Abstract
Objective
To describe clinical signs, pathology, diagnosis and treatment of Cape vultures in which Aspergillus fumigatus (A. fumigatus) and mixed species of bacteria were isolated.
Methods
Six Cape vultures sourced from South Africa for exhibition at Al Ain Zoo developed illness manifesting as anorexia, dyspnea, polyuria and lethargy. Three vultures died manifesting “pneumonia-like syndrome”. These three vultures were necropsied and gross lesions recorded, while organ tissues were collected for histopathology. Internal organs were swabbed for bacteriology and mycology. From live vultures, blood was collected for hematology and biochemistry, oropharyngeal and cloacal swabs were collected for mycology and bacteriology.
Results
A. fumigatus was isolated from the three dead vultures and two live ones that eventually survived. One of the dead vulture and two live vultures were co-infected with A. fumigatus and mixed species of bacteria that included Clostridium perfringens, Pseudomonas, Staphylococcus, Escherichia, Proteus, Enterococcus and Enterbacter. One of the Cape vulture and a Lappet-faced vulture, however, were free of Aspergillus or bacterial infections. At necropsy, intestinal hemorrhages were observed and the lungs were overtly congested with granulomas present on caudal air sac. Histopathological examinations demonstrated granulomatous lesions that were infiltrated by mononuclear cells and giant cells.
Conclusions
Aspergillosis is a persistent threat to captive birds and we recommend routine health assessments so that early diagnosis may prompt early treatment. It is likely that prompt prophylaxis by broad spectrum antibiotics and antifungals medication contributed to the survival of some of the vultures.
Keywords: Aspergillosis, Cape vultures, Wild birds, Aspergillus, Bacteria
1. Introduction
Fungi in the genus Aspergillus are found everywhere and when inhaled by immunocompromised hosts, they cause disease referred to as Aspergillosis. Birds are particularly more susceptible to the disease compared to humans and mammals[1]. The disease causes illness and deaths of captive and wild birds of all ages. Predisposing factors to Aspergillus infection are diverse, unpredictable and tend to suppress host's immune response. Poor husbandry, ventilation, sanitation and warm humid ambience are common conditions that predispose birds to aspergillosis in captivity[2],[3]. Physical trauma, capture, translocation and quarantine also predispose birds to the disease[4]. Among the Aspergillus species, Aspergillus fumigatus accounts for over 95% of the cases of avian infections[1]. Clinical signs that characterize the disease are imprecise while diagnosis is based on a variety of tests that include fungal culture, histology, as well as evaluation of some chemistry and hematological parameters[5]. Definitive diagnosis requires demonstration of the presence of the organism by cytology or histopathology and its identification by culture[6]. Aspergillosis in captive and free-ranging birds remains one of the most frequently encountered and difficult to treat diseases in avian medicine[7]. Moreover, acquired resistance against antimycotics like itraconazole has already become a problem in humans[8]. Aspergillosis causes devastating economic losses in poultry farming[9] while mass mortalities of wild birds remain unresolved challenge in conservation. Cape vulture (Gyps coprotheres) (Figure 1) is listed as vulnerable[10] and among those species of vultures whose survival is at risk due to increased incidences of poisoning, obliteration of breeding sites and electrocution[10],[11]. Al Ain zoo acquired Cape vultures (n=6) from the Republic of South Africa in February 2010 for the purpose of exhibition. However, some of these birds became ill and died. The aim of this study is therefore to describe clinical manifestations, diagnosis, gross and histopathology findings of the Cape vultures.
Figure 1. Alive Cape vulture on a perch at Al Ain Zoo.

2. Materials and methods
Al Ain Zoo is 350 hectares at latitude 24°10′45.37″ N, and longitude 55°44′19.99″ E in Abu Dhabi Emirate. The zoo is open to public and currently holds diverse species of mammals (n=73), reptiles (n=31), and aves (n=92). Among the aves, there were four species of vultures, namely, Egyptian vultures (Neophron percnopterus) (n=3), Ruppell's vulture (Gyps rueppelli) (n=6), Cape vultures (Gyps coprotheres) (n=6), and Lappet-faced vultures (Torgos tracheliotus) (n=3). Upon arrival in the zoo in February 2010, the six Cape vultures were quarantined in an open facility for 30 d. During quarantine period, the birds' body condition was assessed and samples taken to screen for diseases (bacterial, viral and mycology). Laboratory results showed freedom from disease. The birds were later kept together with a Lappet-faced vulture in a wire-meshed enclosure. In September 2010, the six Cape vultures and Lappet-faced vulture were physically restrained and moved to a different enclosure within the zoo. A month later, in October 2010, two of these Cape vultures became ill, manifesting anorexia, dyspnea, polyuria and lethargy.
