Abstract
Arterial spin labeling (ASL) enables the noninvasive, quantitative imaging of cerebral blood flow using standard magnetic resonance imaging (MRI) equipment. Because it requires no contrast injection, ASL can add resting functional information to MRI studies measuring atrophy and signs of ischemic injury. Key features of ASL technology that may affect studies in Alzheimer’s disease are described. The existing literature describing ASL blood flow imaging applied to Alzheimer’s disease and related dementia is reviewed, and the potential role of ASL in treatment and prevention studies of early Alzheimer’s disease is discussed.
Keywords: Cerebral blood flow, diagnosis, imaging, magnetic resonance imaging, perfusion
INTRODUCTION
Imaging provides the primary means for assessing brain structure and function in humans in vivo, and for many clinical situations, brain imaging biomarkers have the potential to provide greater sensitivity and specificity than clinical indices for differential diagnosis and management of brain disorders. Brain imaging methods can also be applied to animal models and can therefore facilitate translational research. While changes in regional gray matter volume have been shown to provide a potential biomarker of Alzheimer’s disease (AD) and can predict conversion to AD from mild cognitive impairment (MCI) over intervals of 6 months or longer [1,2], changes in regional brain function may be more dynamic and provide even greater sensitivity to early disease, disease progression, or responses to therapy.
Functional MRI (fMRI) using the blood oxygen-level dependent (BOLD) technique has emerged as the most popular approach for assessing regional brain function noninvasively in human subjects due to its ease of use, comparatively low cost, and wide availability. A major advantage of fMRI over other modalities is that detailed studies of brain structure can be obtained concurrently. Current research MRI protocols typically include high resolution images for gray and white matter volumes obtained with T1-weighted imaging and now studies of white matter tracts obtained with diffusion tensor imaging. These data facilitate both region-of-interest and voxel-wise comparisons between subjects or cohorts and together allow structure-function correlations to be obtained within a single modality. Research MRI protocols may also include screening for structural lesions with T2-weighted MRI, assessments of brain vasculature with magnetic resonance angiography, and screening for hemorrhage and micro-hemorrhage with gradient echo MRI. Studies of brain function and brain-behavior relationships in clinical or preclinical AD are obviously enhanced by knowledge of regional cortical atrophy, ischemic changes, or the presence of other pathologies.
Nearly all imaging studies of brain function rely on the existence of a coupling between regional changes in brain metabolism and regional cerebral blood flow (CBF) [3]. Changes in blood flow and metabolism occur with both excitatory and inhibitory neurotransmission, both of which are energy consuming processes [4]. Regional blood flow changes co-localize with known functional specialization. Early positron emission tomography (PET) studies comparing regional changes in CBF, CMRGlc, and CMRO2 during visual stimulation suggested a close relationship between changes in CBF and CMRGlc [5]. Accordingly, regional CBF imaging results tend to mirror FDG-PET results except when normal vascular coupling is compromised, as in stroke and potentially other disorders.
Current concepts of brain function in cognitive neuroscience have largely focused on characterizing brain-behavior relationships within the context of specific domains such as language or memory and the majority of fMRI studies have accordingly examined task-evoked changes in regional brain function. While these studies have contributed to a better understanding of how specific cognitive functions are subserved in both health and disease, task-correlated fMRI also has several inherent limitations. Firstly, many cognitive tasks must be contrived to allow administration within the confines of an MRI scanner. Secondly, task-correlated fMRI must be interpreted in the context of task performance, which is often impaired in clinical populations. Thirdly, inferences about regional brain function based on task-activation fMRI data are generally limited to the specific cognitive state recruited by the activation paradigm. Finally, inferences about brain-behavior relationships during a cognitive task may be difficult to interpret without explicit knowledge about baseline brain function since variations in intrinsic brain function certainly occur in most brain disorders.
An alternative approach for studying regional alterations in brain function that occur with disorders such as AD and for elucidating brain-behavior relationships in both patients and controls is to correlate neuroimaging findings made at rest or during a fixed task state with clinical or behavioral measures made outside of the MRI scanner. Unlike task-activation studies, this approach relies on individual differences across the study cohort to provide image contrast, and its most effective use requires quantitative neuroimaging measures that can be effectively compared across subjects and scanning sessions.
Arterial spin labeling (ASL) provides a means of quantifying regional CBF directly by MRI. This class of techniques utilizes magnetically labeled arterial blood water as a nominally diffusible tracer for blood flow measurements in a manner analogous to that used for 15O-PET scanning, except that the tracer is endogenous. ASL methods can also be used for imaging task activation, though their use has been less widespread than BOLD methods because to date these techniques have produced a lower signal change for activation and are more difficult to implement.
