Introduction
Lymphotoxin β receptor (LTβR) was originally discovered in the context of lymph node development, and it has since been increasingly appreciated that the LTβR signaling pathway is involved in inducing apoptosis in tumor cells (1–5). Furthermore, it has been shown that LTβR agonist monoclonal antibody (mAb) effectively inhibits human colorectal tumor growth in a xenograft mouse model (6). LIGHT gene transfer therapy also leads to increased apoptosis and suppression of tumor formation in vivo (2). These data thus suggest that LTβR functions as a death receptor that mediates ligand-dependent tumor suppression. The specific expression of LTα1LTβ2 and LIGHT on immune cells and the expression of LTβR on tumor cells suggest that immune cells might use LTα1LTβ2 and LIGHT to engage the LTβR on tumor cells to execute antitumor cytotoxicity. Indeed, it has been shown that LTα1LTβ2 expressed on dendritic cells and NK cells directly induce apoptotic killing of cancer cells (3,4). We have recently shown that LTβ is constitutively expressed in T lymphocytes, whereas LTα and LIGHT are only expressed in activated T cells (7). Additionally, we and others have shown that LTβR mediates cytotoxic T lymphocyte-exerted antitumor cytotoxicity in vivo (7–9). These observations lead to the proposal to target LTβR to suppress tumor development in human cancer therapy (6).
Although it is well documented that LTβR functions as a death receptor that mediates tumor cell apoptosis, LTβR might be a double-edged sword. Ligation of LTβR also activates NF-κB (10), and sustained LTβR signaling leads to NF-κB-mediated inflammation and hepatocellular carcinoma (HCC) development (11,12). Constitutive expression of LTα and LTβ in liver cells (in LTα and LTβ double transgenic mice) leads to chronic hepatitis at 9 months and HCC at 12 months (11). Therefore, although LTα and LTβ are not expressed in liver cells under physiological conditions, this seminal study indicates that sustained LTβR signaling can also lead to NF-κB-dependent chronic inflammation that promotes HCC development (11). Furthermore, NF-κB also increases LTβ production and promotes prostate cancer development through a B-cell-dependent mechanism (13). In addition, other classical tumor necrosis factor (TNF) death receptors, including TRAIL receptor and Fas, also mediate NF-κB activation. Therefore, it is a general phenomenon that TNF superfamily death receptors mediate both apoptosis and NF-κB activation in tumor cells. These observations prompt the proposal to inhibit TNF death receptor signaling to suppress NF-κB-dependent inflammation and tumor promotion (11).
Currently, it is clear that ligation of the LTβR directly induces tumor cell apoptosis and inflammation-related gene expression. However, the debate is whether the LTβR-initiated signals should be activated or blocked in cancer therapy (6,7,9,11,14–16). This debate is similar to that for TNFα. TNFα is an approved anticancer agent that is currently used in soft tissue sarcoma (STS) therapy in the clinic (17). However, TNFα is also a potent NF-κB activator and inhibiting TNFα function has been proposed in cancer therapy (18). Because of these contrasting observations concerning the role of LTβR in tumor development, it is important to further examine the functions and underlying molecular mechanisms of LTβR in apoptosis and tumor development in both mouse tumor models and in human cancers.
Materials and methods
Mice
BALB/c (H-2d) mice were obtained from the National Cancer Institute (Frederick, MD). LTα knockout (KO) mice on a C57BL/6J background were obtained from the Jackson Laboratory. LIGHT KO and LTβ/LIGHT double knockout (DKO) mice were maintained in Sanford Burnham Medical Research Institute. All mice were housed, maintained and studied in accordance with approved National Institutes of Health and Georgia Health Sciences University guidelines for animal use and handling.
LTβR ligand-deficient chimera mice
Bone marrow was prepared from wild-type (wt), LTα, LIGHT and LTβ/LIGHT KO mice. wt C57BL/6J mice were irradiated with a dose of 950 rad. Bone marrow cells (5×106 cells/mouse) were injected intravenously (i.v.) into the irradiated recipient mice 4h later. Eighteen days later, methylcholanthrene (MCA) was injected into the chimera mice (100 μg/mouse in peanut oil) at the right flank to induce spontaneous sarcoma.
Tumor cells
Human tumor cell lines were obtained from ATCC (Manassas, VA). Mouse colon carcinoma cell line CT26 was also obtained from ATCC. Mouse sarcoma cell line CMS4 was created as described previously (7).
Tumor cell growth measurement
Human tumor cells were seeded in 96-well plates and treated with recombinant interferon-γ (100 units/ml) and humanized tetravalent anti-LTβR mAb [bispecific-1 (BS-1), 100ng/ml or as indicated] for 5 days. Mouse tumor cells were seeded in capture mAb-coated 96-well plates in the presence of anti-mouse LTβR agonist mAb (clone ACH6, 100ng/ml) as described previously (6). Tumor cell growth was measured using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell proliferation assay kit (ATCC). For caspase inhibition assay, Z-VAD (Enzo Life Science, Farmingdale, NY) was added to the cell culture to a final concentration of 25 μM for 1h, followed by addition of BS-1 to the culture.
Anti-LTβR agonist antibody
BS-1 is a tetravalent antibody constructed using humanized forms of the anti-human LTβR mAbs CBE11 and BHA10 as described previously (19). CBE11 formed the basic antibody core and an scFv version of BHA10 was coupled to the C terminus of the CBE11 heavy chain via a (G4S)2 linker. CBE11 and BHA10 bind to different regions of LTβR and therefore BS-1 can create larger oligomeric aggregates of the receptor compared with the parent bivalent antibodies. The tetravalent form was comparable in activity to the decavalent IgM-like forms described previously and is believed to deliver a maximal agonist signal (6). Using an IL-8 release assay with A375 melanoma cells, the ED50 for cytokine release was at 3–4ng/ml. Hamster anti-mouse LTβR mAb (clone ACH6) was described previously (6).
Western blot analysis
Western blot analysis was done essentially as described previously (20,21). Anti-pIκBα antibody (Santa Cruz Biotech) was used at 1:1000 dilution, anti-p100/p52 antibody (Cell Signaling, Danvers, MA) at 1:500 dilution, anticleaved caspase 8 antibody (R&D System) at 1 μg/ml, anticleaved caspase 3 antibody (Cell Signaling) at 1:500 dilution, anti-cytochrome c antibody (BD Biosciences, San Diego, CA) at 1:1000 dilution and anti-β-actin antibody (Sigma, St Louis, MO) was used at 1:8000 dilution.
Analysis of tumor-infiltrating immune cells
Tumor-infiltrating immune cells were analyzed as described previously (22). Briefly, CT26 cells were injected into BALB/c mice i.v. Lungs were collected from tumor-bearing mice ~21 days later and digested with collagenase solution to make a single-cell suspension. The single-cell suspension was stained with LTα- or LTβ-specific mAbs (BD Biosciences) in combination with CD4-, CD8-, CD11b- or NK(DX5)-specific mAbs (Biolegend, San Diego, CA), respectively. The stained cells were analyzed by flow cytometry. Subsets of tumor-infiltrating immune cells were isolated with BioMag® SelectraPure™ anti-mouse CD8a magnetic particles, BioMag® SelectraPure™ anti-mouse CD4 magnetic particles (Polysciences, Warrington, PA) and IMag™ anti-mouse CD11b magnetic particles (BD Biosciences). To purify NK cells, cell digests were incubated with anti-mouse Pan NK cells mAb (clone DX5), washed in phosphate-buffered saline and incubated with BioMag® goat anti-rat IgM magnetic particles (Polysciences).
Cell surface LTβR analysis
Tumor cells were stained with anti-LTβR mAb (Biolegend), followed by fluorescein isothiocyanate-conjugated anti-mouse IgG mAb (Southern Biotech, Birmingham, AL). The cells were analyzed with flow cytometry.
Reverse transcription–polymerase chain reaction analysis
Reverse transcription–polymerase chain reaction (RT–PCR) primer sequences and procedures were as described previously (7,23). Mouse IKKβ primer sequences are forward: 5′-ATTGCTGCTGGCTTGGCG-3′ and reverse: 5′-GCTGTCACCTTCTGTCCTTTGG-3′.
