemia cells were treated with SLV peptide or VLS control. Cell lysates were immunoprecipitated with an antibody to Apc, and immunoprecipitates were analyzed by Western blot analysis.
We found abundant coprecipitation of Fap1 with Apc in control lysates, but this interaction was decreased by SLV peptide treatment (Fig. 1A). This was not unexpected because Fas and Apc interact with Fap1 through the same Fap1-PDZ domain. Consistent with this, we found that a tripeptide representing the Apc C terminus (threonine-serine-valine) equivalently disrupted Fap1/Apc interaction in vitro (not shown). Treatment with SLV peptide did not alter expression of Fap1 or Apc proteins (Fig. 1A).
FIGURE 1.
Fap1 influences Gsk3β activity, βcatenin protein expression, and serine/threonine phosphorylation of βcatenin in myeloid cell lines. A, treatment of U937 myeloid leukemia cells with SLV peptide disrupts interaction between Fap1 and Fas. U937 cells were treated with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were immunoprecipitated (IP) under non-denaturing conditions with an antibody to Apc or irrelevant control antibody. Immunoprecipitates were analyzed by Western blot analysis for coprecipitation of Fap1 with Apc. Non-immunoprecipitated lysates were analyzed as a loading control. B, treatment with of U937 myeloid leukemia cells with SLV peptide increases tyrosine phosphorylation of Gsk3β, decreases total βcatenin protein, and increases serine/threonine phosphorylated βcatenin. U937 cells were treated with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were analyzed by Western blot analyses serially probed with antibodies to total βcatenin, βcatenin phosphorylated on Ser-33/37 or Thr-47, Gsk3β, Gsk3β phosphorylated on Tyr-216, or Gapdh (as a loading control). C, Fap1, Apc, and Gsk3β are present in a single protein complex. U937 cell lysate proteins were immunoprecipitated under non-denaturing conditions with an Apc antibody or irrelevant control antibody. Immunoprecipitates were recovered, and a second round of immunoprecipitation was performed with a Fap1 antibody or irrelevant control antibody. Immunoprecipitates were separated by SDS-PAGE, and Western blot analyses (WB) were probed with antibodies to Apc, Fap1, and Gsk3β. Non-precipitated proteins were used as a loading control.
We hypothesize that interaction of Fap1 with Apc decreased serine/threonine phosphorylation of βcatenin by Gsk3β, thereby increasing βcatenin protein stability. To investigate this, U937 cells were treated with Fap1-blocking SLV peptide or valine-leucine-serine control peptide and lysate proteins were analyzed by Western blot analysis. We found that treatment with SLV peptide decreased total βcatenin protein but increased phosphorylation of serine/threonine residues involved in degradation (Fig. 1B). This could be because Fap1 disrupted the Apc-Gsk3β-βcatenin complex or because Fap1 inactivated (dephosphorylates) Gsk3β. Consistent with the second hypothesis, we found that SLV peptide increased tyrosine phosphorylation of Gsk3β in U937 cells.
These data suggest that Fap1, Apc, and Gsk3β are present in a single protein complex. To investigate this, lysate proteins from U937 cells were first immunoprecipitated with an Apc antibody, collected with Staphylococcus protein A-Sepharose, and then eluted proteins were precipitated with an antibody to Fap1. Western blot analyses of the immunoprecipitates demonstrated Apc, Fap1, and Gsk3β in the double immunoprecipitation eluate (Fig. 1C). This suggests the existence of a trimolecular protein complex that contains all three proteins rather than sets of heterodimers.
Fap1 Influences βCatenin Activity in Icsbp−/− Myeloid Progenitor Cells
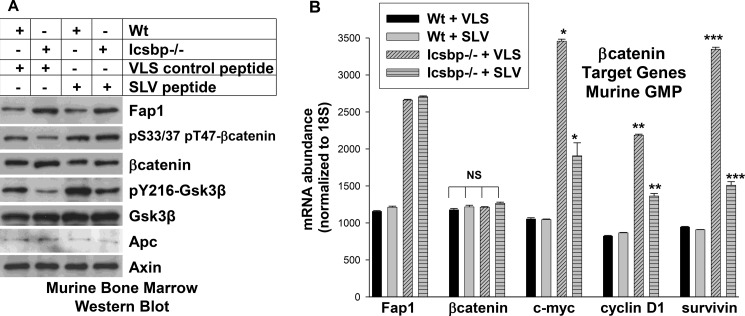
We found previously that Fap1-expression was relatively increased in myeloid progenitor cells from Icsbp−/− mice in comparison to wild-type mice (3). Therefore, we investigated the impact of Fap1 blocking peptide on Gsk3β activity and βcatenin serine/threonine phosphorylation in these cells. For these studies, mononuclear cells were isolated from the bone marrow of WT or Icsbp−/− mice; cultured in GM-CSF, IL3, and Scf; and treated with Fap1-blocking SLV peptide or VLS control. Lysate proteins from CD34+ cells were analyzed by Western blot analysis.
We found that total βcatenin protein was increased in Icsbp−/− cells in comparison to WT cells, consistent with our previous studies (∼35% increase by densitometry of the bands on Western blot analysis) (13). Treatment of Icsbp−/− cells with SLV peptide decreased βcatenin protein (Fig. 2A, ∼42% by densitometry of bands on Western blot analysis). Conversely, serine/threonine phosphorylation of βcatenin was less in Icsbp−/− cells in comparison to WT, and was increased by SLV peptide treatment. Activity (tyrosine phosphorylation) of Gsk3β was less in Icsbp−/− myeloid progenitor cells in comparison to WT but was increased by Fap1-blocking peptide (Fig. 2A). Total Gsk3β protein was equivalent under these four conditions.
FIGURE 2.
Fap1 influences tyrosine phosphorylation of Gsk3β, βcatenin protein expression, and serine/threonine phosphorylation of βcatenin in an Icsbp-dependent manner. A, treatment of primary Icsbp−/− murine myeloid progenitor cells with Fap1-blocking peptide increases tyrosine phosphorylation of Gsk3β, decreases βcatenin protein, and increases βcatenin serine/threonine phosphorylation. Bone marrow mononuclear cells from WT or Icsbp−/− mice were cultured in IL3, GM-CSF, and Scf, and CD34+ cells were separated (considered GMP conditions). Cells were treated with Fap1-blocking SLV peptide or control VLS peptide, and lysates were analyzed by Western blot analysis. Blots were serially probed with antibodies to Fap1, total and serine/threonine phosphorylated βcatenin, total and Tyr-216 phosphorylated Gsk3β, Apc, and Axin (as a loading control). B, treatment of primary Icsbp−/− murine myeloid progenitor cells with SLV peptide decreases βcatenin target gene expression. The cells described above were also analyzed for expression of mRNA representing Fap1, βcatenin, and βcatenin target genes (c-myc, cyclinD1, survivin) by real-time PCR. Statistically significant differences in mRNA abundance with SLV versus VLS peptide are indicated by *, **, or ***. NS, not significant. *, **, and *** represent statistically significant differences.