The first demonstrations that ASL perfusion MRI could quantify regional CBF were obtained in rodents [6,7], but human studies followed closely thereafter [8]. Because arterial transit times in humans are much longer than in rodents, accurate measurement of CBF in humans in ASL requires explicit consideration of both blood and tissue compartments [9]. Several schemes exist for labeling arterial blood water including continuous labeling [7], pseudo continuous labeling [6,10], and pulsed labeling [11,12]. While initial ASL studies were carried out in single slices, whole brain measurements are now routinely performed. The effects of ASL can be sampled using any imaging sequence and, in theory, provides a quantitative perfusion image that is independent of scanning parameters. As such, ASL represents one of the few MRI contrast mechanisms for which the physiological basis is well known and provides a biomarker of brain function that should be portable across scanning platforms or time. Existing data suggest test-retest reliability comparable to other modalities [13–15]. An excellent concordance has been observed between ASL and 15O-PET at rest [16], during functional stimulation [17,18], and in clinical populations [19].
ASL perfusion fMRI is well suited to examining the neural response to pharmacological agents and abstinence states since these are sustained effects lasting hours or longer, and hence benefit from a quantitative functional imaging method that allows comparison of measured values between scanning sessions. Modulation of CBF by vasoactive drugs such as acetazolamide [20], CO2 [21], and caffeine [18] are readily demonstrated. Preliminary studies also suggest that ASL is capable of detecting drug effects in the brain for both parenterally [22,23] and orally administered [24] psychoactive drugs. As such, ASL may provide a sensitive means of detecting the effects of pharmacological treatments for AD or its prevention, and the utility of ASL perfusion MRI in drug development and validation is currently being explored.
TECHNICAL BACKGROUND
Understanding the literature and potential of ASL for AD research and prevention is aided by an understanding of the basic methods used for ASL MRI. Fortunately, an understanding of MRI technology is not particularly essential to a working knowledge of the key issues that affect ASL applications to AD and dementia.
ASL takes advantage of some unique capabilities of MRI. MRI measures signals from the magnetic properties, or magnetization, of hydrogen nuclei, mostly in water. In order to generate the signal, the scanner must first rotate the magnetization from its equilibrium direction along the main magnetic field of the scanner. Fortunately, a combination of time varying magnetic fields can be used to rotate the magnetization from just a localized region of space, such as a slice. ASL takes advantage of this spatially selective rotation to rotate, or “label”, magnetization in a region containing flowing arterial blood. This labeled blood flows into tissue and temporarily changes the magnetization there. In many ways, ASL labeling behaves like an arterial injection of a tracer; magnetic fields replace the syringe and rotated nuclear magnetization of naturally occurring water in the blood replaces any foreign labeling substance.
ASL blood flow sensitive images are created by acquiring two or more images with different labeling [6, 7]. The most common approach is to acquire one “labeled” image with labeling applied to the inflowing arteries some time before the image is acquired and a second “control” image where no labeling is performed. If the labeling timing is performed appropriately, then simple subtraction produces an image that is proportional to blood flow.
Typically, ASL labeling produces a small fractional signal change. This is because only a fraction of the water in tissue is replaced with arterial water in the limited time before the “label” decays away. Labeled magnetization will tend to rotate back to the equilibrium direction with an MRI time constant, known as T1, of approximately 1.5 seconds with the exact time constant determined by hematocrit and field strength [25]. Only about1% of the water can be replaced on this time scale so the ASL labeling induces only a 1% change in a typical MR image. This has two implications. One is that the signal-to-noise ratio of ASL is 100 times lower than for standard anatomic MRI. Recovering sufficient signal-to-noise is usually achieved by reducing spatial resolution approximately four fold in each of the three directions. The second implication is that small motions or instability in either the control or label image can cause errors in the perfusion image. Such motion is a frequent cause of image degradation as techniques are translated from healthy subject testing to application in clinical populations. Fortunately, a technique known as background suppression greatly reduces this source of signal instability [26].
By reducing the MRI signal unrelated to blood flow, background suppression [26] greatly improves the robustness and reliability of ASL [27], especially in less cooperative clinical populations. Background suppression is achieved by applying additional inversions to the imaged region before imaging. If properly timed, the signal from static tissue water can be reduced by up to a factor of 100. Such a reduction means that ASL blood flow signal represents almost 50% of the signal in the label and control images. The reduced signal in the label and control image improves the stability of the difference between the two, creating a much more robust image. Unfortunately, use of background suppression is not yet widespread, so care must be used to exclude motion or other instabilities as possible sources of variance or artifactual signal in many studies.
ASL perfusion images can be corrupted in regions of great interest to dementia research, including the inferior temporal lobes, the orbitofrontal cortex, and even the anterior medial temporal lobes. These regions have less uniform magnetic fields because of proximity to air-tissue or bone-tissue interfaces, and some popular imaging methods for fMRI suffer from signal loss and spatial distortion in such regions. ASL contrast does not depend on using such imaging sequences, however. Spin echo based sequences are particularly insensitive to these non-uniform magnetic fields [28]. Volumetric spin echo based sequences are increasingly being applied to ASL and dementia [29].
