Abstract
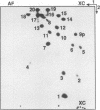
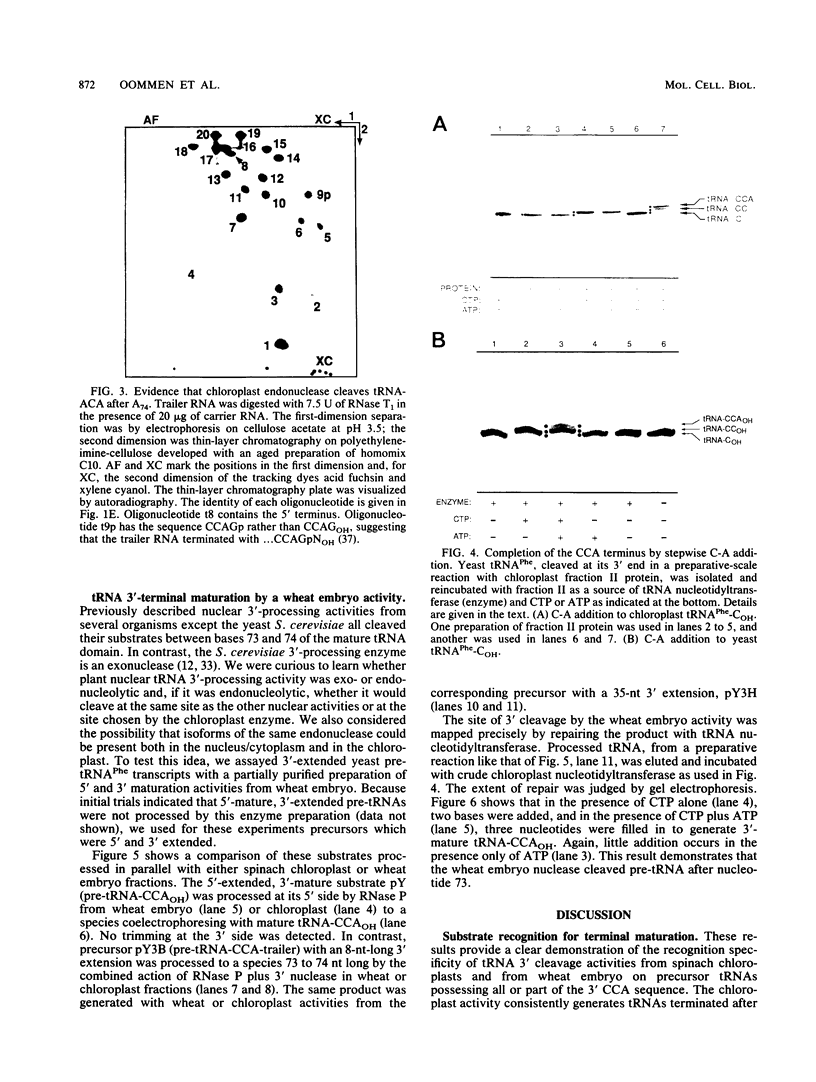
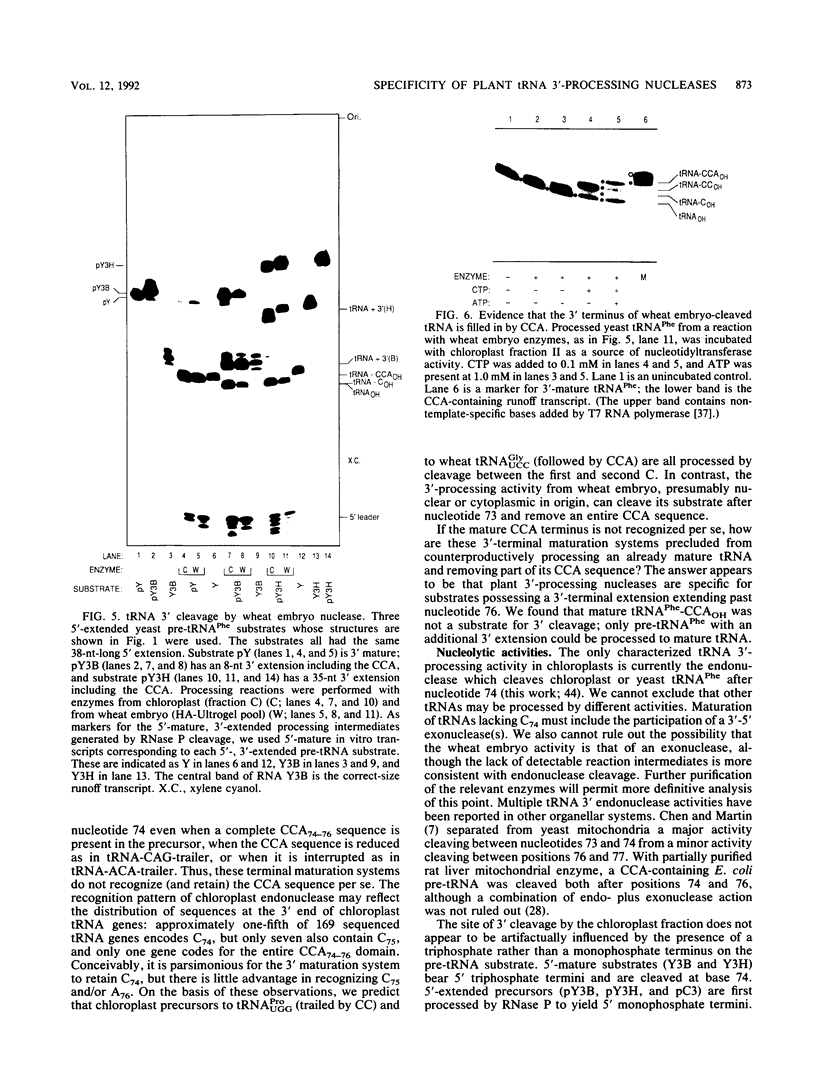
tRNAs in eukaryotic nuclei and organelles are synthesized as precursors lacking the 3'-terminal CCA sequence and possessing 5' (leader) and 3' (trailer) extensions. Nucleolytic cleavage of the 3' trailer and addition of CCA are therefore required for formation of functional tRNA 3' termini. Many chloroplast tRNA genes encode a C at position 74 which is not removed during processing but which can be incorporated as the first base of the CCAOH terminus. Sequences downstream of nucleotide 74, however, are always removed. Synthetic yeast pre-tRNA(Phe) substrates containing the complete CCA74-76 sequence were processed with crude or partially purified chloroplast enzyme fractions. The 3'-extended substrates (tRNA-CCA-trailer) were cleaved exclusively between nucleotides 74 and 75 to give tRNA-COH, whereas a 3'-mature transcript (tRNA-CCAOH) was not cleaved at all. A 5'-, 3'-extended chloroplast tRNA-CAG-trailer was also processed entirely to tRNA-COH. Furthermore, a 5'-mature, 3'-extended yeast pre-tRNA(Phe) derivative, tRNA-ACA-trailer, in which C74 was replaced by A, was cleaved precisely after A74. In contrast, we found that a partially purified enzyme fraction (a nuclear/cytoplasmic activity) from wheat embryo cleaved the 3'-extended yeast tRNA(Phe) precursors between nucleotides 73 and 74 to give tRNA(OH). This specificity is consistent with that of all previously characterized nuclear enzyme preparations. We conclude that (i) chloroplast tRNA 3'-processing endonuclease cleaves after nucleotide 74 regardless of the nature of the surrounding sequences; (ii) this specificity differs from that of the plant nuclear/cytoplasmic processing nuclease, which cleaves after base 73; and (iii) since 3'-mature tRNA is not a substrate for either activity, these 3' nucleases must require substrates possessing a 3'-terminal extension that extends past nucleotide 76. This substrate specificity may prevent mature tRNA from counterproductive cleavage by the 3' processing system.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi-Jones S., Romeo P. H., Zasloff M. Generation of long read-through transcripts in vivo and in vitro by deletion of 3' termination and processing sequences in the human tRNAimet gene. Nucleic Acids Res. 1984 Jan 25;12(2):1101–1115. doi: 10.1093/nar/12.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama K., Tanifuji S. Nucleotide sequence of a methionine initiator tRNA gene of Arabidopsis thaliana. Plant Mol Biol. 1989 Nov;13(5):599–600. doi: 10.1007/BF00027320. [DOI] [PubMed] [Google Scholar]
- Akama K., Tanifuji S. Sequence analysis of three tRNA(Phe) nuclear genes and a mutated gene, and one gene for tRNA(Ala) from Arabidopsis thaliana. Plant Mol Biol. 1990 Aug;15(2):337–346. doi: 10.1007/BF00036919. [DOI] [PubMed] [Google Scholar]