Tumor cell transfection
CT26 cells were stably transfected with pcDNA and pcDNA containing the NF-κB supersuppressor IκBα-AA [kindly provided by Dr Michael Karin, University of California, San Diego, CA and Dr Anning Lin, University of Chicago, IL (24)]. The transfected cells were propagated and maintained in culture medium containing Geniticin (Invitrogen, Grand Island, NY). MES-SA/DX5 and HT29 cells were transfected with pcDNA, pcDNA.IKKα-KM and pcDNA.IKKβ-KA (provided by Dr Warner C.Greene, University of California, San Francisco, CA) to establish stable cell lines as described previously (25).
Gene silencing
CT26 cells were stably transfected with scramble shRNA (GGAATCT- CATTCGATGCATAC) plasmid and IKKβ-specific shRNA (TCCCTTATG- ACACGTAATCCTAA) plasmid (SABiosciences, Germantown, MD), respectively. The transfected cells were propagated and maintained in culture medium containing Geniticin (Invitrogen).
NF-κB activation assay
NF-κB activation was analyzed using electrophoretic mobility shift assay (EMSA) as described previously (26). For specificity controls, unlabeled probe was added to the reaction at a 1:100 molar excess. p65-, p50- and p52-specific antibodies (Santa Cruz Biotech) were also included to identify NF-κB-specific DNA binding. For luciferase reporter assay, tumor cells were transfected with a NF-κB luciferase reporter plasmid (ClonTech) and analyzed using the luciferase assay system (Promega, Madison, WI).
Immunohistochemistry
Human colorectal cancer tissue microarray (TMA) slides were obtained from the National Cancer Institute-sponsored Cooperative Human Tissue Network. The TMA slides were stained with the anti-LTβR mAb [clone BDA8 (6)] as described previously.
mAb and cytotoxic T lymphocyte immunotherapy
Tumor-specific cytotoxic T lymphocytes (CTLs) were generated from perforin-deficient BALB/c mice as described previously (22). CT26 cells were injected i.v. into mouse tail vein. Five days later, tumor-specific CTLs were injected into the tumor-bearing mice. Lung metastasis was analyzed as described previously (20). For mAb therapy, CT26.pcDNA and CT26.IκBα-AA cells were injected into mice i.v. IgG or ACH6 mAb (50 μg/mouse) was injected into tumor-bearing mice at days 3, 6 and 9 after tumor transplantation. Mice were analyzed 14 days after tumor transplantation.
Statistical analysis
All statistical analyses were performed using SAS 9.2 software or with two-tailed t-test. Statistical significance was assessed using an alpha level of 0.05.
Results
LTβR ligands were induced in tumor-infiltrating immune cells
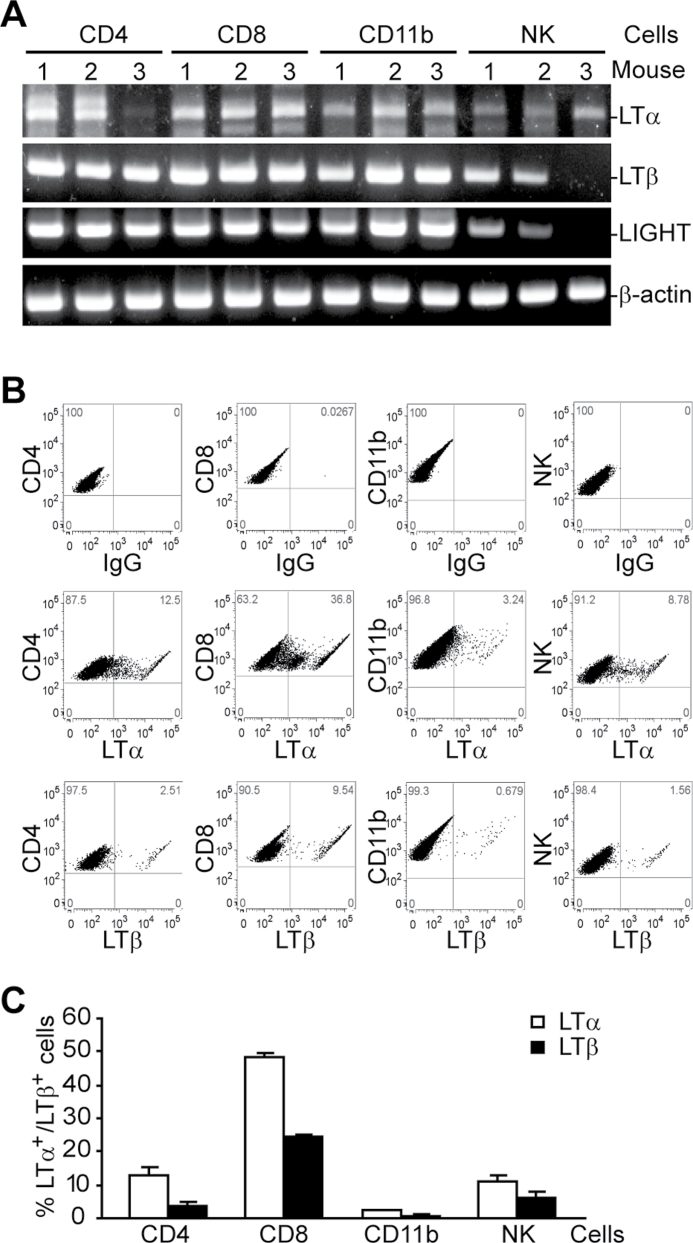
To determine whether the LTβR ligands, LTα1LTβ2 and LIGHT, are expressed in immune cells in the tumor microenvironment, CT26 cells were injected into BALB/c mice i.v. Lungs were collected from tumor-bearing mice and digested with collagenase solution to make a single-cell suspension. CD4+, CD8+, CD11b+ and NK cells were purified from the tumor cells and used for RT–PCR analysis of LTα, LTβ and LIGHT mRNA level. The LTα mRNA level was weak to high in all four subsets of immune cells except in CD4 cells from one mouse, whereas the LTβ mRNA level was high in all four subsets of immune cells except in NK cells from one mouse (Figure 1A). Although it was reported that LIGHT is only expressed in activated T cells (27), it seems that the LIGHT mRNA level is relatively high not only in tumor-infiltrating T cells but also in tumor-infiltrating CD11b+ and NK cells (Figure 1A). To determine LTα and LTβ protein levels on the cell surface, a single-cell suspension as described above was double stained with LTα- or LTβ-specific mAb in combination with CD4-, CD8-, CD11b- or NK-specific mAb, respectively, and analyzed by flow cytometry. Although all four subsets of immune cells express LTα and LTβ on their surfaces, the highest level of LTα and LTβ is found on CD8+ T cell surfaces (Figure 1B and C). Based on these observations, we conclude that the ligands for LTβR are activated in both innate and adaptive immune cells in the tumor microenvironment under pathophysiological conditions.
Fig. 1.
LTβR ligands are expressed in tumor-infiltrating immune cells. (A) RT–PCR analysis of LTβR ligand expression. CT26 tumor cells were injected into naive mice. Lungs were collected from tumor-bearing mice ~21 days later and digested with collagenase solution to make single-cell suspensions. CD4+, CD8+, CD11b+ and NK cells were then isolated from the digest mixtures as described in Materials and methods and lysed to make total RNA for RT–PCR analysis. Shown are results from three mice. (B) LTα and LTβ expression on tumor-infiltrating immune cells. Single-cell suspension as in (A) was stained with LTα- or LTβ-specific mAbs in combination with CD4-, CD8-, CD11b- or NK-specific mAbs, respectively. Isotype control IgGs were used as negative controls. The stained cells were gated for the indicated subsets of immune cells to determine LTα and LTβ expression. The number in each plot indicates percentage of the specific subsets of cells. Shown are representative results of one of three mice. (C) Quantification of percent LTα+ and LTβ+ cells in the four subsets of tumor-infiltrating immune cells as shown in (B). Column: mean; bar: standard deviation (SD).