Labeling of arterial blood can be performed with a number of different MRI approaches. The diversity of approaches can confuse interested users and may complicate multi-site studies. Labeling by rotating arterial spins in a slab of tissue at one time is known as pulsed ASL. Several popular pulsed ASL strategies are known by their acronyms such as FAIR [12] and EPISTAR [30], and the acronyms may sometimes be used in imaging descriptions without further explanation. Continuously rotating arterial spins as they pass a labeling plane just beneath the imaged region is known as continuous ASL [7,31]. Advocates of continuous ASL, such as the authors, find it produces higher signal and signal-to-noise ratio [32]. Others have argued that details of implementation may reduce the advantages of continuous ASL [33]. Certainly, acceptable image quality and quantification can be achieved with a number of different implementations of continuous and pulsed ASL. Probably more critical to successful studies in dementia is the timing of the labeling.
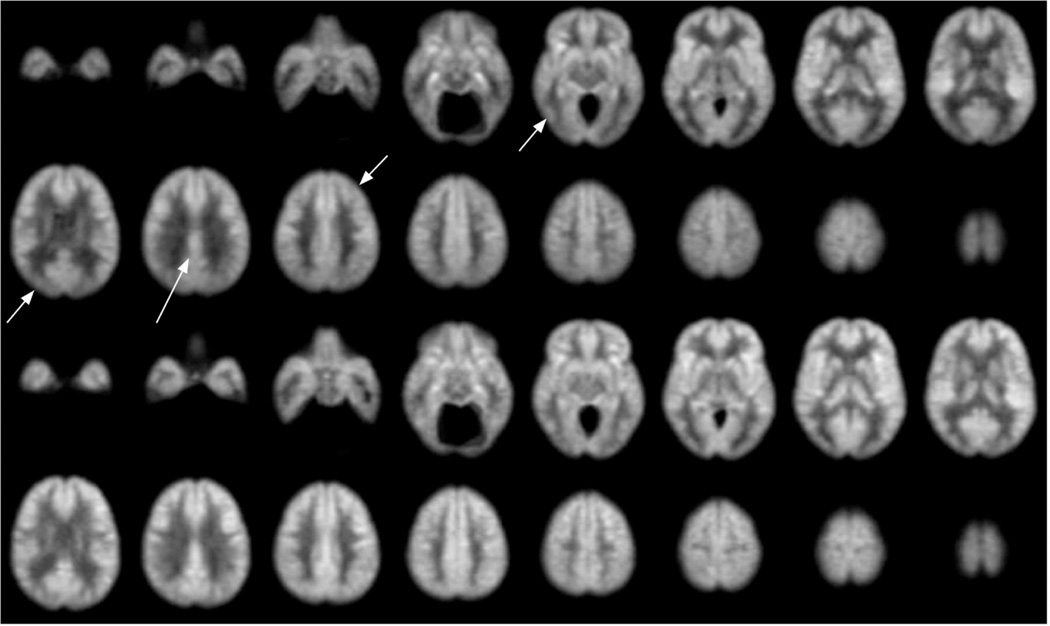
If the timing of labeling is not carefully chosen, then the ASL blood flow measurement may have systematic errors caused by delayed arterial arrival. As mentioned earlier, the label of arterial water decays with a time constant of approximately 1.5. Imaging is often performed as early as possible after labeling to maximize signal change. However, early imaging after labeling enhances vascular structures and will underestimate tissue blood flow in regions with slower vascular supply. Coincidentally, some of the regions with the slowest arrival are those that also show flow decreases in AD, such as parietal and frontal association cortex. Errors from delayed arrival can be largely eliminated by waiting longer after labeling for labeled blood to enter tissue [9]. Some have proposed the use of images acquired at many different delays to quantify both blood flow arterial arrival time [34], but it is not clear such added complication is necessary for quantitative imaging of dementia. If appropriate labeling timing is used, ASL images can be converted to quantitative blood flow maps using a relatively simple model and equations [9, 35,36]. An example of the quality of images that can be obtained in AD is shown in Fig. 1.
Fig. 1.
Average of ASL blood flow images from 24 patients diagnosed with mild to moderate AD, top two rows, compared with images from 17 age-matched controls, bottom two rows. Images were aligned to a standard atlas before averaging. These images, from a dataset analyzed more rigorously elsewhere [29], are shown to illustrate achievable image quality. Example regions with significantly decreased flow are indicated with arrows.
ALZHEIMER’S DISEASE RELATED ASL STUDIES
Since the literature on ASL applied to dementia is not extensive, we summarize each of the reported studies and the key findings.