- Bikoff E. K., LaRue B. F., Gefter M. L. In vitro synthesis of transfer RNA. II. Identification of required enzymatic activities. J Biol Chem. 1975 Aug 25;250(16):6248–6255. [PubMed] [Google Scholar]
- Castaño J. G., Tobian J. A., Zasloff M. Purification and characterization of an endonuclease from Xenopus laevis ovaries which accurately processes the 3' terminus of human pre-tRNA-Met(i) (3' pre-tRNase). J Biol Chem. 1985 Jul 25;260(15):9002–9008. [PubMed] [Google Scholar]
- Chen J. Y., Martin N. C. Biosynthesis of tRNA in yeast mitochondria. An endonuclease is responsible for the 3'-processing of tRNA precursors. J Biol Chem. 1988 Sep 25;263(27):13677–13682. [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. Apparent involvement of ribonuclease D in the 3' processing of tRNA precursors. Proc Natl Acad Sci U S A. 1980 Feb;77(2):837–841. doi: 10.1073/pnas.77.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem. 1984;17(1):45–71. doi: 10.3109/10409238409110269. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Gegenheimer P., Abelson J. Nucleolytic processing of a tRNAArg-tRNAAsp dimeric precursor by a homologous component from Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1271–1279. [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendewey D., Dingermann T., Cooley L., Söll D. Processing of precursor tRNAs in Drosophila. Processing of the 3' end involves an endonucleolytic cleavage and occurs after 5' end maturation. J Biol Chem. 1985 Jan 10;260(1):449–454. [PubMed] [Google Scholar]
- Garber R. L., Altman S. In vitro processing of B. mori transfer RNA precursor molecules. Cell. 1979 Jun;17(2):389–397. doi: 10.1016/0092-8674(79)90165-x. [DOI] [PubMed] [Google Scholar]
- Garber R. L., Gage L. P. Transcription of a cloned Bombyx mori tRNA2Ala gene: nucleotide sequence of the tRNA precursor and its processing in vitro. Cell. 1979 Nov;18(3):817–828. doi: 10.1016/0092-8674(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P. Preparation of extracts from plants. Methods Enzymol. 1990;182:174–193. doi: 10.1016/0076-6879(90)82016-u. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Hallick R. B. Chloroplast gene expression and promoter identification in chloroplast extracts. Methods Enzymol. 1986;118:253–270. doi: 10.1016/0076-6879(86)18077-3. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Larson D., Hall G. I., Sprague K. U. The primary transcription product of a silkworm alanine tRNA gene: identification of in vitro sites of initiation, termination and processing. Cell. 1979 Dec;18(4):1217–1229. doi: 10.1016/0092-8674(79)90234-4. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M. J., Martin N. C. RNase P activity in the mitochondria of Saccharomyces cerevisiae depends on both mitochondrion and nucleus-encoded components. Mol Cell Biol. 1986 Apr;6(4):1058–1064. doi: 10.1128/mcb.6.