We also investigated the impact of Fap1-blocking peptide on expression of βcatenin target genes, including c-myc, cyclinD1, and survivin. For these experiments, WT or Icsbp−/− cells were treated with SLV peptide or VLS control and analyzed by real-time PCR for Fap1, βcatenin, and βcatenin target genes. We found increased Fap1 mRNA expression in Icsbp−/− myeloid progenitor cells in comparison to WT cells (as in Ref. 3). Fap1 mRNA was not altered by treatment with SLV peptide, consistent with protein expression levels (Fig. 2B). In contrast, βcatenin mRNA was equivalent in Icsbp−/− and WT cells. We found increased expression of βcatenin target genes in Icsbp−/− cells relative to WT cells (Fig. 2B) (13). Increased βcatenin target gene expression was partly reversed by treating Icsbp−/− cells with SLV peptide, indicating partial Fap1 dependence for this effect.
In control experiments, treatment with SLV was compared with other Fap1 blocking peptides, including TSV (C-terminal sequence from Apc) or cysteine-leucine-glutamine (murine Fap1 C-terminal sequence) (25). We found that treatment with these peptides had the same effect as SLV peptide but their scrambled control peptides did not. CLE peptide competed more efficiently with Fap1 for either Fas or Apc in studies with murine bone marrow cells by coimmunoprecipitation and Western blot analysis (∼5-fold less peptide required, data not shown). This is consistent with a previously documented higher efficiency of interaction between murine Fap1 and murine Fas in comparison to interaction between murine Fap1 and human Fas (2).
Fap1 Influences βCatenin Activity in Bcr-abl+ Myeloid Leukemia Cells
In previous studies, we identified an Icsbp-dependent increase in expression of Fap1 and Gas2 in Bcr-abl-transfected myeloid cell lines (4, 13). We found that increased βcatenin protein and activity (but not mRNA) in Bcr-abl+ cells was partly due to inhibition of calpain activity by Gas2 (13). In this study, we investigate the contribution of Fap1 to increased βcatenin in Bcr-abl+ cells. U937 myeloid leukemia cells were stably transfected with a vector to express Bcr-abl or empty control vector. U937 is a monocytoid leukemia line that does not have t(9;22) (22).
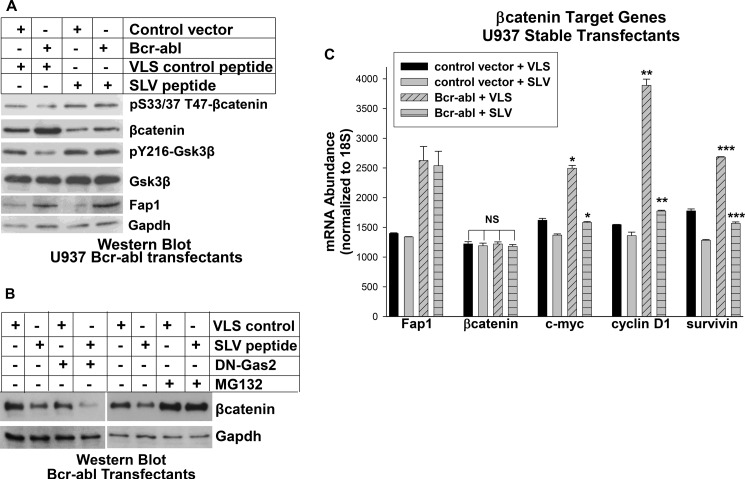
Bcr-abl+ and control cells were treated with Fap1-blocking SLV peptide or VLS control and cell lysates were analyzed by Western blot. We found relatively increased expression of Fap1 and βcatenin in Bcr-abl+ cells in comparison to control cells, as in our previous studies (Fig. 3A) (4, 13). This was associated with decreased serine/threonine phosphorylation of βcatenin, and decreased tyrosine phosphorylation of Gsk3β. Consistent with our hypothesis, SLV treatment decreased total βcatenin protein in Bcr-abl+ cells, but increased serine/threonine phosphorylated βcatenin and tyrosine phosphorylated (active) Gsk3β (Fig. 3A). Total βcatenin and tyrosine phosphorylated Gsk3β were approximately equivalent in SLV peptide treated Bcr-abl+ cells and in VLS treated control U937 cells.
FIGURE 3.
Bcr-abl influences βcatenin expression and activity in a Fap1-dependent manner in myeloid leukemia cells. A, treatment of Bcr-abl+ U937 cells with SLV peptide increases tyrosine phosphorylation of Gsk3β, decreases βcatenin protein, and increases serine/threonine phosphorylation of βcatenin. U937 cells were stably transfected with a Bcr-abl expression vector or empty control vector and treated with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were analyzed by Western blot analyses serially probed with antibodies to Fap1, total and serine/threonine phosphorylated βcatenin, total and Tyr-216 phosphorylated Gsk3β, Apc, and Gapdh (loading control). B, Fap1-blocking SLV peptide decreases βcatenin protein in Bcr-abl+ U937 cells in a manner that was augmented by dominant negative Gas2 but antagonized by the proteasome inhibitor MG132. Bcr-abl+ U937 stable transfectants were cotransfected with a vector to express dominant negative Gas2 (DN-Gas2) or vector control or treated with MG132. The effect of treatment with SLV peptide or VLS control was determined by Western blot analysis for βcatenin. Blots were probed with an antibody to Gapdh as a loading control. C, treatment of Bcr-abl+ U937 cells with SLV peptide decreases βcatenin target gene expression. The cells described in A were also analyzed for expression of mRNA representing Fap1, βcatenin, and βcatenin target genes (c-myc, cyclinD1, survivin) by real-time PCR. Statistically significant differences in mRNA abundance with SLV versus VLS peptide are indicated by *, **, or ***. NS, not significant. *, **, and *** represent statistically significant differences.
To determine the relative contributions of Gas2 and Fap1 to βcatenin protein expression, we cotransfected Bcr-abl+ U937 cells with a vector to express a dominant negative Gas2 (DN-Gas2) or empty control vector. Cells were treated with SLV peptide or VLS control, and lysates were analyzed for βcatenin by Western blot analysis. We found that treatment with SLV peptide decreased βcatenin protein more efficiently than expression of DN-Gas2 (Fig. 3B, decrease of 72% versus 51% by densitometry of Western blot analyses). We also found that SLV peptide cooperated with DN-Gas2 to further decrease βcatenin (90% by densitometry of Western blot analyses). This suggests that Fap1 and Gas2 influence βcatenin through complementary mechanisms.