2.1. Case presentation
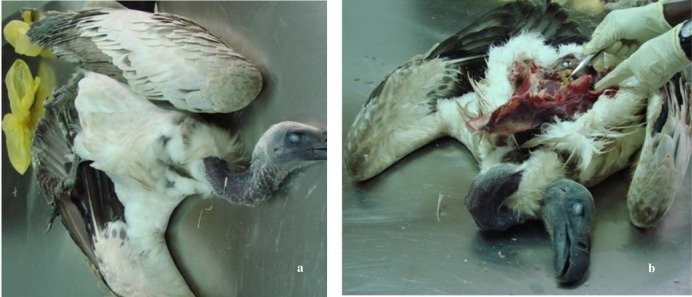
Illness of the two birds were suspected to be due to bacterial pneumonia or aspergillosis and were prescribed medication that consisted of a combination of enrofloxacin (Baytril® 50 mg tablets at a dosage of 15 mg/kg twice daily for 7 d and repeated again after 14 d), itraconazole (Sporanox®, 100 mg at a dosage of 10 mg/kg once daily for a period of 3 months), terbinafine hydrochloride (Lamisil®, 250 mg at a dosage of 15 mg/kg once daily for 3 months) and silymarin (Legalon®, 100 mg at dosage of 10 mg/kg for 3 months). One bird died a day after commencement of treatment. Five days after commencement of medication, another bird developed neurological disorder causing abnormal twisting of the neck (torticollis) that lasted for 3 d before it died. This incidence necessitated immediate screening of the remaining four Cape vultures and one Lappet-faced vulture that were housed together. The same treatment regime and duration as above was extended to the other four Cape vultures and the Lappet-faced vulture. However, a third Cape vulture died two weeks later after commencement of treatment.
2.2. Sampling of live birds
Four Cape vultures and one lappet faced vulture were physically restrained and the following samples collected. Whole blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes for hematology and in plain tubes and prepared sera were aliquoted for mycoplasma serology and biochemistry. The cloaca was swabbed (Citoswab®, Citotest Labware Co. Ltd, China) for bacteriology and Oropharyngeal swabbed (Citoswab®, Citotest Labware Co. Ltd, China) for mycology. Other oropharyngeal swabs were placed in mycoplasma broth (Sigma-Aldrich Chemie GmbH, Germany) for mycoplasma testing. All these samples were placed in cool boxes containing ice packs and taken to the Abu Dhabi Falcon Hospital laboratory within an hour after collection.
2.3. Necropsy and sampling of dead birds
Necropsy of the three Cape vultures were conducted at different times following their varied times of death. Sections of lung, liver and intestines were excised and preserved in 10% (v/v) buffered formalin (Orion Laboratories Pty Ltd, Australia) for histology. Lungs, air sacs, liver and intestines were swabbed for bacteriology, while only lungs and air sacs were swabbed for fungal culture. Both swabs were preserved in charcoal medium (Citoswab®, Citotest Labware Co. Ltd, China). Histopathology samples were sent to Central Veterinary Research Laboratory (CVRL), Dubai, while samples for bacteriology and mycology were analysed at Abu Dhabi Falcon Hospital.
2.4. Laboratory analyses
Oropharyngeal, lungs and air-sac samples were cultured for fungal isolation as described by Versalovic et al[12]. Bacteriological isolation and characterization was done as described in Bergeys' manual of determinative bacteriology[13]. Tissues for histology were processed routinely and embedded in paraffin. Sections were cut at 5 mm and serial sections from each block were stained with haematoxylin and eosin. Hematology parameters were determined manually. Biochemistry parameters were analysed by an ACE Chemistry analyzer (Alfa Wassermann®, New Jersey, USA). The specific parameters determined were red blood cells, white blood cells, hemoglobin, hematocrit, lymphocytes, heterophils, monocytes, eosinophils, basophils, aspartate amino transferase, alanine amino transferase, alkaline phosphatase, total protein, creatinine kinase, albumin, cholesterol, urea, uric acid and lactate dehydrogenase.