LTα1LTβ2 and LIGHT played critical roles in cancer immune surveillance against spontaneous sarcoma
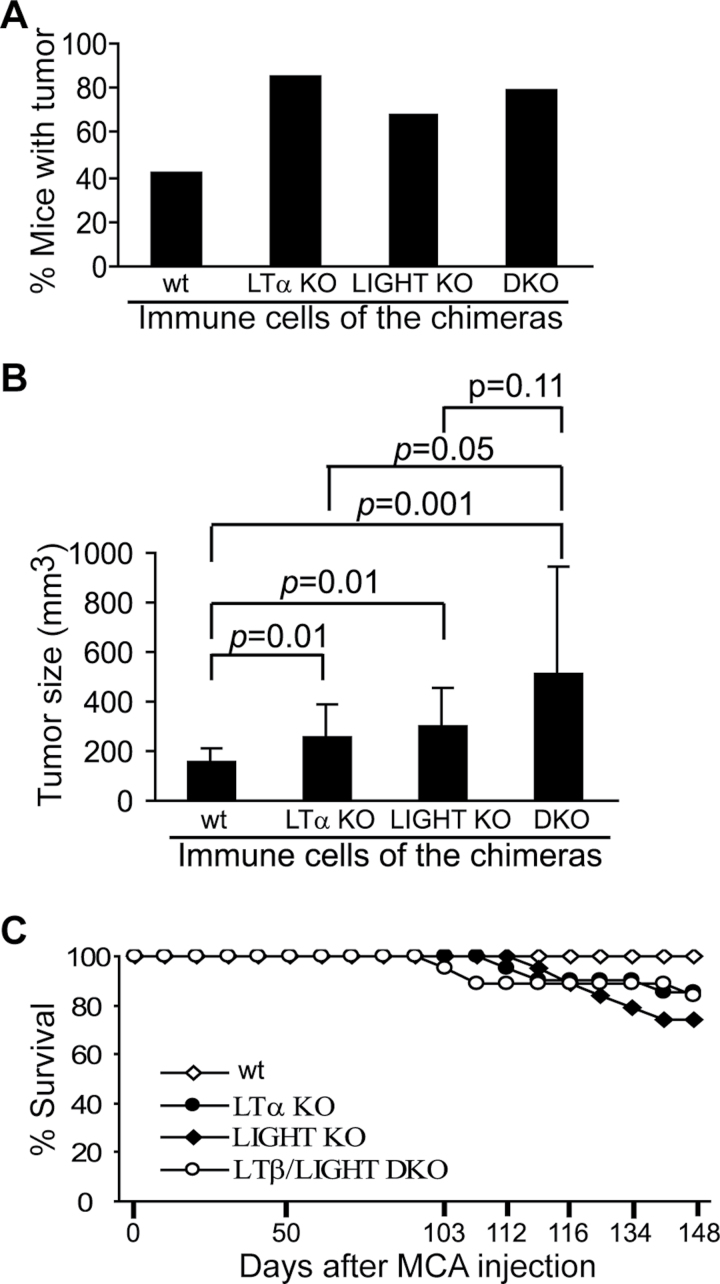
Based on the above observations, we reasoned that tumor-infiltrating immune cells might target tumor cells through LTβR-mediated cytotoxicity in vivo. To test this hypothesis, we made use of LTβR ligand-deficient mice. The rationale is that if immune cells utilize their LTβR ligands to bind to the LTβR on tumor cells to suppress tumor development, then LTβR ligand-deficient mice should be more prone to tumor induction. Because LTβR ligands are only expressed in immune cells under physiological conditions (3,4,9,27,28), we generated chimera mice with LTβR ligand deficiency only in the immune cells. Bone marrow cells were prepared from wt, LTα KO, LIGHT KO and LTβ/LIGHT DKO mice and transplanted into lethally irradiated wt recipient mice. The chimera mice were then injected with MCA to induce sarcomas. Both the tumor incidence and tumor size are significantly greater in LTα KO, LIGHT KO and LTβ/LIGHT DKO chimera mice as compared with that in wt chimera mice (Figure 2). Our data thus suggest that both ligands of the LTβR, LTα1LTβ2 and LIGHT, play critical roles in suppressing spontaneous sarcoma development under physiological conditions.
Fig. 2.
LTβR ligands play a critical role in cancer immune surveillance. Bone marrow cells from LTα KO, LIGHT KO and LTβ/LIGHT DKO mice were transferred to lethally irradiated wt C57BL/6J mice. The chimera mice were then injected with MCA 18 days later (wt: n = 19, LTα KO: n = 20, LIGHT: n = 19 and LTβ/LIGHT DKO: n = 19). Tumor incidence (A) and size (B) were analyzed 96 days after MCA injection. Column: mean; bar: SD. (C) Survival curve of the wt and LTβR ligand-deficient chimera mice after MCA injection.
Ligation of the LTβR resulted in human tumor cell growth inhibition in vitro
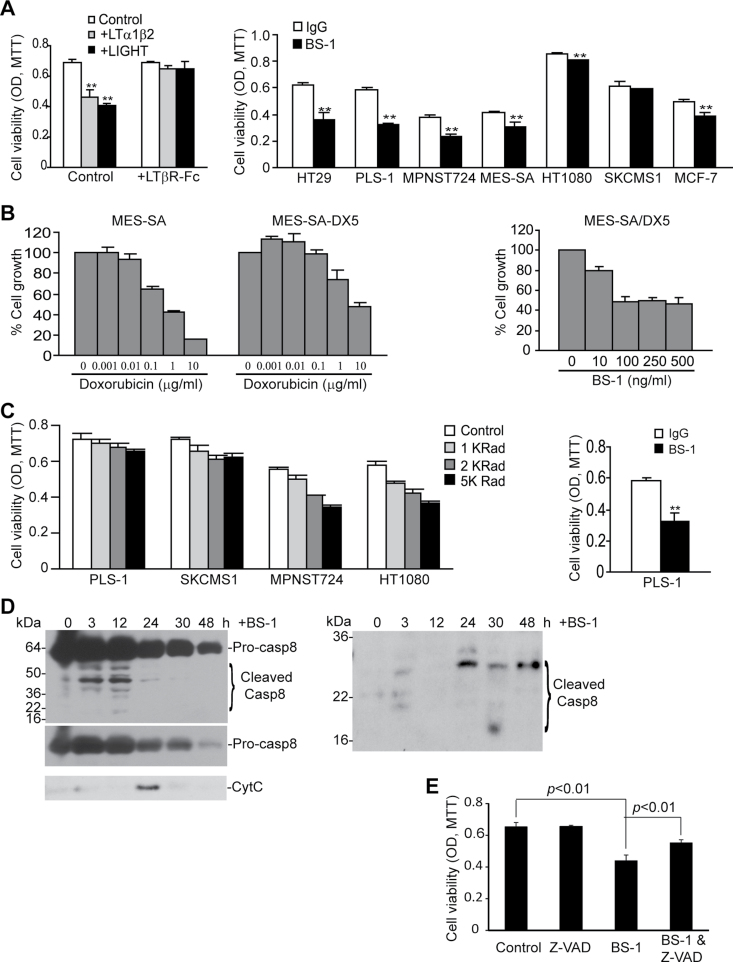
There are contrasting observations regarding the role of LTβR signaling pathways in tumor cell apoptosis and growth promotion. Compelling experimental data have demonstrated that agonist LTβR mAb or recombinant ligand proteins induce apoptosis in tumor cells (1–5,19,29–32). However, it was also observed that recombinant LTα1LTβ2 and LIGHT protein promote tumor cell growth (16). Our above observations clearly indicate that immune cell–tumor cell interactions through the LTβR suppress spontaneous tumor development under pathophysiological conditions (Figure 2). To define whether LTβR mediates tumor cell apoptosis or growth promotion, we cultured human colon carcinoma cells in the presence of recombinant LTα1LTβ2 and LIGHT proteins and observed that both LTα1LTβ2 and LIGHT significantly inhibit human colon carcinoma cell growth in vitro (Figure 3A). To determine whether LTα1LTβ2 and LIGHT specifically interact with the LTβR, excess amount of recombinant LTβR-Fc chimera protein was added to the cell culture. It is clear that LTβR-Fc chimera protein blocked LTα1LTβ2- and LIGHT-mediated human colon carcinoma cell growth inhibition (Figure 3A), suggesting that LTα1LTβ2 and LIGHT specifically bind to the LTβR in tumor cells to induce apoptosis. Next, we expanded our study to seven human cancer cell lines (one colon carcinoma, five soft tissue carcinomas and one mammary carcinoma). Incubation of these human tumor cells with a humanized LTβR agonist mAb (clone BS-1) significantly inhibited the growth of six of the seven human tumor cell lines (Figure 3A).
Fig. 3.