To investigate whether increased serine/threonine phosphorylation of βcatenin protein in SLV treated increases degradation by the proteasome, we treated some Bcr-abl+ U937 cells MG132 (a proteasome inhibitor). We found an increase in βcatenin protein in Mg132-treated cells that was not altered by treatment with SLV peptide (Fig. 3B, decrease of 61% versus 56% without MG132 by densitometry of Western blot analyses).
We investigated βcatenin activity in Bcr-abl+ cells by analyzing the expression of βcatenin target genes. We found increased expression of c-myc, cyclinD1, and survivin in Bcr-abl+ transfectants in comparison to control cells, and this was partly reversed by SLV peptide treatment (Fig. 3C). In control experiments, we determined that mRNA expression for Fap1, but not βcatenin, was increased by Bcr-abl−expression in U937 cells, consistent with our previous studies (4, 13). Fap1 expression was not altered by treatment with SLV peptide (Fig. 3C).
Three independent stable transfectants pools were selected for each construct, and Bcr-abl transgene expression was confirmed by real-time PCR and Western blot analysis (data not shown, as in Refs. 4, 13). Western blot analyses are representative studies, and real-time PCR results represent data from all transfectant pools.
Fap1 Influences βCatenin Activity in Bcr-abl+ Myeloid Progenitor Cells
U937 is a transformed cell line with abnormalities in cell proliferation and survival prior to introduction of the Bcr-abl oncogene. To study Bcr-abl in the absence of other transforming events, we transduced primary murine bone marrow cells with a Bcr-abl retroviral expression vector or with empty control vector. Transduced cells were cultured in GM-CSF, IL3, and Scf, and CD34+ cells were isolated (considered GMP conditions for these studies) (3, 4, 13). Cells were treated with SLV peptide or VLS control. Previous studies by other investigators determined that a Bcr-abl+ GMP with increased βcatenin activity functions as the leukemia stem cell in chronic-phase CML (8).
We found increased Fap1 and βcatenin protein and decreased serine/threonine phosphorylated βcatenin in Bcr-abl+ myeloid progenitor cells in comparison with control cells (Fig. 4A). The increase in Fap1 protein was not altered by treatment with SLV peptide. In contrast, the increase in βcatenin protein in Bcr-abl+ cells was reversed by SLV peptide (58% decrease by densitometry of bands on Western blot analysis), and βcatenin serine/threonine phosphorylation was increased. Expression and serine/threonine phosphorylation of βcatenin in SLV-treated Bcr-abl+ myeloid progenitor cells was equivalent to expression and serine/threonine phosphorylation in control vector-transduced, VLS-treated cells (Fig. 4A). Tyrosine-phosphorylated (but not total) Gsk3β was less in Bcr-abl-transduced cells in comparison with control cells, and this was reversed by treatment with SLV peptide.
FIGURE 4.
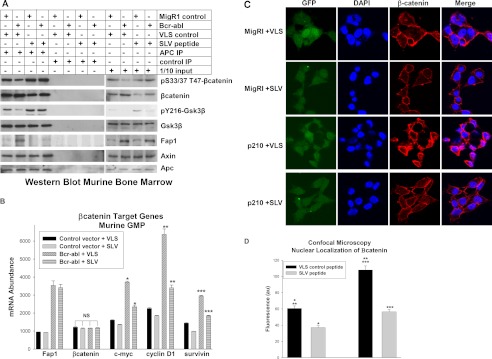
Bcr-abl influences βcatenin expression and activity in a Fap1-dependent manner in primary murine myeloid progenitor cells. A, treatment of Bcr-abl+ primary murine myeloid progenitor cells with SLV peptide decreases Fap1/Apc interaction, decreases tyrosine phosphorylation of Gsk3β, decreases βcatenin protein, and increases serine/threonine phosphorylation of βcatenin. Bone marrow mononuclear cells from WT mice were transduced with a Bcr-abl-expression vector or empty control vector. The GMP population was cultured (as described above) and treated (IP) with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were immunoprecipitated with an antibody to Apc or irrelevant control antibody, and immunoprecipitates were analyzed by Western blot analysis. Non-precipitated lysate was used to control for total protein abundance. Blots were serially probed with antibodies to total and serine/threonine phosphorylated βcatenin, total and Tyr-216 phosphorylated Gsk3β, Fap1, Apc, and Axin. B, treatment of Bcr-abl+ primary murine myeloid progenitor cells with SLV peptide decreases βcatenin-target-gene expression. The cells described above were also analyzed for abundance of mRNA representing Fap1, βcatenin, and βcatenin target genes (c-myc, cyclinD1, survivin) by real-time PCR. Statistically significant differences in mRNA abundance with SLV versus VLS peptide are indicated by *, **, or ***. NS, not significant. C, SLV peptide treatment decreases nuclear βcatenin in Bcr-abl+ murine myeloid progenitor cells. Murine bone marrow cells were transduced with a Bcr-abl-expression vector or empty MigR1 control vector and treated with Fap1-blocking SLV peptide or VLS control. Cells were analyzed by confocal microscopy for nuclear localization of βcatenin. Transduced cells are green (green fluorescent protein label in vector), nuclei are indicated in blue (DAPI stain), βcatenin is shown in red (Cy3-labeled secondary antibody), and purple indicates merged DAPI + Cy3 signals. D, nuclear βcatenin is significantly decreased by SLV peptide treatment of Bcr-abl+ murine bone marrow cells. The cells described above were analyzed for fluorescent intensity of the merged signal. At least 30 cells were counted in three fields for each sample. Statistically significant differences are indicated by *, **, or ***.
These lysates were also immunoprecipitated with an Apc-antibody and analyzed for association of Fap1 with Apc, Axin, Gsk3β, and βcatenin by Western blot analysis. We found increased Fap1 in Apc coimmunoprecipitates from Bcr-abl-transduced cells in comparison with control cells (Fig. 4A). We also found increased total βcatenin protein in Apc coimmunoprecipitates from Bcr-abl+ cells but decreased serine/threonine phosphorylated βcatenin. We also found that Gsk3β tyrosine phosphorylation was less in Apc coimmunoprecipitates from Bcr-abl+ cells in comparison with control cells. Consistent with our hypothesis, these Bcr-abl-related differences were reversed by treatment with SLV peptide (Fig. 4A).