3. Results
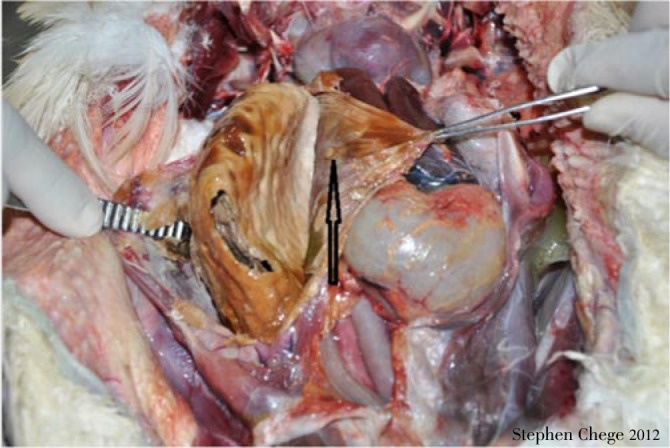
Out of the six Cape vultures introduced into Al Ain Zoo, five were infected with A. fumigatus following fungal culture. Of these five A. fumigatus-infected vultures, three died at different times after medication was commenced. The first two vultures died on Days 1 and 5, respectively after commencement of medication. The third vulture that died two weeks after commencement of medication was co-infected with A. fumigatus (diagnosed by culture of air-sac samples) and mixed species of bacteria (diagnosed by culture of the lung, liver and intestinal swabs. Two Cape vultures under prophylaxis for 30 d survived in spite of being co-infected with A. fumigatus and the mixed bacterial species. The bacterial mix included Clostridium perfringens (alpha toxin producer), Pseudomonas aeruginosa, Staphylococcus xylosus, Staphylococcus sciuri Escherichia coli (E. coli), Proteus mirabilis, Enterococcus fecalis and Enterobacter cloacae. Nevertheless, two birds (a Cape vulture and a Lappet-faced vulture) were free of A. fumigatus but harbored the above mentioned mix of bacteria. Mycoplasma sp. was not detected in any of the samples from live birds. Necropsies showed that the three dead birds had similar gross pathology characterized by congestion of lungs, large yellowish granulomas in caudal air-sacs (4-5 cm in length by 1.5-2.0 cm in width) (Figure 2) and diffuse hemorrhages in the small intestines. Histopathological examinations of all three dead vultures showed granulomatous lesions infiltrated with mononuclear cells (lymphocytes, macrophages) and giant cells (Figure 3). Some of the hematological and biochemistry values of the live birds were elevated as shown in Table 1. Yellowish granulomas in the caudal abdominal air sacs is shown in Figure 4.
Figure 2. Yellowish granuloma in the caudal abdominal airsacs of a dead Cape vulture at Al Ain Zoo.
Figure 3. Necrotic center of the granuloma with few fungal structures visible in the centre and some giant cells (arrows) at the periphery (HE-staining).
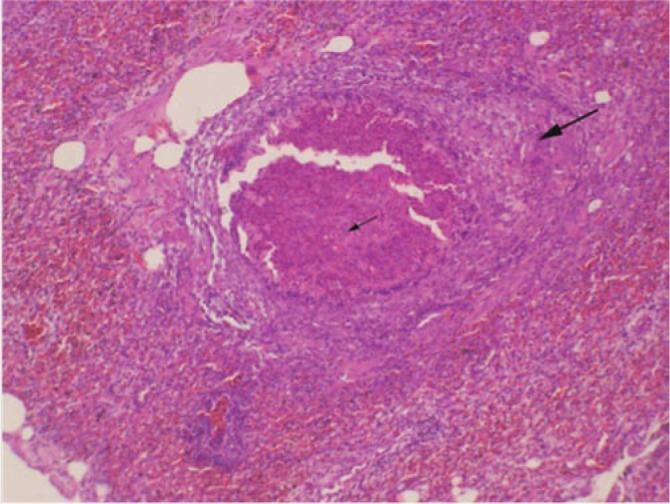
Table 1. Biochemical and hematological values of sick vultures at the Al Ain Zoo.