LTβR agonist mAb suppresses human cancer cell growth in vitro through inducing apoptosis. (A) Left panel: human colon carcinoma HT29 cells were incubated with recombinant LTα1LTβ2 (100ng/ml) and LIGHT (50ng/ml) protein in the absence or presence of excess LTβR-Fc chimera protein (1 μg/ml) for 5 days. Cell growth was measured by MTT assay. Right panel: human colon carcinoma (HT29), STS (PLS-1, MPNST724, MES-SA, HT1080, SKCMS1) and mammary carcinoma (MCF-7) cells were cultured in the presence of LTβR agonist mAb (BS-1) for 5 days and analyzed for growth by MTT assay. (B) BS-1 inhibits doxorubicin-resistant STS cell growth. Left panel: MES-SA and MES-SA/DX5 cells were incubated in the presence of various concentrations of doxorubicin for 5 days and analyzed by MTT assay. Right panel: doxorubicin-resistant MES-SA/DX5 cells were incubated with various concentrations of BS-1 mAb for 5 days and analyzed for cell growth by MTT assay. (C) BS-1 inhibits radiation-resistant STS cell growth. Left panel: four STS cell lines were irradiated with the indicated doses and analyzed for cell growth by MTT assays. Right panel: the radiation-resistant PLS-1 cells were incubated with BS-1 mAb for 5 days and analyzed for cell growth by MTT assay. Column: mean; bar: SD. **P < 0.01. (D) BS-1 induced caspase activation and cytochrome c release. MES-SA/DX5 cells were incubated with BS-1 for the indicated times and analyzed for cleaved caspases 8 and 3, as well as for cytosolic cytochrome c by western blot analysis. (E) Caspase inhibitor blocked BS-1-induced tumor cell death. MES-SA/DX5 cells were cultured in the presence of Z-VAD for 1h, followed by the addition of BS-1. Cell viability was quantified as in (B) and analyzed by two-tailed t-test. The t-test was done with three replicates in each group. Shown are results of one representative experiment of two independent experiments. Column: mean; bar: SD.
LTβR agonist mAb suppressed doxorubicin-resistant STS growth
Doxorubicin is the standard treatment drug for human STS. However, STS cells often develop resistance to doxorubicin, which is currently a major problem in human STS chemotherapy (33). To determine whether LTβR-based therapy is effective in suppressing doxorubicin-resistant STS, we made use of an existing doxorubicin-resistant STS cell line MES-SA/DX5. We confirmed that MES-SA/DX5 is indeed resistant to doxorubicin as compared with the parent cell line (Figure 3B). Treatment of MES-SA/DX5 cells with BS-1 significantly inhibited tumor cell growth in vitro (Figure 3B). Therefore, our data suggest that LTβR mAb therapy might be effective in cancer therapy against doxorubicin-resistant human STS.
LTβR agonist mAb suppressed radiation-resistant STS cell growth
Adjuvant radiotherapy after surgical resection of tumor is another standard treatment for STS patients. However, resistance to radiation often occurs and results in recurrence and metastasis (33). To determine whether LTβR agonist mAb is effective in suppressing radiation-resistant STS cells, we first tested the sensitivity of four STS cell lines to ionizing radiation. PLS-1 is a STS cell line that exhibits significant resistance to radiation (Figure 3C). SKCMS1, a primary STS cell line, is also resistant to radiation. Two other primary STS cell lines are relatively sensitive to radiation (Figure 3C). We treated the radiation-resistant PLS-1 cells with BS-1 and measured tumor growth rate. BS-1 significantly inhibited PLS-1 cell growth (Figure 3C), suggesting that LTβR-based therapy is potentially an effective approach to treat radiation-resistant human STS.
Ligation of the LTβR-induced caspase activation and mitochondrion-dependent apoptosis
MES-SA/DX5 cells were then treated with BS-1 and analyzed for caspase activation and cytochrome c release. Analysis of the cytosolic fractions of the tumor cells revealed that BS-1 induces caspase 8 activation that peaked at 12h (Figure 3D). Caspase 8 activation was followed by cytochrome c release and caspase 3 activation (Figure 3D). To functionally determine whether BS-1-induced cell death is caspase dependent, we incubated MES-SA/DX5 cells with the caspase inhibitor Z-VAD, followed by addition of BS-1 to the culture. Analysis of cell death showed that Z-VAD blocked BS-1-induced cell death (Figure 3E). Thus, our data suggest that BS-1 suppresses STS cell growth at least partially through inducing caspase-dependent apoptosis.
Ligation of the LTβR also induced NF-κB activation in human tumor cells in vitro
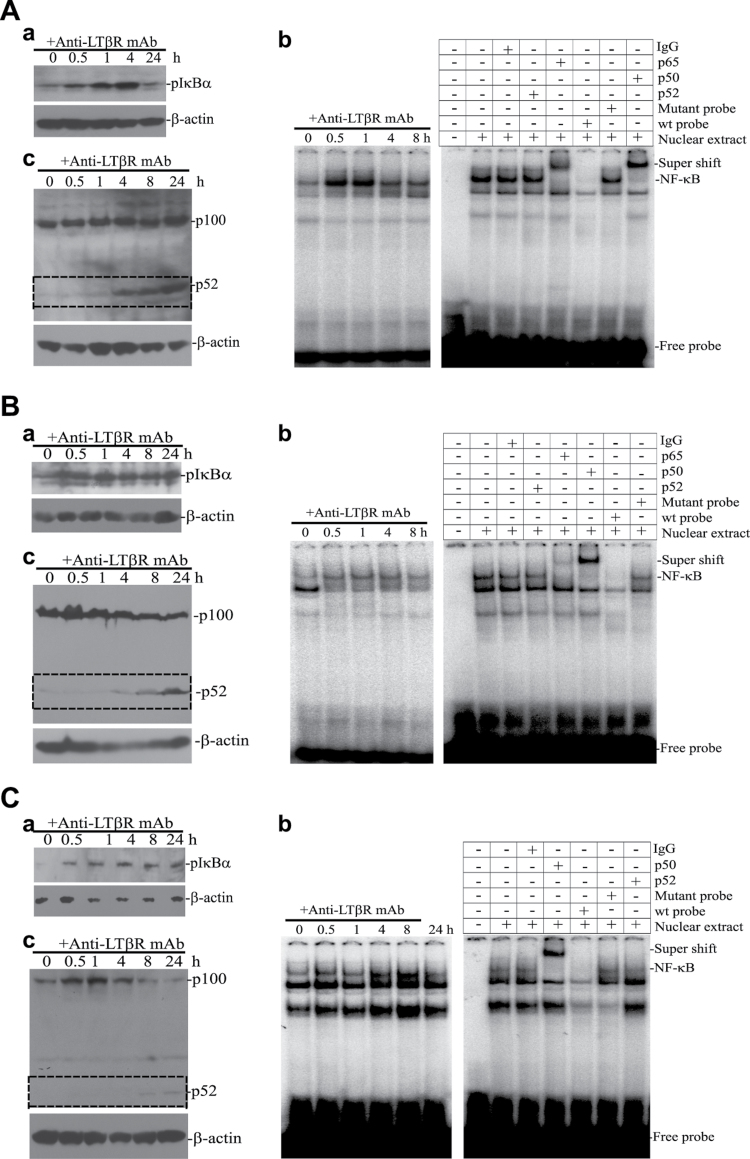
It is known that ligation of the LTβR also leads to NF-κB activation (10). To determine whether LTβR agonist mAb simultaneously induces apoptosis and NF-κB activation in human cancer cells, we measured two signatures of the canonical NF-κB activation pathway: IκBα phosphorylation (pIκBα) and NF-κB electrophoresis mobility shift in human colon carcinoma, mammary carcinoma and STS cells. Western blot analysis indicated that a low level of pIκBα is constitutively present in tumor cells; however, IκBα phosphorylation was quickly increased after BS-1 treatment (Figure 4Aa, 4Ba and 4Ca). EMSA also revealed that the canonical NF-κB (p65/p50) is quickly activated (Figure 4Ab, 4Bb and 4Cb). Therefore, the canonical NF-κB is constitutively active, albeit at a low level, and LTβR agonist mAb induces rapid canonical NF-κB activation in human colon carcinoma, mammary carcinoma and STS cells.
Fig. 4.