Sandson et al. [37]
This study used single slice EPISTAR and relative blood flow measures in 11 AD patients and 8 controls. Importantly a Hachinski Ischemia score of less than 4 was required for entry. Regions in temporo-occipital and parieto-occipital association cortex showed reduced CBF relative to whole slice flow. Regions in medial occipital, lateral prefrontal, motor/sensory, and head of the caudate did not show significant difference. Only parieto-occipital perfusion significantly correlated with dementia severity, as measured by a subset of the Blessed Dementia Scale, but not with the Matthis Dementia Rating Scale.
Alsop et al. [38]
This study used a multi-slice technique to cover a larger portion of the brain and a post-labeling delay approach to minimize transit time related errors. Continuous ASL and echoplanar acquisition were used. 18 AD subjects were studied, though 1 was excluded for motion artifacts, and 11 age-matched controls were imaged for comparison. Significant flow decreases were detected in parietal, temporal, occipital, precuneus/posterior cingulate, and prefrontal cortex. Again, correlations with severity, here using the Mini Mental State Exam (MMSE) score, showed only significance with parietal cortex, along with the precuneus/posterior cingulate, a region not covered by the Sandson et al. study. Subjects were required to have Hachinski Ischemia score less than 2. The medial temporal lobes, including the hippocampus, were at the edge of the covered volume in this study.
Johnson et al. 2005 [39]
This paper employed pulsed arterial spin labeling in 20 subjects with AD, 18 diagnosed with MCI, and 23 matched controls. This multi-slice technique provided coverage of the upper half of the brain. This study provided confirmation of the AD associated decreases of ASL blood flow in precuneus/posterior cingulate, parietal association cortex, and lateral prefrontal cortex reported earlier. In addition, a less significant decrease in the parietal cortex was detected in MCI related to controls in the right inferior parietal lobe. Additionally, correction for atrophy was performed in the AD group. Right parietal lobe and bilateral middle frontal gyrus regions remained significantly decreased after correction of atrophy. Though no severity correlation was performed, the comparison of MCI, of which at least half are early stage AD, and AD showed more decreased flow in the bilateral precuneus/posterior cingulate, and the bilateral inferior parietal lobe. The study also explored the relative benefits of using absolute blood flow values or normalization to global blood flow. Generally unnormalized results showed slightly higher significance.
Du et al. 2006. [40]
This study performed ASL in 24 subjects with AD, 21 subjects with frontotemporal dementia (FTD), and 25 control subjects. Though not clearly stated, this study may include data from the subjects in Johnson et al. [39], as the imaging methods are identical and the study is only one year later. Hence any meta-analysis should probably not include the AD data as an independent study. FTD patients with motor neuron symptoms were excluded. Analysis was only performed for FTD relative to AD and FTD relative to cognitively normal, so the independent effect of AD is not determined. Still, significantly lower flow in the precuneus, inferior parietal cortex, and posterior cingulate was detected in the AD relative to the FTD patients. FTD patients showed reduced flow relative to controls in the right superior and middle frontal cortex. Correlations between cognitive impairment and perfusion were minimal in the FTD group.
Xu et al. [41]
This study performed ASL CBF imaging in 12 subjects with amnestic MCI and 14 matched controls. A pulsed multi-slice technique was employed, but multiple volumes were acquired to provide greater coverage than in the study of Johnson and colleagues [39]. In addition to the larger coverage, the study added the use of an activation state where blood flow ASL was also measured during a memory encoding task. Significant differences between MCI and control were only detected in the right precuneus and cuneus in the baseline study. During the task, the significant region expanded to include the posterior cingulate. When comparing task to baseline, a significant increase in flow was observed in the parahippocampal gyrus in the controls but not in the MCI subjects. As commented in an accompanying editorial [42], this study provides the first link to the growing literature on two areas of brain activity modulation: the use of BOLD MRI activation to detect early AD and the growing interest in the hypothesized default network that is found to be more active at rest and seems to be more active in AD. An important conclusion of this work is that all comparisons were more significant during the task than at rest.
Asllani et al. [43]
This study compared ASL blood flow studies in 12 subjects with AD and 20 age-matched controls. A multi-slice continuous ASL method was used. All analysis was performed using absolute, unnormalized, measures of blood flow. The most significant finding in this study was a global decrease in flow (averaged 40%) in AD compared to controls. Consistent with this global decrease in flow, regional analysis found significant decreases in flow essentially in every region. A novel co-variance pattern analysis showed decreases in many areas of interest to AD including the posterior cingulate, the parahippocampal gyrus and the hippocampus.
Alsop et al. [29]
This study employed a more sophisticated imaging method using background suppression and a 3D acquisition to provide imaging of the entire supratentorial brain. 22 patients with AD and 16 control subjects were imaged. In addition to the blood flow images, maps of gray matter density were constructed to perform atrophy correction for neuronal loss or shrinkage. In addition to confirming prior studies, this study provided the first atrophy corrected, whole brain analysis with ASL. Interestingly, after correction for atrophy, a number of areas, including hippocampus, parahippocampal gyrus, temporal pole, superior temporal, and anterior cingulate, saw elevated blood flow. Without atrophy correction, there was a trend toward increased flow in the hippocampus, p < 0.2, the amygdala, p < 0.32, and the parahippocampus, p < 0.24. Only the hippocampal atrophy was significantly correlated with MMSE, though a trend towards decreased tissue volume and flow (p < 0.08) was seen in the precuneus.