We used these transduced cells to investigate the effects of Bcr-abl and Fap1-blocking peptide on expression of βcatenin target genes. We found increased expression of c-myc, cyclinD1, and survivin in Bcr-abl-transduced cells relative to control cells, consistent with our previous studies (13). We also found that treatment of these cells with SLV peptide partly reversed the increase in βcatenin target gene expression in Bcr-abl+ cells (Fig. 4B). As in the studies above, mRNA expression of Fap1, but not βcatenin, was increased in Bcr-abl+ cells versus control cells, and this was not influenced by treatment with SLV peptide (Fig. 4B).
These studies were repeated in at least two independent transduction experiments, and Bcr-abl-transgene expression was confirmed by real-time PCR and Western blot analysis (data not shown). Representative Western blot analyses are shown, and real-time PCR represents data from all transduction experiments.
We also used these cells to study the role of Fap1 blockade on βcatenin nuclear localization. For these experiments, transduction was determined by expression of green fluorescent protein by the MigR1 vector used to express Bcr-abl (versus control MigR1). Cells were stained with antibodies to colocalize βcatenin with nuclear structures. Transduced murine GMP were studied after treatment with Fap1-blocking SLV peptide or VLS control. We found that nuclear βcatenin was significantly increased in Bcr-abl-transduced cells in comparison to control vector-transduced cells (Fig. 4C). We also found that SLV peptide significantly decreased nuclear βcatenin abundance in Bcr-abl-transduced cells (Fig. 4C). These data were quantified by measuring the fluorescent intensity of the nuclear colocalization signal for 30 cells in three different high-power fields (Fig. 4D).
Fap1 Influences βCatenin-induced Transactivation in Bcr-abl+ Myeloid Leukemia Cells
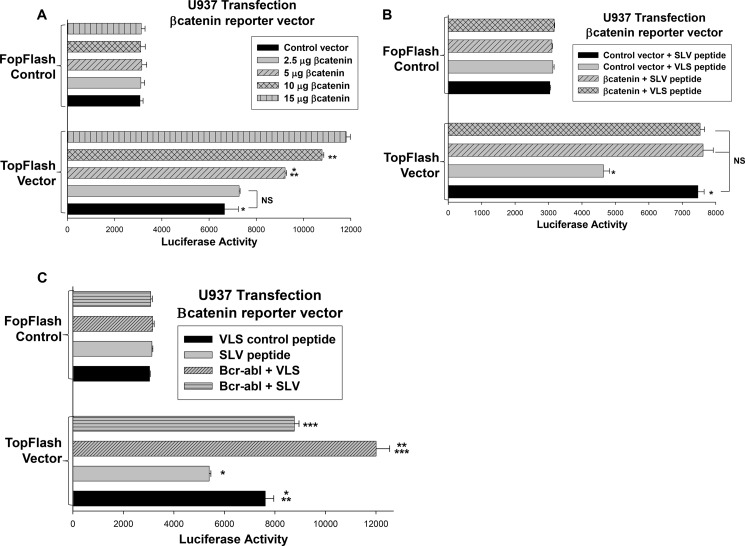
In the studies above, we used βcatenin target gene expression as a measure of βcatenin activity. βCatenin activity is more directly measured by determining activation of an artificial promoter/reporter construct with a βcatenin/Tcf-binding consensus sequence (26). We used this construct (referred to as TopFlash) and a non-βcatenin binding control construct (FopFlash) in U937 transfection studies. For initial experiments, cells were cotransfected with a dose titration of a βcatenin expression vector. We found that TopFlash reporter activity increased in a βcatenin dose-dependent manner but that control FopFlash activity did not (Fig. 5A). The goal of this study was to identify the plasmid doses that result in maximal or minimal effects of overexpressing βcatenin in these cells.
FIGURE 5.
Fap1 contributes to βcatenin-induced transcriptional activation in Bcr-abl+ cells. A, βcatenin-overexpression in U937 cells activates a reporter construct with a βcatenin/Tcf binding consensus sequence in a dose-dependent manner. U937 cells were cotransfected with a construct with a βcatenin/Tcf-binding consensus sequence linked to a minimal promoter and reporter (TopFlash) or a construct with mutation of the βcatenin binding site (FopFlash) and a vector to express βcatenin (or empty control vector). Reporter activity was determined and statistically significant differences are indicated by * and **. NS, not significant. B, decreased activity of a βcatenin-binding reporter construct in SLV peptide-treated U937 transfectants is reversed by βcatenin overexpression. U937 cells were cotransfected with TopFlash or FopFlash (control) reporter constructs and a vector to express βcatenin (2.5 μg, the lowest dose that does not increase TopFlash activity above background). Transfectants were treated with Fap1-blocking SLV peptide or control VLS peptide, and reporter gene activity was determined. Statistically significant differences with SLV versus VLS peptide are indicated by *. C, treatment of U937 transfectants with SLV peptide blocks Bcr-abl-induced activation of a βcatenin-binding reporter construct. U937 cells were cotransfected with TopFlash or control FopFlash reporter vector and a vector to express Bcr-abl (or control vector). Transfectants were treated with Fap1-blocking SLV peptide or control VLS peptide, and reporter gene activity was determined. Statistically significant differences in TopFlash reporter activity are indicated by *, **, or ***.
Using these results, we cotransfected U937 cells with a vector to express TopFlash or FopFlash and a vector to express βcatenin (2.5 μg) or empty control vector. Transfectants were treated with Fap1-blocking SLV peptide or control peptide, and reporter activity was determined. We found that SLV peptide significantly decreased activity of the TopFlash reporter vector in control transfectants (p < 0.01, n = 4) (Fig. 5B). In contrast, SLV peptide had a minimal effect on this reporter construct in cells that were cotransfected with increasing amounts of a βcatenin expression vector (p = 0.8, n = 4).
We also tested the effect of blocking Fap1 on βcatenin activity in Bcr-abl-expressing transfectants. U937 cells were cotransfected with the TopFlash or FopFlash reporter vector and a vector to express Bcr-abl. Cells were treated with SLV or VLS (control) peptide, and reporter activity was determined. We found that TopFlash reporter activity was significantly greater in Bcr-abl-transfectants in comparison with control transfectants (p < 0.01, n = 4) (Fig. 5C). SLV peptide significantly decreased TopFlash activity in Bcr-abl-expressing transfectants (p < 0.03, n = 4). These studies suggest a Fap1-dependent increase in βcatenin activity in Bcr-abl-expressing cells. In control studies, we found that FopFlash reporter activity was not significantly influenced by expression of βcatenin, Bcr-abl, or treatment with SLV or VLS peptides.