| Parameters | Individual Identities |
Reference Intervals** | ||||
| CV1 | CV2 | CV3 | CV4 | LFV* | ||
| Red blood cells × (1012/L) | 2.6 | 2.8 | 2.6 | 3.0 | 2.6 | 2.34-3.26 |
| White blood cells × (109/L) | 13.4 | 18.2 | 9.4 | 8.4 | 9.2 | 5.0-24 |
| Hemoglobin (g/dL) | 12.6a | 13.3 | 14.7 | 17.5 | 15.8 | 14.4-18.6 |
| Hematocrit (%) | 38 | 40 | 48 | 54 | 48 | 38-56 |
| Heterophils (%) | 56 | 54 | 52 | 54 | 56 | 51-72 |
| Lymphocytes (%) | 37 | 40 | 44 | 41 | 40 | 24-49 |
| Monocytes (%) | 4b | 3b | 3b | 3b | 2b | 0-1 |
| Eosinophils (%) | 2 | 2 | 1 | 1 | 1 | 0-4 |
| Basophils (%) | 1 | 1 | 0 | 1 | 1 | 0 |
| Aspartate amino transferase (µ/L) | 107 | 372c | 186c | 213c | 317c | 93.5-156.0 |
| Alanine amino transferase (µ/L) | 24d | 79d | 80d | 25d | 86d | 3.9-19.8 |
| Alkaline phosphatase (µ/L) | 47 | 94 | 53 | 55 | 112e | 21-98 |
| Total protein (g/dL) | 3.34 | 3.08 | 3.44 | 2.79f | 2.86f | 3.0-5.1 |
| Albumin (g/dL) | 1.22g | 1.13g | 1.15g | 1.06g | 1.07g | 1.3-2.23 |
| Lactate dehydrogenase (µ/L) | 420 | 1213h | 275 | 251 | 638h | 103-586 |
| Creatine kinase (µ/L) | 170i | 310 | 203 | 327 | 842 | 172-1 485 |
| Cholesterol (mg/dL) | 93j | 130 | 111j | 114j | 126 | 119-274.6 |
| Uric acid (mg/dL) | 2.92 | 3.82 | 5.32 | 3.67 | 5.33 | 2.8-11.4 |
| Urea (mg/dL) | 3.1k | 7.8 | 2.7k | 4.5k | 8 | 6.05-31.9 |
CV: Cape vulture; LFV*: Lappet faced vulture; **: Polo et al. 1992; a,b,c,d,e,f,g,h,I,j,k: Parameters outside of reference intervals.
Figure 4. Granuloma (arrow) in the caudal abdominal airsacs of a dead Cape vulture at Al Ain Zoo.
4. Discussion
Aspergillosis is an opportunistic non-contagious infection, yet it has persisted among the important diseases in poultry farming where it causes heavy economic losses[9]. The disease is now emerging as an important factor in the conservation of wild birds following wide spread cases of morbidity and mortality. In the present case, pathologic lesions that were consistent with pulmonary aspergillosis were observed in the dead vultures corresponding to the positive fungal culture results. In particular, yellowish granulomas that were confined in the caudal abdominal air sacs and the histological lesions were suggestive of pulmonary aspergillosis[11]. Frequent causal organism of aspergillosis is A. fumigatus[1]. However, factors that drive its predominance over other species of Aspergillus are equivocal despite postulations that it is able to overwhelm avian respiratory immune cells[14]. In the present case, A. fumigatus was the only Aspergillus spp. isolated from the vultures, which was the likely cause for the observed pathologic lesions, morbidity and deaths. When birds inhale fungal spores, they are deposited in lungs where they germinate and cause pulmonary aspergillosis. However, fungal spores may disseminate to other organs including brain where their presence cause neurological disorders such as encephalitis or torticollis as was observed in the present case in one vulture[6]. Variations in hematology and biochemistry values are usually indicative of infectious disease including aspergillosis or physiological stress[15]. In our case, we compared the blood parameters with those of Polo et al.[16], and the variations were not conclusively suggestive of aspergillosis. However, we noted the elevation of alanine amino transferase and aspartate amino transferase, enzymes that are often associated with physiological harm such as trauma, stress, toxicity or hepatocellular injury[17]. The vultures in the present case underwent a series of physical capture events within the zoo during sampling. We believe that cumulative effect of stress during these disturbances and liver impairment following A. fumigatus toxin production could have triggered elevation of the two enzymes.