Dai et al. [44]
This study performed 2D continuous labeling ASL in 38 control subjects, 29 MCI patients, and 37 AD patients. As with Alsop et al., gray matter segmentation was performed. All analyses were after atrophy correction for gray matter loss. In AD, the key findings were reduced blood flow in the poster cingulate extending into the precuneus, the inferior parietal cortex, the left inferior lateral frontal and orbitofrontal cortex. Elevated flow was only seen in the anterior cingulate. MCI subjects showed decreased atrophy corrected blood flow in the posterior cingulate with extension to the medial precuneus and increased blood flow to the left hippocampus, right amygdala and the basal ganglia on the right side.
Fleisher et al. [45]
This study considered 13 subjects at risk for AD because of a positive family history and at least one copy of the apolipoprotein E ε4 gene and compared them to 10 without such a history. Subjects were between 50–65 with mean age of 58.5. 6 mm slices were acquired with a multi-slice pulsed ASL sequence. The acquired volume was placed parallel to the axis of the hippocampus and included the entirety of the medial temporal lobes. Regional analysis was used to limit the data to a region defined in the hippocampus. No other regions were considered. The results showed elevated blood flow in the hippocampus in the at risk group, approximately 25% higher, at baseline. During task activation with a memory task, the flow activation was a comparable amount lower in the at risk group. Activation as measured with blood oxygenation contrast was also lower in the high risk group. Hence, similar to Xu et al., the activation was reduced. There are several caveats to this study: The low risk group was 50% female but the high risk group was 78% female and no other regions were measured to control for global blood flow effects.
Yoshiura et al. [46]
Nineteen patients with AD and 22 healthy controls were studied with a multi-delay pulsed ASL imaging technique that acquired 7, 6 mm thick slices to create a 54 mm thick slab entirely above the anterior and posterior commissures. In addition to blood flow, arterial transit delay and a measure of arterial blood volume were obtained. Significantly decreased flow was detected in the precuneus and posterior cingulate bilaterally. No other significant regions were found. No significant regions of decreased transit delay or arterial blood volume were found.
Yoshiura et al. [47]
In a related paper with 20 patients with AD and 23 healthy controls (presumably mostly the same as the above paper), the diagnostic accuracy was assessed for detection of AD with posterior cingulate/precuneus blood flow as a marker. Sensitivity and specificity of 91/80 were achieved.
Chiao et al. [48]
This study applied similar methods to Johnson et al. and Du et al. to the subdivision of MCI subjects using ASL. ASL imaging of 12 subjects diagnosed with amnestic MCI, 12 subjects diagnosed with dysexecutive MCI, and 12 elderly control subjects was performed. The upper half of the brain was studied with a multi-slice echoplanar approach. A method to correct for atrophy was used as part of the analysis. Lower posterior cingulate CBF than controls was found in both MCI groups. Dysexecutive MCI had significantly lower flow in the left middle frontal, left posterior cingulate, and left precuneus relative to amnestic MCI. An analysis of sensitivity and specificity for diagnosis of MCI subgroups showed modest values of 60–70% using combinations of regions derived from the data without a validation sample. Significant correlations between regional CBF and two neuropsychological test scores were found.
Raji et al. [49]
This study focused on the qualitative, diagnostic use of ASL to detect patients with mild AD. A whole brain, multi-slice technique very similar to Dai et al. [44] was used. Neuroradiologists were trained in the qualitative analysis of ASL studies. Images were presented using an absolute perfusion color scale. Four readers were evaluated for their diagnostic separation of 13 AD subjects and 19 cognitively normal elderly. AD subjects were better separated from normals by their ASL, average sensitivity/specificity of 85/54%, than by T1 weighted structural scans, average 56/70%.
SUMMARY OF ASL FINDINGS
The most consistent finding across the studies of AD is decreased precuneus and/or posterior cingulate blood flow. Lateral parietal cortex also frequently shows decreases. These changes cannot be explained by simple loss of tissue as they remain strong effects after atrophy correction. Posterior cingulate effects were observed in MCI, so this would appear a promising region for early detection. Posterior cingulate and especially parietal effects appear moderately related to disease severity. These blood flow decreases are broadly consistent with metabolism studies using PET, where far more extensive studies have been performed [50]. Since only a few of the studies observed the temporal lobes, the results are more limited, but signs of decreased flow in inferior-lateral temporal cortex are suggested.