Gsk3β Mediates a Fap1-dependent Increase in βCatenin Activity in Bcr-abl+ Cells
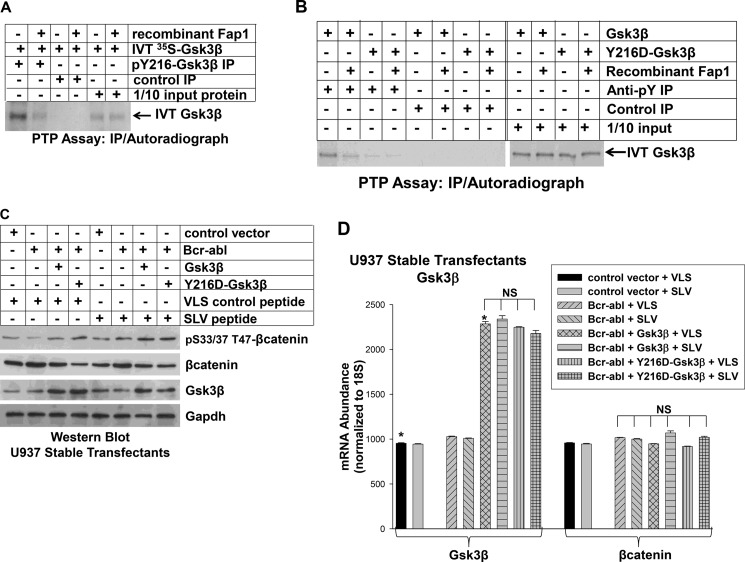
The studies above support the hypothesis that Apc recruits Fap1 to the Apc/Gsk3β/βcatenin complex and leads to inactivation of Gsk3β. Because Fap1 is a PTP and Gsk3β is activated by tyrosine phosphorylation, we considered the possibility that Gsk3β is a Fap1 substrate. We used an in vitro PTP assay with Gsk3β expressed in rabbit reticulocyte lysate to investigate this hypothesis. We first confirmed that in vitro-translated Gsk3β is phosphorylated on Tyr-216 by immunoprecipitation with a phospho-Tyr-216-Gsk3β-specific antibody (Fig. 6A). This residue is the key to Gsk3β activity (27).
FIGURE 6.
Fap1 decreases activity and tyrosine phosphorylation of Gsk3β. A, recombinant Fap1 dephosphorylates in vitro-translated (IVT) Gsk3β. Gsk3β was translated in vitro in rabbit reticulocyte lysate (in the presence of [35S]methionine) and incubated with recombinant Fap1 or with buffer control. Proteins were immunoprecipitated (IP) with an antibody to phospho-Tyr-216-Gsk3β or irrelevant control antibody, separated by SDS-PAGE, and identified by autoradiography. 1/10 input (non-immunoprecipitated) Gsk3β was used as a loading control. B, recombinant Fap1 dephosphorylates Gsk3β primarily at Tyr-216. Gsk3β or a form of Gsk3β with mutation of Tyr-216 (Y216D) were in vitro-translated in rabbit reticulocyte lysate as above. Proteins were incubated with recombinant Fap1 or buffer control and immunoprecipitated with an antibody to phospho-tyrosine residues (4G10) or with irrelevant control antibody. Immunoprecipitates were separated by SDS-PAGE and detected by autoradiography. C, expression of constitutively active Gsk3β (Y216D) in Bcr-abl+ U937 cells decreases βcatenin protein and increases serine/threonine phosphorylation of βcatenin in a manner that is not influenced by SLV peptide. U937 cells were cotransfected with a Bcr-abl expression vector and a vector to express Y216D-Gsk3β or WT Gsk3β (or empty control vector). Cells were treated with Fap1-blocking SLV peptide or VLS control peptide and lysates and analyzed by Western blot analysis. Blots were serially probed with antibodies to total and serine/threonine phosphorylated βcatenin, Gsk3β, and Gapdh (as a loading control). D, expression of constitutively active Gsk3β (Y216D) in Bcr-abl+ U937 cells does not alter expression of βcatenin mRNA. The stably transfected U937 cells described above were also analyzed for mRNA expression of Gsk3β and βcatenin by real-time PCR. Statistically significant differences are indicated by *. NS, not significant.
We incubated in vitro-translated Gsk3β with recombinant Fap1 or buffer control under standard PTP assay conditions (as in Ref. 21). Following incubation, proteins were immunoprecipitated with an antibody to phospho-Tyr-216-Gsk3β, separated by SDS-PAGE, and identified by autoradiography. We found that incubation with recombinant Fap1 decreased phospho-Tyr-216 of Gsk3β but did not alter total Gsk3β (Fig. 6A).
To investigate the possibility that Fap1 dephosphorylated other Tyr residues in Gsk3β, we generated in vitro-translated Gsk3β with mutation of Tyr-216 to an amino acid that cannot be phosphorylated (Y216D-Gsk3β). WT and Y216D-Gsk3β were treated with recombinant Fap1 (or control, as described above), and lysates were immunoprecipitated with an antibody that recognizes phospho-tyrosine residues (4G10). Although the amounts of input protein were equal, far less Y216F-Gsk3β was immunoprecipitated by anti-phospho-tyrosine antibody in comparison with WT Gsk3β (Fig. 6B).
After Fap1 treatment, the amount of WT Gsk3β that immunoprecipitated with anti-phospho-tyrosine antibody decreased (Fig. 6B, as in A). However, Fap1 treatment resulted in little change in the amount of Y216D-Gsk3β that was immunoprecipitated by anti-phospho-tyrosine antibody. The amount of precipitating Fap1-treated WT Gsk3β and Y216D-Gsk3β were similar to each other and to untreated Y216D-Gsk3β (Fig. 6B).
If Fap1 influences βcatenin protein stability and activity by inactivating (dephosphorylating) Gsk3β, expressing a constitutively active form of Gsk3β will increase serine/threonine phosphorylation of βcatenin and destabilize βcatenin protein in Bcr-abl+ cells. To test this hypothesis, we used Y216D-Gsk3β because Asp is a charged amino acid that mimics phospho-tyrosine residues but is not influenced by PTP activity. If our hypothesis is correct, treatment of Bcr-abl+Y216D-Gsk3β-expressing cells with SLV peptide will not influence βcatenin serine/threonine phosphorylation or stability.
We generated U937 stable transfectants with a vectors to coexpress Bcr-abl and WT Gsk3β or Y216D-Gsk3β (or empty vector control). Cells were treated with SLV peptide or control VLS peptide, and lysate proteins were analyzed by Western blot analysis. We found that expression of Y216D-Gsk3β decreased βcatenin protein in Bcr-abl+ cells in a manner that was not influenced by SLV peptide (Fig. 6C, decrease of 43% versus 39% by densitometry of bands on Western blot analyses). In contrast, SLV treatment further decreased βcatenin expression (decrease of 32% by densitometry of bands on Western blot analyses) and increased serine/threonine phosphorylation of βcatenin in control cells. Expression of βcatenin mRNA was not altered by overexpression of either form of Gsk3β or by treatment with SLV peptide (Fig. 6D).