The infection and pathogenicity of aspergillosis have been widely studied in diverse host taxa, and the common factor is that susceptibility depends on the immunity of the host[18], which could be compromised by previous stressful conditions or events[2]–[6]. Vultures could have been immunocompromised by disturbance caused by the sequential capture and translocation from South Africa and again within the zoo. In addition, adverse climatic conditions in the Middle East, which have been identified as a predisposing factor to aspergillosis in birds from other geographic regions[19], could have driven the infections in the vultures.
Prevalence and diversity of bacteria in aspergillosis-infected hosts are usually very high[20],[21], but the pathophysiological effect of the co-infections are deemed multiple and complex[21]. Bacterial species such as E. coli, Pasteurella multocida, Staphyloccocus spp. may predispose birds as well as humans to aspergillosis[20],[22],[23]. In our case, the diversity of bacteria included E. coli, Staphyloccocus spp., Clostridium perfringens that have been associated with severe aspergillosis[20]. Specifically, Staphylococcus spp. bacteria have been associated with chronic fatigue and immune dysfunction that predispose birds to opportunistic aspergillosis[23].
Early diagnosis is strongly recommended towards effective treatment of aspergillosis[24]. It is likely that prophylactic treatment in our case was timely administered and thus protected the two vultures that survived. Even so, treatment of aspergillosis is usually difficult due to the paucity on pharmacokinetics of antifungal drugs in different avian species and the presence of granuloma that shield off drugs from target fungus[25]. The emergence of avian A. fumigatus strains that are resistant to both itraconazole and voriconazole, the first line drugs against aspergillosis, is a challenge to be considered in the treatment of avian aspergillosis[18]. Further, in A. fumigatus infected birds, treatment by voriconazole have failed due to chronic fatigue syndrome usually induced by underlying Staphylococci infections[26].
In this study, we have described clinical case of aspergillosis in Cape vultures and add knowledge to the limited available literature on aspergillosis in Cape vultures, although aspergillosis has been studied extensively in other captive birds. We deduce that early detection of aspergillosis is critical to management and possible recovery of infected birds. As blood examinations and general screening samples are not conclusive to rule out early stages of aspergillosis, an endoscopic examination during quarantine period could have provided useful results, and increase chances of detecting the infection early.
Acknowledgments
The project was funded and supported by the Al Ain Wildlife Park and Resort through Grant No. 10/917001. The capture and veterinary team helped in capture and sample collection. We would also like to thank the Abu Dhabi Falcon Hospital and the Central Veterinary Research Laboratory in Dubai for performing laboratory tests.
Comments
Background
Aspergillosis forms a major problem in birds and especially in raptors as well as in parrots. If this mycotic disease is detected in an advanced stage with already manifest clinical signs, it may prove fatal. Predisposing factors are stress, transport, new environment as well as any kind of immunosuppression. In the beginning stages, aspergillosis can be treated very well. In the literature, aspergillosis is covered extensively but mainly in parrots or raptors used for falconry whereas not so much research focus is put on birds of prey used for exhibits in zoological collections.
Research frontiers
Studies are being performed in order to determine the cause of death of very rare Cape cultures that had been recently imported from South Africa for the use of exhibit. The paper tries to establish a correlation between the high mortality of the vultures and respective clinical symptoms, laboratory findings and histopathological results for the diseased and dead vultures.
Related reports
The presented data is not in agreement with the statement of Beernaert et al. (2010) that aspergillosis is a “major cause of mortality in captive birds and, less frequently, in free-living birds”. This contrast might probably be due to the fact that the diseased vultures were kept for a short time in captivity and underwent major changes of their environment thus leading to immunosuppression and stress being predisposing factors for aspergillosis.