Though medial temporal regions have been less frequently studied with ASL, changes in blood flow appear to be more complex. No study has yet detected a straightforward decrease, as was readily detected in the precuneus and parietal cortex. Several studies in AD [29,44] and one in an at risk population [45] have pointed towards elevated hippocampal blood flow, at least as measured with ASL.
DISCUSSION
ASL remains a promising but unproven technique for early detection and characterization of AD. Of the existing studies, only one studied more than 25 patients and none employed longitudinal designs or pathology to confirm the clinical diagnosis. Hence it is virtually impossible to infer superiority of detection relative to clinical criteria. These studies do provide tantalizing suggestions of the potential of the ASL technique in the study and treatment of AD.
The correspondence between functional deficits detected with ASL MRI and PET techniques in AD suggests that ASL may provide similar detection sensitivity to PET but using an MRI scanner. Since structural imaging, white matter hyperintensity measurement, and potentially diffusion tensor imaging can be powerful tools in the diagnosis and longitudinal monitoring of AD, MRI will remain an important part of the study of AD. Blood flow MRI may help to add to the power of such studies. As evidenced by several of the reviewed studies, employing MRI atrophy measures to highlight functional rather than atrophic changes in the brain is an important approach to the interpretation of functional imaging measures. Since regional atrophy can be readily measured using MRI techniques [51] within the same scanner and without moving the subject, correction of ASL blood flow changes for atrophy are arguably more convenient and accurate than functional studies performed with other modalities.
In the absence of longitudinal studies or pathologic validation, one potentially valuable way to validate the changes observed in the reported ASL studies of AD is to relate increases or decreases with measures of cognitive functioning thought to be associated with these brain regions. Measures such as MMSE are not thought to be specific enough to be informative. However, the precuneus region has been associated with episodic memory, and a regression analysis relating reduced precuneus perfusion to episodic memory difficulty might be useful in demonstrating that precuneus change is not a spurious finding. Likewise, studies relating the hippocampus to episodic memory functioning would be able to establish the validity of observed changes, particularly if co-varied for disease duration. Finally, in cortical association areas where perfusion change is less clear, such as parietal and frontal regions, other cognitive measures that are compromised in AD can be related to these changes like confrontation naming and spatial relations.
ASL findings in the medial temporal lobe and nearby regions remain the most controversial and potentially exciting. Since these hippocampal findings are relatively preliminary and factors such as limited spatial resolution and sensitivity to magnetic field variation could make the variance of ASL higher in the temporal lobes, it would be prudent to await further confirmation before over interpreting hyperperfusion. Unlike the cingulate and association cortex hypoperfusion, the signs of preserved or elevated flow with ASL are not broadly consistent with the FDG-PET literature. Hypometabolism in the medial temporal lobes on PET has been questioned [52,53], but the most recent studies continue to indicate a decrease in hippocampal metabolism in AD, e.g., [54]. One potential reason for disagreement would be decoupling of flow and glucose consumption, as has been reported in medial temporal epilepsy [55]. The similar location and recent interest in the relationship between AD and epilepsy [56] make this possibility more compelling. Certainly the demonstrated relationship between baseline blood flow and fMRI responses in an at risk population [45] suggests baseline ASL should be a component of MRI activation studies in AD. Task-dependent BOLD signal changes observed in preclinical AD may reflect true functional differences or altered sensitivity to activation due to changes in baseline blood flow [42,57], or a combination of these two effects.
The analyses in these studies have emphasized regional differences or across subject differences within individual studies. In principle, a technique capable of quantifying absolute CBF should produce numerical values that can be compared across studies and potentially used to guide diagnostic decisions. In practice, different implementations of ASL and different assumptions or approaches in the image analysis used for quantification contribute to considerable variation in absolute values between different published papers. Identifying errors in different approaches, developing a consensus on methods, and validating the consensus measures are important objectives of current research.
One important role for ASL and MRI may be in exploring the relationship between cerebrovascular disease and AD. Though the reviewed studies show little sign of ischemia, most of the studies included exclusions for cerebrovascular disease. A quantitative marker of blood flow, such as ASL, in a broader population of patients with dementia could address the added risk or impairment that may accompany moderate hypoperfusion [58].
In evaluating the role of ASL in the characterization of early dementia, it is important to appreciate that advancements in ASL, and MRI in general, are still rapidly proceeding. Improvements in sensitivity from higher magnetic field strengths [59,60] and specialized array coils [61] have not been fully appreciated in the reviewed studies. Faster scans and reduced motion sensitivity by using parallel imaging techniques can also improve the sensitivity and resolution of ASL images. Improved analysis methods for atrophy correction, for identifying spatial patterns of diffuse disease [43,62], or potentially using higher resolution structural images to guide high resolution reconstructions of low resolution ASL images [63] may further enhance studies. Finally, using modified ASL approaches to characterize arterial transit times [64], arterial blood volume [46], capillary permeability [65] and oxygenation [66], and vascular reactivity [20] could provide new insights into early AD pathophysiology.