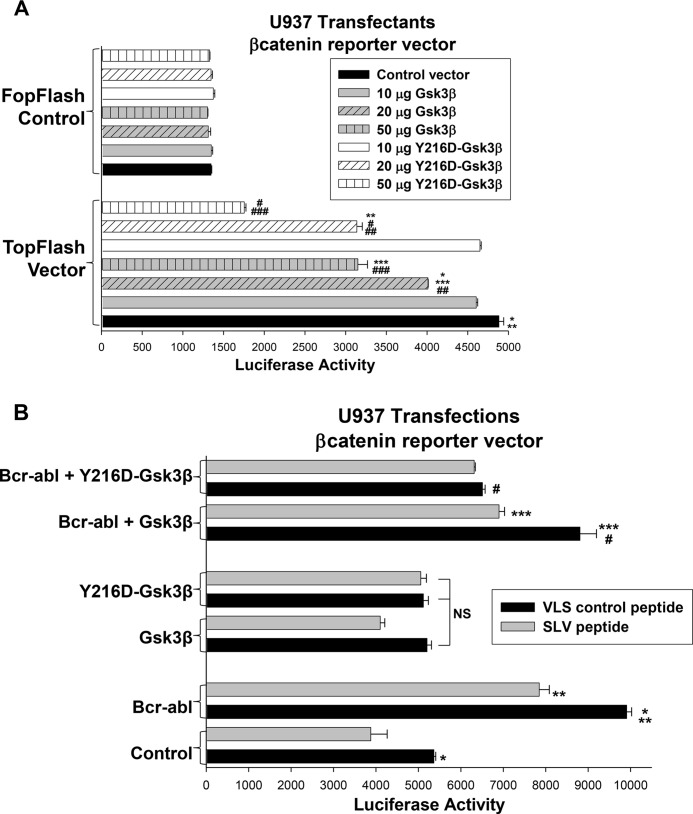
We next determined whether Y216D-Gsk3β influenced βcatenin activity. For these studies, U937 cells were cotransfected with the TopFlash βcatenin-binding reporter vector or FopFlash control vector and vectors to express dose titrations of WT Gsk3β or Y216D-Gsk3β. We found that both WT and Y216D-Gsk3β significantly decreased TopFlash activity in a dose dependent manner (Fig. 7A) (p < 0.01, n = 4). We also found that the effect of Y216D-Gsk3β was significantly greater than the effect of the wild-type Gsk3β at most doses (p < 0.005, n = 3 for transfectants with 20 or 50 μg of vector to express WT or Y216D-Gsk3β).
FIGURE 7.
Constitutive activation of Gsk3β bypasses Fap1. A, expression of Y216D-Gsk3β in U937 cells decreases activity of a βcatenin-binding reporter construct more efficiently than WT Gsk3β. U937 cells were cotransfected with the βcatenin-activated TopFlash reporter vector or FopFlash control vector and a dose titration of vectors to overexpress WT or Y216D-Gsk3β (or control vector). Statistically significant differences in reporter expression are indicated by *, **, ***, #, ##, or ###. B, Y216D-Gsk3β blocks Bcr-abl-induced activation of a βcatenin-binding reporter construct in U937 transfectants in a manner that is not influenced by SLV peptide. U937 cells were cotransfected with the TopFlash or control FopFlash reporter vector, a vector to express Bcr-abl (or control vector), and a vector to express WT or Y216D-Gsk3β (or vector control). Transfectants were treated with Fap1-blocking SLV peptide or control VLS peptide, and reporter gene activity was determined. Statistically significant differences are indicated by *, **, ***, or #. NS, not significant.
We also investigated the effect of constitutive Gsk3β activation on Fap1-dependent βcatenin activity. U937 cells were cotransfected with the TopFlash or control FopFlash reporter vector, a vector to express WT or Y216D-Gsk3β (or control vector), and a vector to express Bcr-abl (or control vector). Transfectants were treated with Fap1-blocking SLV peptide or VLS control. We found that SLV peptide significantly decreased reporter activity in Bcr-abl+ transfectants in a manner that was not influenced by overexpression of WT Gsk3β (p ≤ 0.03, n = 4) (Fig. 7B). In contrast, Y216D-Gsk3β expression significantly decreased Bcr-abl-induced TopFlash reporter activity (p < 0.001, n = 4) in a manner that was not altered by SLV peptide treatment (p = 0.6, n = 4) (Fig. 7B). In control experiments, expression of the FopFlash control vector was not influenced by transfection with vectors to express WT Gsk3β, Y216D-Gsk3β, or Bcr-abl, or by treatment with SLV peptide.
Fap1 Influences Cytokine Hypersensitivity of Bcr-abl+ Myeloid Progenitor Cells
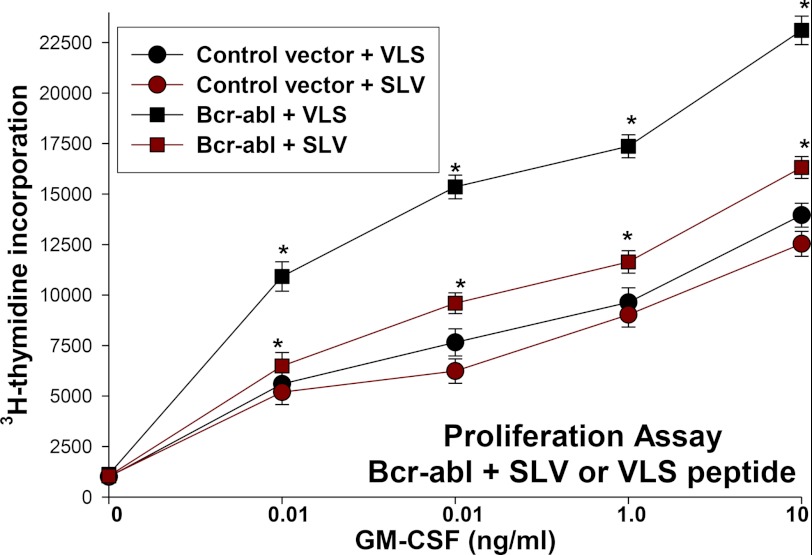
These studies established a functional connection between increased Fap1 expression and increased βcatenin activity in Bcr-abl+ myeloid cells. Because βcatenin target genes include c-myc and cyclinD1, this suggests the possibility that increased Fap1 contributes to cytokine hypersensitivity of Bcr-abl+ myeloid progenitor cells. To investigate this, we used Bcr-abl-transduced murine GMP as described above. These cells (or control vector-transduced cells) were deprived of cytokines for 24 h, stimulated with a dose titration of GM-CSF in the presence of Fap1-blocking SLV peptide or VLS control, and then proliferation was evaluated by incorporation of 3H-thymidine (as in Ref. 13).