LTβR agonist mAb induces both canonical and alternate NF-κB activation in human cancer cells. Human colon carcinoma (HT29, A), mammary carcinoma (MCF-7, B) and STS (MPNST724, C) cells were cultured in the presence of BS-1 and analyzed for NF-κB activation. (a) Western blot analysis of IκBα activation (pIκBα). Tumor cells were incubated with BS-1 for the indicated time and analyzed with pIκBα-specific antibody. β-Actin was used as loading controls. (b) EMSA of canonical NF-κB activation kinetics. Tumor cells were treated with BS-1 for the indicated times and nuclear extracts were prepared. Nuclear extracts were incubated with NF-κB DNA probe and analyzed with 6% polyacrylamide gel electrophoresis. Right panel: specificity control of EMSA. Nuclear extracts were prepared from BS-1-treated cells [30min for HT29 cells (A), 60min for MCF-7 cells (B) and 4h for MPNST724 cells (C), respectively] and incubated with 32P-labelled NF-κB probe in the presence of IgG, p52, p65, p50, excess cold NF-κB probe and excess cold mutant NF-κB probe. The DNA–protein complexes were analyzed by 6% polyacrylamide gel electrophoresis. The canonical NF-κB (NF-κB1) complex is indicated. (c) Western blot analysis of alternate NF-κB activation. Tumor cells were incubated with BS-1 for the indicated times and analyzed with p100/p52-specific antibody. β-Actin was used as loading controls.
We also analyzed alternate NF-κB activation with one of the signature markers of the alternate NF-κB pathway: NF-κB2 (p100) processing to p52. Western blot analysis revealed that p52 was weakly detectable in the untreated cells, suggesting a low level of constitutive alternate NF-κB activation in the tumor cells. A significant p52 increase occurred at ~4–8h after BS-1 stimulation and was sustained beyond 24h (Figure 4Ac, 4Bc and 4Cc). Therefore, it seems that ligation of the LTβR also activates alternate NF-κB, and NF-κB activation occurs after canonical NF-κB activation. In summary, our data indicate that ligation of the LTβR induces tumor cell growth inhibition, as well as both canonical and alternate NF-κB activation in human colon carcinoma, mammary carcinoma and STS cells.
LTβR was highly expressed in human cancer cells
A previous study has shown that LTβR is expressed in human cancer cells (6). To determine the relative LTβR levels in various stages of colorectal carcinoma and whether LTβR level is correlated with clinical outcome, we made use of a colorectal progression TMA and used immunohistochemical methods to analyze the LTβR protein level. This TMA contains 14 normal colon tissues, 14 colorectal adenocarcinomas, 7 lymph node metastases of colorectal adenocarcinoma and 7 distal site metastases (4 liver and 3 lung metastases) of colorectal adenocarcinoma. LTβR protein level ranged from ‘undetectable’ to ‘weak’ in 13 of the 14 normal colonic mucosa specimens and in 10 of the 14 adenoma specimens. Only one adenoma specimen exhibited high LTβR level. In contrast, the majority of adenocarcinomas (86%), lymph node metastases (100%) and distal metastases (86%) expressed high levels of LTβR protein (Supplementary Figure 1A, available at Carcinogenesis Online). Flow cytometry analysis revealed that LTβR is highly expressed on colon carcinoma cell surfaces (Supplementary Figure 1B, available at Carcinogenesis Online).
We then extended the above study to a large cohort of human colorectal cancer tissues in a TMA containing 341 colorectal adenoma/adenocarcinomas, 27 adenoma and 71 normal colon tissues. Consistent with the above observation, LTβR is weakly expressed in normal human colon tissues and adenoma, but is highly expressed in the majority of adenocarcinomas. Twenty-five percent of adenoma cells are LTβR-positive, whereas 89.5% adenocarcinoma cells are LTβR-positive (Supplementary Table 1, available at Carcinogenesis Online). Statistical analysis revealed that the expression level of LTβR is not correlated with patient survival and recurrence (Supplementary Figure 2, available at Carcinogenesis Online), suggesting that LTβR alone does not suppress or promote human colorectal cancer development.
We also analyzed LTβR protein levels in STS specimens. Malignant fibrous histiocytoma derived from three STS patients was stained for LTβR protein. All three specimens showed high levels of LTβR protein (Supplementary Figure 3A, available at Carcinogenesis Online). To quantitatively determine the LTβR protein level, we then analyzed the cell surface LTβR protein in four human STS cell lines (three primary and one recurrent cell line). Overall, as observed in human cancer specimens, LTβR is highly expressed on the surfaces of human STS cells (Supplementary Figure 3B, available at Carcinogenesis Online).
LTβR agonist mAb decreased colon carcinoma metastatic potential in vivo
LTβR ligation can induce apoptosis and NF-κB activation in human tumor cells (Figures 3 and 4). We reasoned that LTβR-mediated NF-κB activation might interfere with the LTβR-mediated apoptosis, resulting in decreased tumor cell death. Therefore, blocking NF-κB activation should increase the efficacy of LTβR-mediated tumor growth inhibition. To test this hypothesis, HT29 and MES-SA/DX5 cells were stably transfected with the dominant-negative IKKα (IKKα-KM) and IKKβ (IKKβ-KA) mutant plasmids, respectively, to block the alternate and canonical NF-κB pathways. Analysis of sensitivity of these cells to BS-1-induced growth inhibition indicated that blocking the alternate or canonical NF-κB pathways does not significantly affect BS-1-induced growth inhibition in human tumor cells (Supplementary Figure 4, available at Carcinogenesis Online).
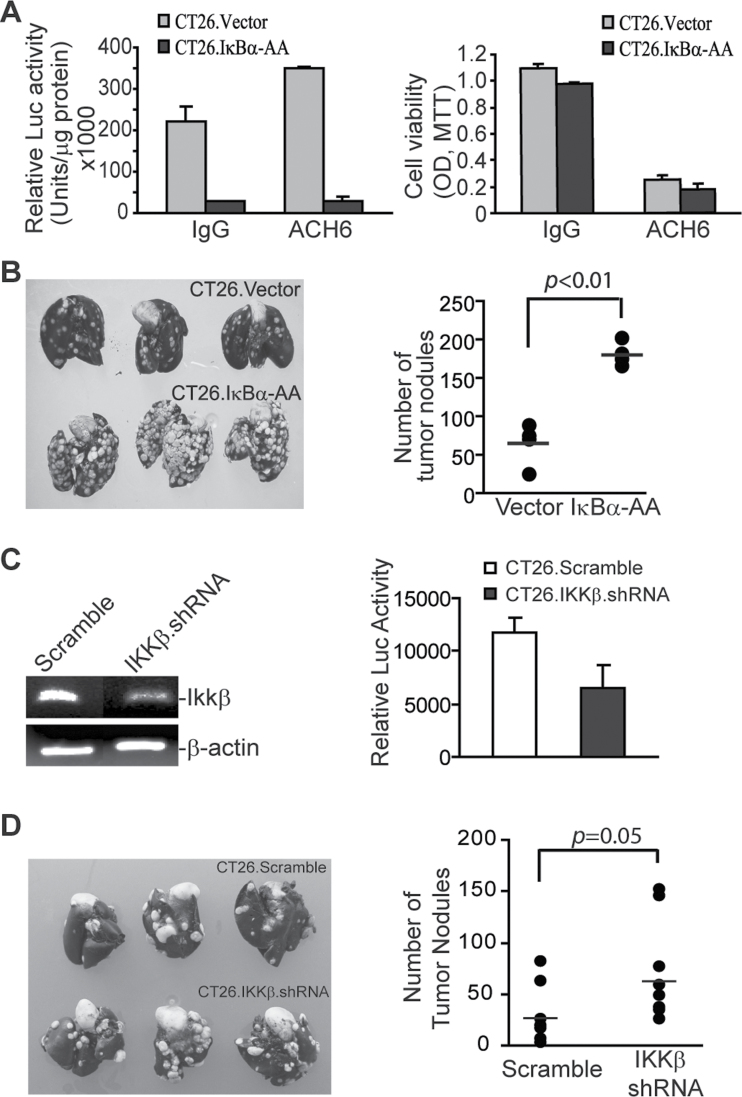
Next, we sought to determine whether NF-κB interferes with LTβR-mediated tumor cell apoptosis in vivo in a mouse tumor model. We first analyzed the role of LTβR in inhibition of mouse tumor cell growth and in induction of NF-κB activation in mouse sarcoma (CMS4-met), melanoma (B16F1), mammary carcinoma (4T1) and colon carcinoma (MC38 and CT26) cells. Tumor cells were cultured in the absence or presence of LTβR agonist mAb (clone ACH6). It is clear that ACH6 significantly inhibits the proliferation of all five cell lines in vitro (P < 0.01) (Supplementary Figure 5A, available at Carcinogenesis Online). NF-κB reporter assay of CMS4-met and CT26 cells revealed that, as in human tumor cells, LTβR agonist mAb also induced NF-κB activation in mouse tumor cells (Supplementary Figure 5B, available at Carcinogenesis Online). It has been shown previously that ACH6 induces necrosis and T cell infiltration in established CT26 tumors in vivo (6). Next, we used this CT26 tumor model, but in an experimental metastasis model system, to determine whether ACH6 suppresses CT26 tumor growth in vivo and whether blocking NF-κB activation increases ACH6-mediated tumor suppression in vivo. CT26 cells were stably transfected with an empty vector of the NF-κB suppressor IκBα-AA (24). NF-κB reporter assay indicated that IκBα-AA effectively suppresses both constitutive and ACH6-induced NF-κB activation in CT26 cells in vitro (Figure 5A). However, IκBα-AA did not significantly alter ACH6-induced growth inhibition in CT26 cells in vitro (Figure 5A). Surprisingly, blocking NF-κB activation with IκBα-AA significantly and reproducibly (in five independent experiments) increased CT26 cell metastatic potential in this experimental metastasis mouse model (Figure 5B).