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Sirdeskmukh R., Schlessinger D. Nucleolytic processing of ribonucleic acid transcripts in procaryotes. Microbiol Rev. 1986 Dec;50(4):428–451. doi: 10.1128/mr.50.4.428-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manam S., Van Tuyle G. C. Separation and characterization of 5'- and 3'-tRNA processing nucleases from rat liver mitochondria. J Biol Chem. 1987 Jul 25;262(21):10272–10279. [PubMed] [Google Scholar]
- Micard D., Sobrier M. L., Couderc J. L., Dastugue B. Purification of RNA-free plasmid DNA using alkaline extraction followed by Ultrogel A2 column chromatography. Anal Biochem. 1985 Jul;148(1):121–126. doi: 10.1016/0003-2697(85)90636-0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Stråby K. B. Processing of transcripts of a dimeric tRNA gene in yeast uses the nuclease responsible for maturation of the 3' termini upon 5 S and 37 S precursor rRNAs. FEBS Lett. 1989 Jul 3;250(2):311–316. doi: 10.1016/0014-5793(89)80745-8. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Altman S., Smith J. D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid presursor. J Biol Chem. 1972 Aug 25;247(16):5243–5251. [PubMed] [Google Scholar]
- Rooney R. J., Harding J. D. Processing of mammalian tRNA transcripts in vitro: different pre-tRNAs are processed along alternative pathways that contain a common rate-limiting step. Nucleic Acids Res. 1986 Jun 25;14(12):4849–4864. doi: 10.1093/nar/14.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Contreras R., Takeya T., Khorana H. G. Total synthesis of a tyrosine suppressor transfer RNA gene. XVII. Transcription, in vitro, of the synthetic gene and processing of the primary transcript to transfer RNA. J Biol Chem. 1979 Jul 10;254(13):5802–5816. [PubMed] [Google Scholar]
- Solari A., Deutscher M. P. Identification of multiple RNases in Xenopus laevis oocytes and their possible role in tRNA processing. Mol Cell Biol. 1983 Oct;3(10):1711–1717. doi: 10.1128/mcb.3.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Gross H. J., Beier H. Wheat germ splicing endonuclease is highly specific for plant pre-tRNAs. EMBO J. 1988 Dec 1;7(12):3823–3828. doi: 10.1002/j.1460-2075.1988.tb03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Wang M. J., Davis N. W., Gegenheimer P. Novel mechanisms for maturation of chloroplast transfer RNA precursors. EMBO J. 1988 Jun;7(6):1567–1574. doi: 10.1002/j.1460-2075.1988.tb02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Romeo P., Rosenberg M. Transcription and precursor processing of normal and mutant human tRNAiMet genes in a homologous cell-free system. J Biol Chem. 1982 Jul 10;257(13):7857–7863. [PubMed] [Google Scholar]
- Zhang J. R., Deutscher M. P. Transfer RNA is a substrate for RNase D in vivo. J Biol Chem. 1988 Dec 5;263(34):17909–17912. [PubMed] [Google Scholar]