We found that treatment with SLV peptide significantly decreased proliferation of Bcr-abl+ cells at all tested concentrations of GM-CSF (p < 0.001, n = 6) (Fig. 8). In control experiments, expression of Bcr-abl was confirmed by Western blot analysis and real-time PCR (data not shown). These studies identify a functional consequence to increased Fap1 in Bcr-abl+ cells.
FIGURE 8.
Treatment with Fap1 blocking peptide decreases cytokine hypersensitivity of Bcr-abl+ primary murine myeloid progenitor cells. Primary murine bone marrow cells were transduced with a Bcr-abl expression vector or empty control vector. Cells were cultured under GMP conditions, deprived of cytokines, and stimulated with a dose titration of GM-CSF in the presence of Fap1-blocking SLV peptide or VLS control peptide. Proliferation was determined by incorporation of 3H-thymidine. Statistically significant differences between proliferation at a given GM-CSF dose are indicated by * (p < 0.001, n = 3).
DISCUSSION
The leukemia stem cell in CML has been functionally characterized as a GMP with increased βcatenin activity (8). We found previously that increased expression of Gas2 (a calpain inhibitor) stabilized βcatenin in Bcr-abl+ myeloid progenitor cells in an Icsbp-dependent manner (13). In this study, we identified another Icsbp-dependent mechanism for stabilizing βcatenin protein in Bcr-abl+ cells. We found previously that decreased Icsbp-expression in Bcr-abl+ myeloid progenitor cells increased expression of Fap1 and impaired Fas-induced apoptosis (3, 4). In this study, we found that increased Fap1 in Bcr-abl+ cells also stabilized βcatenin protein by participating in a multiprotein complex that includes Apc, Gsk3β, and βcatenin. We found that Fap1 dephosphorylated (inactivated) Gsk3β. Inactivation of Gsk3β decreased inhibitory serine/threonine phosphorylation of βcatenin, thereby decreasing degradation of βcatenin by the proteasome. Therefore, we have identified at least two Icsbp-dependent mechanisms that stabilize βcatenin in CML.
Increased βcatenin activity is associated with poor prognosis and disease progression in human CML. Because neither CTNNB1 transcription nor Wnt signaling are increased consistently in CML (18), βcatenin stabilization because of increased expression of Fap1 and Gas2 may be important to disease pathogenesis. Other investigators identified inactivating mutations of the gene encoding Gsk3β that arose during disease progression in a murine CML model (28). These mutations were associated with decreased serine/threonine phosphorylation and degradation of βcatenin. However, although decreased serine/threonine phosphorylation of βcatenin was frequently associated with increased βcatenin activity in human CML, GSK3B mutations were infrequent (28). These previous studies and our work suggest that multiple mechanisms stabilize βcatenin in CML.
We previously identified Icsbp-binding negative cis elements in the PTPN13 and GAS2 promoters (3, 4, 10, 13). These homologous cis elements bind a protein complex that includes Tel and histone deacetylase 3 (Hdac3) (3, 13). Assembly of this complex on DNA required prebinding of Tel, which recruits the other two proteins. Interestingly, expression of Tel mRNA and protein was slightly increased in cells transduced with a Bcr-abl expression vector4. In human CML, Tel expression is not altered chronic phase, but decreases significantly with relapse or blast crisis3. This would facilitate an increase in Fap1 and Gas2 during disease progression in CML and stabilization of βcatenin protein.
Increased βcatenin activity facilitates the proliferative response of myeloid progenitor cells to hematopoietic cytokines by increasing expression of target genes such as c-myc and cyclinD1. βCatenin also contributes to Fas-resistance through increased expression of survivin, an inhibitor of apoptosis protein that inhibits caspase 3. Therefore, the net effect of increased Fap1 in CML is cytokine hypersensitivity and Fas resistance. This may contribute to persistence of CML LSC during tyrosine kinase treatment. Persistent CML LSC are hypothesized to be quiescent and, therefore, relatively indifferent to the antiproliferative effects of tyrosine kinase inhibitors. These cells may slowly expand over time, providing a reservoir for ongoing mutation and disease progression in CML.
Our studies suggest that blocking the action of Fap1 may decrease βcatenin activity and overcome Fas resistance of Bcr-abl+ bone marrow cells. This might have specific implications for eliminating the CML LSC in conjunction with a tyrosine kinase inhibitor. The observation that protein-protein interactions by Fap1 can be blocked by a tripeptide suggests that Fap1-PDZ domains might be a rationale target for small molecule development. This will be the focus of future studies in the laboratory.
Oncomine, Research Addition, University of Michigan, Ann Arbor, MI.
W. Huang, L. Bei, and E. A. Eklund, unpublished observations.
- PTP
- protein tyrosine phosphatase
- CML
- chronic myeloid leukemia
- LSC
- leukemia stem cell(s)
- GMP
- granulocyte-monocyte progenitor
- Icsbp
- interferon consensus sequence binding protein.