CONCLUSION
ASL Blood Flow MRI is a promising marker of early disease in AD and other dementias. ASL currently lags some other imaging methods in the maturity of the technology and the evaluation in AD. With widespread distribution of the technique in progress, we anticipate these challenges will shortly be overcome and ASL will become an essential tool in AD treatment and prevention research.
Footnotes
DISCLOSURE STATEMENTS
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=333).
REFERENCES
- 1.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR. MRI and CSF biomarkers in normal, MCI, and AD subjects Predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR. MRI and CSF biomarkers in normal, MCI, and AD subjects Diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- 5.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 7.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts DA, Detre JA, Bolinger L, Insko EK, Leigh JS., Jr Quantitative magnetic resonance imaging of human brain perfusion at 1.5 T using steady-state inversion of arterial water. Proc Natl Acad Sci U S A. 1994;91:33–37. doi: 10.1073/pnas.91.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsop DC, Detre JA. Reduced transit-time sensitivity in non-invasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 13.Petersen ET, Mouridsen K, Golay X. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage. 49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahng GH, Song E, Zhu XP, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–916. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 16.Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, Yang Y, Duyn J, Smith AM, Frank JA, Weinberger DR, McLaughlin AC. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44:450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, Zhu F, Tan LH, Fox PT, Gao JH. CBF changes during brain activation: fMRI vs. PET. Neuroimage. 2004;22:443–446. doi: 10.1016/j.neuroimage.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Parrish TB. Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage. 2009;46:577–583. doi: 10.1016/j.neuroimage.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim YM, Cho YW, Shamim S, Solomon J, Birn R, Luh WM, Gaillard WD, Ritzl EK, Theodore WH. Usefulness of pulsed arterial spin labeling MR imaging in mesial temporal lobe epilepsy. Epilepsy Res. 2008;82:183–189. doi: 10.1016/j.eplepsyres.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detre JA, Samuels OB, Alsop DC, Gonzalez-At JB, Kasner SE, Raps EC. Noninvasive magnetic resonance imaging evaluation of cerebral blood flow with acetazolamide challenge in patients with cerebrovascular stenosis. J Magn Reson Imaging. 1999;10:870–875. doi: 10.1002/(sici)1522-2586(199911)10:5<870::aid-jmri36>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Floyd TF, Clark JM, Gelfand R, Detre JA, Ratcliffe S, Guvakov D, Lambertsen CJ, Eckenhoff RG. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol. 2003;95:2453–2461. doi: 10.1152/japplphysiol.00303.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. Remifentanil-induced cerebral blood flow effects in normal humans: dose and ApoE genotype. Anesth Analg. 2007;105:167–175. doi: 10.1213/01.ane.0000266490.64814.ff. [DOI] [PubMed] [Google Scholar]
- 23.Bruns A, Kunnecke B, Risterucci C, Moreau JL, von Kienlin M. Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in rats. Magn Reson Med. 2009;61:1451–1458. doi: 10.1002/mrm.21779. [DOI] [PubMed] [Google Scholar]
- 24.O’Gorman RL, Mehta MA, Asherson P, Zelaya FO, Brookes KJ, Toone BK, Alsop DC, Williams SC. Increased cerebral perfusion in adult attention deficit hyperactivity disorder is normalised by stimulant treatment: a non-invasive MRI pilot study. Neuroimage. 2008;42:36–41. doi: 10.1016/j.neuroimage.2008.04.169. [DOI] [PubMed] [Google Scholar]
- 25.Dobre MC, Ugurbil K, Marjanska M. Determination of blood longitudinal relaxation time (T1) at high magnetic field strengths. Magn Reson Imaging. 2007;25:733–735. doi: 10.1016/j.mri.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Garcia D, Duhamel G, Alsop D. Efficiency of Inversion Pulses for Background Suppressed Arterial Spin Labeling. Magn Reson Med. 2005;54:366–372. doi: 10.1002/mrm.20556. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Li L, Roc AC, Alsop DC, Tang K, Butler NS, Schnall MD, Detre JA. Reduced susceptibility effects in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magn Reson Imaging. 2004;22:1–7. doi: 10.1016/S0730-725X(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 29.Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage. 2008;42:1267–1274. doi: 10.1016/j.neuroimage.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelman RR, Siewert B, Darby DG, Thangaraj V, Nobre AC, Mesulam MM, Warach S. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513–520. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- 31.Dixon WT, Du LN, Faul DD, Gado M, Rossnick S. Projection Angiograms of Blood Labeled by Adiabatic Fast Passage. Magn Reson Med. 1986;3:454–462. doi: 10.1002/mrm.1910030311. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 33.Wong EC, Buxton RB, Frank LR. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn Reson Med. 1998;40:348–355. doi: 10.1002/mrm.1910400303. [DOI] [PubMed] [Google Scholar]