Fig. 5.
Blocking NF-κB activation increases colon carcinoma metastatic potential in vivo. (A) Blocking NF-κB did not significantly alter CT26 cell growth in vitro. Left panel: CT26.pcDNA and CT26.IκBα-AA cells were cultured in the presence of IgG control mAb and ACH6, respectively, and NF-κB activation was analyzed by NF-κB reporter assay. Right panel: CT26.pcDNA and CT26.IκBα-AA cells were cultured as in the right panel for 5 days and analyzed by MTT assay. (B) Blocking NF-κB activation significantly increased the metastatic potential of colon carcinoma cells in vivo. CT26 cells were stably transfected with the pcDNA plasmid (Vector) and pcDNA-containing IκBα-AA (IκBα-AA) (0.5×105 cells/mouse). Cells were injected i.v. into syngeneic mice. Lung metastasis was analyzed 21 days after tumor injection. Left panel: images of tumor-bearing lungs. Right panel: the number of lung tumor nodules in each mouse was enumerated. Shown are results of one representative experiment of five independent experiments. (C) Left panel: RT–PCR analysis of IKKβ expression. CT26 cells were stably transfected with scramble shRNA (Scramble) or IKKβ-specific shRNA (IKKβ.shRNA) plasmid and analyzed for IKKβ mRNA expression. β-Actin was used as normalization control. Right panel: NF-κB activation in CT26 cells. CT26.Scramble and CT26.IKKβ.shRNA cells were transiently transfected with a NF-κB-luciferase reporter overnight and then treated with ACH6 mAb for 8h for luciferase reporter assay. (D) Silencing IKKβ significantly increased the metastatic potential of colon carcinoma cells in vivo. CT26.Scramble and CT26.IKKβ.shRNA cells were injected i.v. into syngeneic mice. Lung metastasis was analyzed 21 days after tumor injection. Left panel: images of tumor-bearing lungs. Right panel: the number of lung tumor nodules in each mouse was enumerated. Each dot represents the number of tumor nodules of a single mouse.
A complementary approach was then used to validate the finding that blocking NF-κB promotes tumor development. CT26 cells were stably transfected with either a scramble shRNA or IKKβ-specific shRNA. It has been shown that the canonical NF-κB is activated through IKKβ and blocking LKKβ inhibits the canonical NF-κB activation (11). Indeed, silencing IKKβ diminished NF-κB activity in CT26 cells (Figure 5C). Consistent with what was observed in CT26.IκBα-AA cells, silencing IKKβ significantly enhanced the metastatic potential of CT26 cells in vivo (Figure 5D). Taken together, our data suggest that NF-κB acts as a tumor suppressor in this CT26 experimental lung metastasis model.
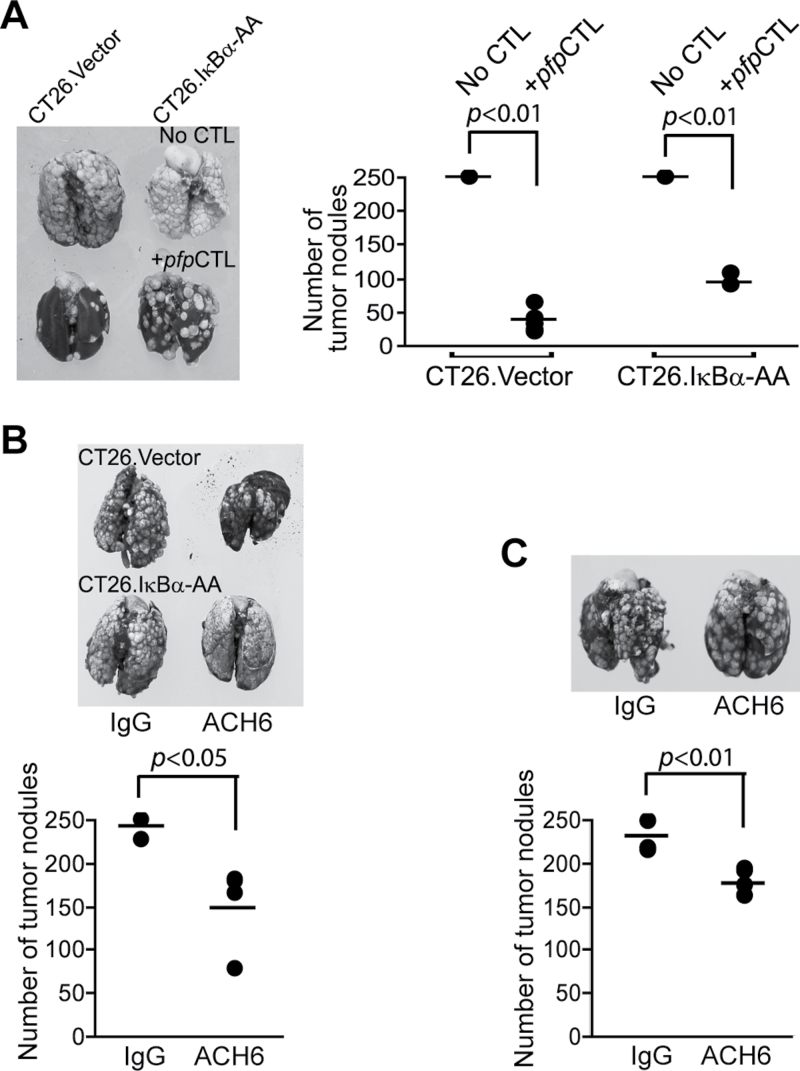
We then sought to determine whether blocking NF-κB enhances the efficacy of CTL adoptive transfer immunotherapy. To better evaluate the LTβR-mediated cytotoxicity, a perforin-deficient CTL line was used. CT26 tumor cells are resistant to Fas-mediated apoptosis. Therefore, the perforin and Fas-mediated effector mechanisms are impaired in this system. Consistent with the above observation that NF-κB actually functions to suppress colon carcinoma metastasis in vivo, blocking NF-κB did not increase the efficacy of CTL adoptive transfer immunotherapy against colon carcinoma metastasis in this experimental metastasis mouse model (Figure 6A). In the next experiment, CT26.Vector and CT26.IkBa-AA tumors were treated with isotype control mAb or ACH6 and lung metastasis was analyzed. ACH6 significantly decreased CT26 cell metastatic potential, and blocking NF-κB activation did not increase ACH6 efficacy in suppression of CT26 metastatic potential in this experimental metastasis mouse model (Figure 6B and C). Taken together, our data suggest that the LTβR-mediated apoptosis signaling pathway and the NF-κB signaling pathway might act in concert to suppress tumor development in vivo.
Fig. 6.
LTβR agonist mAb suppresses colon carcinoma metastatic potential in vivo. (A) Blocking NF-κB in tumor cells did not increase the efficacy of CTL adoptive transfer immunotherapy. CT26.Vector and CT26.IκBα-AA cells (2×105 cells/mouse) were transplanted to mice to establish lung metastases. Perforin-deficient CTLs (pfpCTL) were then adoptively transferred to the tumor-bearing mice (n = 5 each group). Left panel: representative images of tumor-bearing lungs. Right panel: the number of lung tumor nodules in each mouse was enumerated and compared. Shown are results of one representative experiment of two independent experiments. (B) ACH6 significantly suppressed CT26 lung metastasis in vivo. Top panel: CT26.Vector and CT26.IκBα-AA cells (2×105 cells/mouse) were injected i.v. into mice. IgG or ACH6 (50 μg/mouse) were injected i.v. into tumor-bearing mice at days 3, 6 and 9 after tumor injection. Mice were sacrificed 14 days after tumor injection and analyzed for lung metastasis. Bottom panel: quantification of lung metastasis in CT26.Vector tumor-bearing mice as in (A). The tumor nodules in CT26.IκBα-AA tumor-bearing mice exceeded 250 and were not counted. (C) CT26.IκBα-AA cells (0.75×105 cells/mouse) were injected into mice and treated with ACH6 as in the left panel (n = 5, each group). Shown are results of one representative experiment of two independent experiments. Bottom panel: quantification of lung metastasis as in (A).