REFERENCES
- 1. Saras J., Claesson-Welsh L., Heldin C. H., Gonex L. J. (1994) Cloning and characterization of PTPL1, a PTP with similarities to cytoskeletal-associated proteins. J. Biol. Chem. 269, 24082–24089 [PubMed] [Google Scholar]
- 2. Yanagisawa J., Takahashi M., Kanki H., Yano-Yanagisawa H., Tazunoki T., Sawa E., Nishitoba T., Kamishohara M., Kobayashi E., Kataoka S., Sato T. (1997) The molecular interaction of Fas and Fap1. J. Biol. Chem. 272, 8539–8545 [DOI] [PubMed] [Google Scholar]
- 3. Huang W., Zhu C., Wang H., Horvath E., Eklund E. A. (2008) The interferon consensus sequence binding protein (ICSBP/IRF8) represses PTPN13 gene transcription in differentiating myeloid cells. J. Biol. Chem. 283, 7921–7935 [DOI] [PubMed] [Google Scholar]
- 4. Huang W., Bei L., Eklund E. A. (2012) Fas-Associated Phosphatase 1 (Fap1) mediates Fas-resistance in myeloid progenitor cells expressing the Bcr-abl oncogene. Leuk Lymphoma 54, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kozlov G., Banville D., Gehring K., Ekiel I. (2002) Solution structure of the PDZ2 domain from cytosolic human phosphatase hPTP1E complexed with a peptide reveals contribution of the B2-B3 loop to PDZ domain-ligand interactions. J. Mol. Biol. 320, 813–820 [DOI] [PubMed] [Google Scholar]
- 6. Zhang J., Sapienza P. J., Ke H., Chang A., Hengel S. R., Wang H., Phillips G. N., Lee A. L. (2010) Crystallographic and nuclear magnetic resonance evaluation of the impact of peptide binding to the second PDZ domain of protein tyrosine phosphatase 1E. Biochemistry 49, 9280–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamieson C. H., Ailles L. E., Dylla S. J., Muijtjens M., Jones C., Zehnder J. L., Gotlib J., Li K., Manz M. G., Keating A., Sawyers C. L., Weissman I. L. (2004) Granulocyte-macrophage progenitors as candidate leukemia stem cells in blast crisis CML. NEJM 351, 657–667 [DOI] [PubMed] [Google Scholar]
- 8. Minami Y., Stuart S. A., Ikawa T., Jiang Y., Banno A., Hunton I. C., Young D. J., Naoe T., Murre C., Jamieson C. H., Wang J. Y. (2008) Bcr/abl-transformed GMP as myeloid leukemia stem cells. Proc. Natl. Acad. Sci. U.S.A. 105, 17967–17972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt M., Nagel S., Proba J., Thiede C., Ritter M., Waring J. F., Rosenbauer F., Huhn D., Wittig B., Horak I., Neubauer A. (1998) Lack of interferon consensus sequence binding protein transcripts in human myeloid leukemias. Blood 91, 22–29 [PubMed] [Google Scholar]
- 10. Huang W., Hu L., Bei L., Hjort E., Eklund E. A. (2012) The leukemia-associated fusion-protein Tel-platelet derived growth factor receptor B (Tel-PdgfRB) inhibits transcriptional repression of the PTPN13 gene by the interferon consensus sequence binding protein (Icsbp). J. Biol. Chem. 287, 8110–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X., Ren R. (1998) Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice. A novel model for chronic myelogenous leukemia. Blood 92, 3829–3840 [PubMed] [Google Scholar]
- 12. Hao S. X., Ren R. (2000) Expression of ICSBP is down-regulated in bcr-abl-induced murine CML-like disease and forced co-expression of ICSBP inhibits Bcr-abl-induced MPD. Mol. Cell. Biol. 20, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W., Zhou W., Saberwal G., Konieczna I., Horvath E., Katsoulidis E., Platanias L. C., Eklund E. A. (2010) The interferon consensus sequence binding protein (ICSBP) decreases βcatenin-activity in myeloid cells by repressing GAS2 transcription. Mol. Cell. Biol. 30, 4575–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holtschke T., Löhler J., Kanno Y., Fehr T., Giese N., Rosenbauer F., Lou J., Knobeloch K. P., Gabriele L., Waring J. F., Bachmann M. F., Zinkernagel R. M., Morse H. C., 3rd, Ozato K., Horak I. (1996) Immuno-deficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 [DOI] [PubMed] [Google Scholar]
- 15. Konieczna I., Horvath E., Wang H., Lindsey S., Saberwal G., Bei L., Huang W., Platanias L., Eklund E. A. (2008) Constitutive activation of SHP2 cooperates with ICSBP-deficiency to accelerate progression to acute myeloid leukemia. J. Clin. Invest. 118, 853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272, 1023–1026 [DOI] [PubMed] [Google Scholar]
- 17. Barker N., Morin P. J., Clevers H. (2000) The Yin-Yang of TCF/β-catenin signaling. Adv. Cancer Res. 77, 1–24 [DOI] [PubMed] [Google Scholar]
- 18. Simon M., Grandage V. L., Linch D. C., Khwaja A. (2005) Constitutive activation of the Wnt/B catenin signaling pathway in myeloid leukemia. Oncogene 24, 2410–2420 [DOI] [PubMed] [Google Scholar]
- 19. Benetti R., Copetti T., Dell'Orso S., Melloni E., Brancolini C., Monte M., Schneider C. (2005) The calpain system is involved in the constitutive regulation of βcatenin signaling functions. J. Biol. Chem. 280, 22070–22080 [DOI] [PubMed] [Google Scholar]
- 20. Zhu C., Saberwal G., Lu Y., Platanias L. C., Eklund E. A. (2004) The interferon consensus sequence binding protein (ICSBP) activates transcription of the gene encoding Neurofibromin 1 (NF1). J. Biol. Chem. 279, 50874–50885 [DOI] [PubMed] [Google Scholar]
- 21. Kautz B., Kakar R., David E., Eklund E. A. (2001) SHP1 protein tyrosine phosphatase inhibits transcription of the CYBB and NCF2 genes in undifferentiated myeloid cell lines by inhibiting interaction of PU. 1, IRF1, ICSBP and CBP. J. Biol. Chem. 276, 37868–37878 [DOI] [PubMed] [Google Scholar]
- 22. Larrick J. W., Fischer D. G., Anderson S. J., Koren H. S. (1980) Characterization of a human macrophage-like cell line stimulated to differentiate in vitro. A model of macrophage functions. J. Immunol. 125, 6–12 [PubMed] [Google Scholar]
- 23. Eklund E. A., Jalava A., Kakar R. (1998) PU. 1, interferon regulatory factor 1, and interferon consensus sequence binding protein cooperate to increase gp91phox expression. J. Biol. Chem. 273, 13957–13965 [DOI] [PubMed] [Google Scholar]
- 24. Eklund E. A., Kakar R. (1999) Recruitment of CBP by PU. 1, IRF1 and ICSBP is necessary for gp91phox and p67phox expression. J. Immunol. 163, 6095–6105 [PubMed] [Google Scholar]
- 25. Saras J., Engström U., Góñez L. J., Heldin C. H. (1997) Characterization of the interactions between PDZ domains of the protein-tyrosine phosphatase PTPL1 and the carboxyl-terminal tail of Fas. J. Biol. Chem. 272, 20979–20981 [DOI] [PubMed] [Google Scholar]
- 26. Kim K., Pang K. M., Evans M., Hay E. D. (2000) Overexpression of β-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol. Biol. Cell 11, 3509–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhat R. V., Shanley J., Correll M. P., Fieles W. E., Keith R. A., Scott C. W., Lee C. M. (2000) Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3β in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. U.S.A. 97, 11074–11079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abrahamsson A. E., Geron I., Gotlib J., Dao K. H., Barroga C. F., Newton I. G., Giles F. J., Durocher J., Creusot R. S., Karimi M., Jones C., Zehnder J. L., Keating A., Negrin R. S., Weissman I. L., Jamieson C. H. (2009) Glycogen synthase kinase 3β missplicing contributes to leukemia stem cell generation. Proc. Natl. Acad. Sci. U.S.A. 106, 3925–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]