Innovations and breakthroughs
Data about the significance of aspergillosis as mortality cause in wild vultures being kept in a zoological exhibit is relatively rare. The research study proved that a much higher mortality in wild vultures being newly brought in captivity can be established than for example in other raptors like falcons.
Applications
It is significant to understand the importance of aspergillosis in newly acquired vultures or birds of prey especially in case of rare species that are vulnerable or threatened by extinction. This report highlights the mandatory clinical screening at the time of adding new raptors into an existing collections and points out the importance of quarantine controls. This case study contributes to the subject of preventive aspergillosis treatment as this would have been possibly a way to avoid the deaths of the previously healthy vultures.
Peer review
This case study is an interesting report about aspergillosis in vultures with matching clinical, laboratory and histopathological results to establish this diagnosis. It will hopefully bring more emphasis on the issue of preventive treatment for aspergillosis in zoological collections and thus lead to a rethinking of the way how newly acquired raptors and other birds are screened and preventively treated.
Footnotes
Foundation Project: Supported by the Al Ain Wildlife Park and Resort through. Grant No. 10/917001.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Olias P, Hauck R, Windhaus H, Grinten E, Gruber AD, Hafez HM. Articular aspergillosis of hip joints in turkeys. Avian Dis. 2010;54:1098–1101. doi: 10.1637/9232-011110-Case.1. [DOI] [PubMed] [Google Scholar]
- 2.Oglesbee BL. Mycotic diseases. In: Altman RB, editor. Avian medicine and surgery. 1st ed. Philadelphia: WB Saunders Company; 1997. pp. 323–361. [Google Scholar]
- 3.Phalen DN. Respiratory medicine of cage and aviary birds. Vet Clin North Am Exot Anim Pract. 2000;3:423–452. doi: 10.1016/s1094-9194(17)30080-4. [DOI] [PubMed] [Google Scholar]
- 4.Abrams GA, Paul-Murphy J, Ramer JC, Murphy CJ. Aspergillus blepharitis and dermatitis in a peregrine falcon-gyrfalcon hybrid (Falco peregrinus × Falco rusticolus) J Avian Med Surg. 2001;15:114–120. [Google Scholar]
- 5.Ruhnke M, Bohme A, Buchheidt D, Donhuijsen K, Einsele H, Enzensbereger R, et al. et al. Diagnosis of invasive fungal infections in hematology and oncology-guidelines of the infectious diseases working party (AGIHO) of the German society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S141–S148. doi: 10.1007/s00277-003-0768-0. [DOI] [PubMed] [Google Scholar]
- 6.Jensen HE, Christensen JP, Bisgaard M, Nielsen OL. Immunohistochemistry for the diagnosis of aspergillosis in turkey poults. Avian Pathol. 1997;26:5–18. doi: 10.1080/03079459708419189. [DOI] [PubMed] [Google Scholar]
- 7.Westerhof I. Treatment of tracheal obstruction in psittacine birds using a suction technique: a retrospective study of 19 birds. J Avian Med Surg. 1995;9:45–49. [Google Scholar]
- 8.Morio F, Aubin GG, Danne-Boucher I, Haloun A, Sacchetto E, Garcia-Hermoso D, et al. et al. High prevalence of triazoles resistance in Aspergillus fumigatus, especially mediated by TR/L89H, in a French cohort of patients with cystic fibrosis. J Antimicrob Chemother. 2012;67(8):1870–3. doi: 10.1093/jac/dks160. [DOI] [PubMed] [Google Scholar]
- 9.Zafra R, Perez J, Perez-Ecija RA, Borge C, Bustamante R, Carbonero A, et al. et al. Concurrent aspergillosis and ascites with high mortality in a farm of growing broiler chickens. Avian Dis. 2008;52:711–713. doi: 10.1637/8283-031208-Case.1. [DOI] [PubMed] [Google Scholar]
- 10.IUCN Red List of Threatened Species.. Version 2011. 2. [Online] Available from: http://www.iucnredlist.org. [Accessed on 18th May, 2012].