- 34.Günther M, Bock M, Schad L. Arterial spin labeling in combination with a look-locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR) Magn Reson Med. 2001;46:874–884. doi: 10.1002/mrm.1284. [DOI] [PubMed] [Google Scholar]
- 35.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 36.Buxton RB. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging. 2005;22:723–726. doi: 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- 37.Sandson TA, O’Connor M, Sperling RA, Edelman RR, Warach S. Noninvasive perfusion MRI in Alzheimer’s disease: a preliminary report. Neurology. 1996;47:1339–1342. doi: 10.1212/wnl.47.5.1339. [DOI] [PubMed] [Google Scholar]
- 38.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- 39.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin KP, Miller BL, Weiner MW, Schuff N. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–1220. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69:1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- 42.Alsop DC, Press DZ. Activation and baseline changes in functional MRI studies of Alzheimer disease. Neurology. 2007;69:1645–1646. doi: 10.1212/01.wnl.0000265395.87983.66. [DOI] [PubMed] [Google Scholar]
- 43.Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, Stern Y. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. J Cereb Blood Flow Metab. 2008;28:725–736. doi: 10.1038/sj.jcbfm.9600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai WY, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshiura T, Hiwatashi A, Yamashita K, Ohyagi Y, Monji A, Takayama Y, Nagao E, Kamano H, Noguchi T, Honda H. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. Am J Neuroradiol. 2009;30:1388–1393. doi: 10.3174/ajnr.A1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshiura T, Hiwatashi A, Noguchi T, Yamashita K, Ohyagi Y, Monji A, Nagao E, Kamano H, Togao O, Honda H. Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur Radiol. 2009;19:2819–2825. doi: 10.1007/s00330-009-1511-6. [DOI] [PubMed] [Google Scholar]
- 48.Chao LL, Pa J, Duarte A, Schuff N, Weiner MW, Kramer JH, Miller BL, Freeman KM, Johnson JK. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis Assoc Disord. 2009;23:245–252. doi: 10.1097/WAD.0b013e318199ff46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raji CA, Lee C, Lopez OL, Tsay J, Boardman JF, Schwartz ED, Bartynski WS, Hefzy HM, Gach HM, Dai W, Becker JT. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A1955. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80(Spec No 2):S160–S167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 51.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 52.Jagust WJ, Eberling JL, Richardson BC, Reed BR, Baker MG, Nordahl TE, Budinger TF. The cortical topography of temporal-lobe hypometabolism in early Alzheimer’s-disease. Brain Res. 1993;629:189–198. doi: 10.1016/0006-8993(93)91320-r. [DOI] [PubMed] [Google Scholar]
- 53.Ishii K, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, Mori E. Relatively preserved hippocampal glucose metabolism in mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:317–322. doi: 10.1159/000017083. [DOI] [PubMed] [Google Scholar]
- 54.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[F-18]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fink GR, Pawlik G, Stefan H, Pietrzyk U, Wienhard K, Heiss WD. Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesial structures. J Neurol Sci. 1996;137:28–34. doi: 10.1016/0022-510x(95)00323-t. [DOI] [PubMed] [Google Scholar]
- 56.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 58.De La Torre JC. Vascular basis of Alzheimer’s pathogenesis. Ann N Y Acad Sci. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang JJ, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- 60.Gardener AG, Gowland PA, Francis ST. Implementation of quantitative perfusion imaging using pulsed arterial spin labeling at ultra-high field. Magn Reson Med. 2009;61:874–882. doi: 10.1002/mrm.21796. [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, Wang J, Connick TJ, Wetmore GS, Detre JA. Continuous ASL (CASL) perfusion MRI with an array coil and parallel imaging at 3T. Magn Reson Med. 2005;54:732–737. doi: 10.1002/mrm.20574. [DOI] [PubMed] [Google Scholar]
- 62.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kornak J, Young K, Schuff N, Du A, Maudsley AA, Weiner MW. K-Bayes Reconstruction for Perfusion MRI I: Concepts and Application. J Digit Imaging. 2009 doi: 10.1007/s10278-009-9183-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang JJ, Alsop DC, Song HK, Maldjian JA, Tang K, Salvucci AE, Detre JA. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST) Magn Reson Med. 2003;50:599–607. doi: 10.1002/mrm.10559. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Fernandez-Seara MA, Wang S, St Lawrence KS. When perfusion meets diffusion: in vivo measurement of water permeability in human brain. J Cereb Blood Flow Metab. 2007;27:839–849. doi: 10.1038/sj.jcbfm.9600398. [DOI] [PubMed] [Google Scholar]
- 66.St Lawrence KS, Wang J. Effects of the apparent transverse relaxation time on cerebral blood flow measurements obtained by arterial spin labeling. Magn Reson Med. 2005;53:425–433. doi: 10.1002/mrm.20364. [DOI] [PubMed] [Google Scholar]