Discussion
Mice with a targeted mutation in LTα exhibited enhanced tumor growth and metastasis in a melanoma tumor model (34). However, LTα and p53 double-deficient mice showed no significant difference in lymphoma development as compared with p53-deficient mice (35). In addition, targeted mutation of LTα dramatically decreased tumor growth and metastasis in a prostate cancer mouse model (36). More recently, it has been shown that LTβR plays a critical role in NF-κB-dependent promotion of HCC and prostate cancer (11,13). The ligands for LTβR, LTα and LIGHT, are expressed on activated T cells but not on quiescent T cells (7,27). Dysregulation of LIGHT expression on T cells resulted in hypertriglyceridemia and hypercholesterolemia, and constitutive expression of LIGHT via a transgene on T cells and resultant intimate contact between lymphocytes and hepatocytes resulted in altered lipid metabolism and dyslipidemia (37). On the other hand, although under physiological conditions, LTα and LTβ are not expressed on hepatocytes, constitutive forced expression of LTα and LTβ on hepatocytes mimics hepatitis virus-induced liver inflammation and NF-κB-dependent HCC development (11). These observations thus suggest that the function of LTβR, as either a tumor suppressor or promoter, might be tumor type and cellular context dependent.
In this study, we used LTβR ligand-deficient chimera mice in which the LTβR ligand deficiencies are only in the immune cells of the tumor-bearing mice. We observed that knocking down either LTα or LIGHT in the immune cells significantly increases carcinogen-induced spontaneous tumor development (Figure 2). Furthermore, LTβR agonist mAb significantly decreased colon carcinoma metastatic potential (Figure 6). However, although the MCA-induced tumor sizes are significantly larger in LTβ/LIGHT DKO mice than those in LTα KO mice (Figure 2B), the tumor incidence in LTβ/LIGHT DKO mice is not significantly different from that in LTα or LIGHT KO mice (Figure 2A), suggesting that there are no additive effects of the two LTβR ligands and the LTα might also function through the TNF receptor. However, it is also possible that the tumor incidence reached a plateau in the LTα and LIGHT single gene KO mice since MCA is a potent carcinogen (Figure 2A). Further studies are needed to determine the relative contributions of these two ligands in LTβR-mediated tumor suppression. Nevertheless, our data thus suggest that the LTβR-ligand system plays a critical role in host immune cell-mediated suppression of spontaneous sarcoma and colon carcinoma development in the experimental system used in our studies.
The molecular mechanism of LTβR-mediated tumor cell growth inhibition is largely unknown. Previous studies have revealed that TNF receptor-associated factor (TRAF) 2 and TRAF3 (38–40), caspase-independent mechanisms (41), superoxide radical formation (42), kinase activation (31), survivin, IAP1 and Smac (5,39) and downregulation of Bcl-2 (43) as consequences of LTβR signaling. In this study, we observed that ligation of LTβR in human sarcoma cells induces caspases 8 and 3 activation and cytochrome c release. Therefore, our data demonstrated, for the first time, that LTβR signaling activates caspases to induce mitochondrion-mediated apoptosis. It has also been shown that LTαβ of tumor-specific effector T cells crosslinks the LTβR on tumor cells to stimulate them to secrete chemokines that are chemotactic for macrophages, which might contribute to tumor elimination in vivo (9). Therefore, LTβR might also mediate tumor suppression through an indirect mechanism.
Because ligation of the LTβR simultaneously activates the apoptosis signaling pathway and induces NF-κB activation and because NF-κB is a potent tumor promoter (12), we hypothesized that blocking NF-κB activation should shift the LTβR-mediated signaling toward the apoptosis pathway. In an attempt to test this hypothesis, we surprisingly but reproducibly observed that blocking NF-κB activation increases colon carcinoma metastatic potential in vivo (Figure 5), suggesting that NF-κB signaling actually functions to suppress colon carcinoma development in vivo. This observation, although unexpected, may not be surprising. Although the prosurvival and tumor promotion roles of canonical NF-κB signaling are well demonstrated (12), it has also been shown that NF-κB promotes apoptosis in multiple types of cells (15,44–56). More recently, the proapoptotic function of NF-κB in the death receptor-mediated apoptosis pathway has been well demonstrated in tumor cells (53,55). Additionally, even though constitutive expression of LTα and LTβ (via transgenes) in the non-hematopoietic liver cells leads to chronic inflammation and HCC (11), targeted mutation of IKKβ, the kinase that activates canonical NF-κB, actually inhibits hepatocyte apoptosis and promotes carcinogen-induced and spontaneous HCC development (57–59). These studies indicate that in the context where prosurvival signals derive from other oncogenes, NF-κB functions as a tumor suppressor (15).
The molecular mechanism underlying the tumor suppression function of NF-κB in vivo observed in this study (Figure 5) is not clear. Our data indicate that blocking NF-κB activation in colon carcinoma cells does not alter tumor cell response to LTβR agonist mAb-induced growth inhibition in vitro but increases colon carcinoma cell metastatic potential in vivo (Figures 5A and 6). Although both the canonical and alternate NF-κB pathways do not affect LTβR-mediated tumor growth inhibition in vitro (Supplementary Figure 4, available at Carcinogenesis Online), we have recently shown that the canonical NF-κB functions as a transcription activator of death receptor Fas in both human colon carcinoma cells and mouse embryonic fibroblasts and promotes Fas-mediated apoptosis (25). Our recent study has also shown that CT26 cells induce FasL activation on tumor-infiltrating CTLs in vivo (60). Therefore, it is possible that blocking NF-κB activation in CT26 cells might diminish CT26 cell sensitivity to apoptosis induction by FasL on tumor-specific CTLs, resulting in increased tumor metastatic potential, which remains to be determined.
Supplementary material
Supplementary Table 1 and Figures 1–5 can be found online at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (CA133085 to K.L.); the American Cancer Society (RSG-09-209-01-TBG to K.L.).
Supplementary Material
Acknowledgement
We thank Ms Kimberly K.Smith for assistance in immunohistochemical staining of tumor tissues.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocyte
- DKO
double knockout
- HCC
hepatocellular carcinoma
- i.v.
intravenously
- KO
knockout
- LTβR
lymphotoxin β receptor
- mAb
monoclonal antibody
- MCA
methylcholanthrene
- MTT
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
- pIκBα
IκBα phosphorylation
- RT–PCR
reverse transcription–polymerase chain reaction
- SD
standard deviation
- STS
soft tissue sarcoma
- TMA
tissue microarray
- TNF
tumor necrosis factor
- wt
wild-type.