- 11.Jung K, Kim Y, Lee H, Kim JT. Aspergillus fumigatus infection in two wild Eurasian black vultures (Aegypius monachus Linnaeus) with carbofuran insecticide poisoning: a case report. Vet J. 2009;179:307–312. doi: 10.1016/j.tvjl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Versalovic J, Karen C, Funkey GC, Jorgensen HJ, Landry IM, Warnock WD. Manual of clinical microbiology. 10th ed. Washingtion DC: ASM Press; 2011. pp. 110–118. [Google Scholar]
- 13.Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST. Bergey's manual of determinative bacteriology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 1994. pp. 175–200. [Google Scholar]
- 14.Van Waeyenberghe L, Pasmans F, D'Herde K, Ducatelle R, Favoreel H, Li S, et al. et al. Germination of Aspergillus fumigatus inside avian respiratory macrophages is associated with cytotoxicity. Vet Res. 2012;43(1):32. doi: 10.1186/1297-9716-43-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beernaert LA, Pasmans F, Van Waeyenberghe L, Haesebrouck F, Martel A. Aspergillus infections in birds: a review. Avian Pathol. 2010;39(5):325–331. doi: 10.1080/03079457.2010.506210. [DOI] [PubMed] [Google Scholar]
- 16.Polo FJ, Celdran JF, Peinado VI, Viscor G, Palomeque J. Hematological values for four species of birds of prey. Condor. 1992;94:1007–1013. [Google Scholar]
- 17.Naidoo V, Diekmann M, Wolters K, Swan GE. Establishment of selected baseline blood chemistry and hematologic parameters in captive and wild-caught African white-backed vultures (Gyps africanus) J Wildl Dis. 2008;44(3):649–654. doi: 10.7589/0090-3558-44.3.649. [DOI] [PubMed] [Google Scholar]
- 18.Beernaert LA, Van Waeyenberghe L, Pasmans F, Dorrestein GM, Verstappen F, Vercammen F, et al. et al. Avian Aspergillus fumigatus strains resistant to both itraconazole and voriconazole. Antimicrob Agents Chemother. 2009;53(5):2199–2201. doi: 10.1128/AAC.01492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Somma A, Bailey T, Silvanose C, Garcia-Martinez C. The use of voriconazole for treatment of aspergillosis in falcons (Falco species) J Avian Med Surg. 2007;21:307–316. doi: 10.1647/1082-6742(2007)21[307:TUOVFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Tarello W. Etiologic agents and diseases found associated with clinical aspergillosis in falcons. Int J Microbiol. 2011 doi: 10.1155/2011/176963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiadou SP, Kontoyiannis DP. Concurrent lung infections in patients with hematological malignancies and invasive pulmonary aspergillosis: How firm is the aspergillus diagnosis? J Infect. 2012 doi: 10.1016/j.jinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkle RA, Rimler RB. Early pulmonary lesions in turkeys produced by nonviable Aspergillus fumigatus and/or Pasteurella multocida lipopolysaccharide. Avian Dis. 1998;42(4):770–780. [PubMed] [Google Scholar]
- 23.Tarello W. Chronic fatigue and immune dysfunction syndrome associated with Staphylococcus spp. bacteraemia responsive to thiacetarsamide sodium in eight birds of prey. J Vet Med Series B. 2001;48(4):267–281. doi: 10.1046/j.1439-0450.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- 24.Kreisel W, Köchling G, Von Schilling C, Azemar M, Kurzweil B, Dölken G, et al. et al. Therapy of invasive aspergillosis with itraconazole: improvement of therapeutic efficacy by early diagnosis. Mycoses. 1991;34:385–394. doi: 10.1111/j.1439-0507.1991.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 25.Orosz SE, Frazier DL. Antifungal agents: a review of their pharmacology and therapeutic indications. J Avian Med Surg. 1995;9:8–18. [Google Scholar]
- 26.Silvanose CD, Bailey TA, Di Somma A. Susceptibility of fungi isolated from the respiratory tract of falcons to amphotericin B, itraconazole and voriconazole. Vet Rec. 2006;159(9):282–284. doi: 10.1136/vr.159.9.282. [DOI] [PubMed] [Google Scholar]