References
- 1. Rooney I.A., et al. (2000). The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J. Biol. Chem., 275, 14307–14315 [DOI] [PubMed] [Google Scholar]
- 2. Zhai Y., et al. (1998). LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J. Clin. Invest., 102, 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kashii Y., et al. (1999). Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J. Immunol., 163, 5358–5366 [PubMed] [Google Scholar]
- 4. Lu G., et al. (2002). Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-α1β2, Fas ligand, and TNF-related apoptosis-inducing ligand. J. Immunol., 168, 1831–1839 [DOI] [PubMed] [Google Scholar]
- 5. You R.I., et al. (2006). Inhibition of lymphotoxin-beta receptor-mediated cell death by survivin-DeltaEx3. Cancer Res., 66, 3051–3061 [DOI] [PubMed] [Google Scholar]
- 6. Lukashev M., et al. (2006). Targeting the lymphotoxin β receptor with agonist antibodies as a potential cancer therapy. Cancer Res., 66, 9617–9624 [DOI] [PubMed] [Google Scholar]
- 7. Yang D., et al. (2007). Targeting lymphotoxin beta receptor with tumor-specific T lymphocytes for tumor regression. Clin. Cancer Res., 13, 5202–5210 [DOI] [PubMed] [Google Scholar]
- 8. Dobrzanski M.J., et al. (2004). Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res., 64, 406–414 [DOI] [PubMed] [Google Scholar]
- 9. Winter H., et al. (2007). Tumor-specific T cells signal tumor destruction via the lymphotoxin beta receptor. J. Transl. Med., 5, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ware C.F. (2005). Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol., 23, 787–819 [DOI] [PubMed] [Google Scholar]
- 11. Haybaeck J., et al. (2009). A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell, 16, 295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pikarsky E., et al. (2004). NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature, 431, 461–466 [DOI] [PubMed] [Google Scholar]
- 13. Ammirante M., et al. (2010). B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature, 464, 302–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen F., et al. (2007). Nuclear factor-κB, an unappreciated tumor suppressor. Cancer Res., 67, 11093–11098 [DOI] [PubMed] [Google Scholar]
- 15. Klein U., et al. (2011). The two faces of NF-κB signaling in cancer development and therapy. Cancer Cell, 20, 556–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daller B., et al. (2011). Lymphotoxin-β receptor activation by lymphotoxin-α1β2 and LIGHT promotes tumor growth in an NFκB-dependent manner. Int. J. Cancer, 128, 1363–1370 [DOI] [PubMed] [Google Scholar]
- 17. van Horssen R., et al. (2006). TNF-α in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist, 11, 397–408 [DOI] [PubMed] [Google Scholar]
- 18. Balkwill F. (2009). Tumour necrosis factor and cancer. Nat. Rev. Cancer, 9, 361–371 [DOI] [PubMed] [Google Scholar]
- 19. Mackay F., et al. (1997). Cytotoxic activities of recombinant soluble murine lymphotoxin-α and lymphotoxin-αβ complexes. J. Immunol., 159, 3299–3310 [PubMed] [Google Scholar]
- 20. Liu F., et al. (2011). TNFα cooperates with IFN-γ to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS ONE, 6, e16241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu X., et al. (2011). IRF8 regulates acid ceramidase expression to mediate apoptosis and suppresses myelogeneous leukemia. Cancer Res., 71, 2882–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang D., et al. (2008). Downregulation of IFN-γR in association with loss of Fas function is linked to tumor progression. Int. J. Cancer, 122, 350–362 [DOI] [PubMed] [Google Scholar]
- 23. Zimmerman M., et al. (2010). IFN-γ upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PLoS ONE, 5, e14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo J.L., et al. (2004). Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell, 6, 297–305 [DOI] [PubMed] [Google Scholar]
- 25. Liu F., et al. (2012). NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem., 287, 25530–25540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGough J.M., et al. (2008). DNA methylation represses IFN-γ-induced and signal transducer and activator of transcription 1-mediated IFN regulatory factor 8 activation in colon carcinoma cells. Mol. Cancer Res., 6, 1841–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mauri D.N., et al. (1998). LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity, 8, 21–30 [DOI] [PubMed] [Google Scholar]
- 28. Janjic B.M., et al. (2002). Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J. Immunol., 168, 1823–1830 [DOI] [PubMed] [Google Scholar]
- 29. Browning J.L., et al. (1996). Signaling through the lymphotoxin beta receptor induces the death of some adenocarcinoma tumor lines. J. Exp. Med., 183, 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu M.Y., et al. (1999). The cytoplasmic domain of the lymphotoxin-beta receptor mediates cell death in HeLa cells. J. Biol. Chem., 274, 11868–11873 [DOI] [PubMed] [Google Scholar]
- 31. Chen M.C., et al. (2003). The role of apoptosis signal-regulating kinase 1 in lymphotoxin-beta receptor-mediated cell death. J. Biol. Chem., 278, 16073–16081 [DOI] [PubMed] [Google Scholar]
- 32. Force W.R., et al. (1997). Dominant negative mutants of TRAF3 reveal an important role for the coiled coil domains in cell death signaling by the lymphotoxin-beta receptor. J. Biol. Chem., 272, 30835–30840 [DOI] [PubMed] [Google Scholar]
- 33. Clark M.A., et al. (2005). Soft-tissue sarcomas in adults. N. Engl. J. Med., 353, 701–711 [DOI] [PubMed] [Google Scholar]
- 34. Ito D., et al. (1999). Mice with a targeted mutation in lymphotoxin-alpha exhibit enhanced tumor growth and metastasis: impaired NK cell development and recruitment. J. Immunol., 163, 2809–2815 [PubMed] [Google Scholar]
- 35. Kuprash D.V., et al. (2008). Ablation of TNF or lymphotoxin signaling and the frequency of spontaneous tumors in p53-deficient mice. Cancer Lett., 268, 70–75 [DOI] [PubMed] [Google Scholar]
- 36. Zhou P., et al. (2009). Targeting lymphotoxin-mediated negative selection to prevent prostate cancer in mice with genetic predisposition. Proc. Natl Acad. Sci. U.S.A., 106, 17134–17139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lo J.C., et al. (2007). Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science, 316, 285–288 [DOI] [PubMed] [Google Scholar]
- 38. VanArsdale T.L., et al. (1997). Lymphotoxin-beta receptor signaling complex: role of tumor necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor κB. Proc. Natl Acad. Sci. U.S.A., 94, 2460–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuai J., et al. (2003). Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J. Biol. Chem., 278, 14363–14369 [DOI] [PubMed] [Google Scholar]
- 40. Kim Y.S., et al. (2005). TRAF2 plays a key, nonredundant role in LIGHT-lymphotoxin beta receptor signaling. Mol. Cell. Biol., 25, 2130–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson C.A., et al. (2002). Death of HT29 adenocarcinoma cells induced by TNF family receptor activation is caspase-independent and displays features of both apoptosis and necrosis. Cell Death Differ., 9, 1321–1333 [DOI] [PubMed] [Google Scholar]
- 42. Chen M.C., et al. (2000). Overexpression of bcl-2 enhances LIGHT- and interferon-gamma-mediated apoptosis in Hep3BT2 cells. J. Biol. Chem., 275, 38794–38801 [DOI] [PubMed] [Google Scholar]
- 43. Zhang M., et al. (2003). LIGHT sensitizes IFNγ-mediated apoptosis of MDA-MB-231 breast cancer cells leading to down-regulation of anti-apoptosis Bcl-2 family members. Cancer Lett., 195, 201–210 [DOI] [PubMed] [Google Scholar]
- 44. Brady R.R., et al. (2011). c-Src dependency of NSAID-induced effects on NF-κB-mediated apoptosis in colorectal cancer cells. Carcinogenesis, 32, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 45. Stark L.A., et al. (2007). Aspirin activates the NF-κB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis, 28, 968–976 [DOI] [PubMed] [Google Scholar]
- 46. Vince J.E., et al. (2007). IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell, 131, 682–693 [DOI] [PubMed] [Google Scholar]
- 47. Petersen S.L., et al. (2007). Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell, 12, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varfolomeev E., et al. (2007). IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell, 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y., et al. (2002). NF-κB2 p100 is a pro-apoptotic protein with anti-oncogenic function. Nat. Cell Biol., 4, 888–893 [DOI] [PubMed] [Google Scholar]
- 50. Ishikawa H., et al. (1997). Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J. Exp. Med., 186, 999–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chien Y., et al. (2011). Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev., 25, 2125–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jing H., et al. (2011). Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev., 25, 2137–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crescenzi E., et al. (2011). NF-κB-dependent cytokine secretion controls Fas expression on chemotherapy-induced premature senescent tumor cells. Oncogene, 30, 2707–2717 [DOI] [PubMed] [Google Scholar]
- 54. Karl S., et al. (2009). Identification of a novel pro-apopotic function of NF-κB in the DNA damage response. J. Cell. Mol. Med., 13, 4239–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jennewein C., et al. (2012). Identification of a novel pro-apoptotic role of NF-κB in the regulation of TRAIL- and CD95-mediated apoptosis of glioblastoma cells. Oncogene, 31, 1468–1474 [DOI] [PubMed] [Google Scholar]
- 56. Thoms H.C., et al. (2010). Nucleolar targeting of RelA(p65) is regulated by COMMD1-dependent ubiquitination. Cancer Res., 70, 139–149 [DOI] [PubMed] [Google Scholar]
- 57. He G., et al. (2010). Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell, 17, 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luedde T., et al. (2007). Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell, 11, 119–132 [DOI] [PubMed] [Google Scholar]
- 59. Maeda S., et al. (2005). IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell, 121, 977–990 [DOI] [PubMed] [Google Scholar]
- 60. Yang D., et al. (2012). Decitabine and vorinostat cooperate to sensitize colon carcinoma cells to Fas ligand-induced apoptosis in vitro and tumor suppression in vivo . J. Immunol., 188, 4441–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.