Abstract
The Wnt/β-catenin signaling pathway controls several biological processes throughout development and adult life. Dysregulation of Wnt/β-catenin signaling underlies a wide range of pathologies in animals and humans, including cancer in different tissues. In this review, we provide an update of the Wnt/β-catenin signaling pathway and the possible roles of the Wnt/β-catenin signaling in the biology of testis, epididymis and prostate. Data from our laboratory suggest the involvement of 17β-estradiol and estrogen receptors (ERs) on the regulation of β-catenin expression in rat Sertoli cells. We also provide emerging evidences of the involvement of Wnt/β-catenin pathway in testis and prostate cancer. Our understanding of the role of Wnt/β-Catenin signaling in male reproductive tissues is still evolving, and several questions are open to be addressed in the future.
Keywords: AR, ER, Sertoli cells, Wnt/β-catenin, cancer, epididymis, prostate, testis
The term Wnt derives from a contraction of the gene name Wingless, first identified in the development of the fruit fly Drosophila, and the proto-oncogene Int-1 (Integration 1) which was first isolated in mammary tumor in the mouse1 (for a review, see ref. 2). Wnt/β-catenin signaling pathway controls a myriad of biological phenomena throughout development and adult life. In addition, aberrant Wnt signaling underlies a wide range of pathologies in animals and human, including testis3,4 and prostate cancer (for a review, see refs. 2 and 5). The cross-regulation of Wnt/β-catenin, kinases, and transcription factors with members of the nuclear receptor family, including androgen receptor (AR) and estrogen receptors (ERs), has been reported (for a review, see refs. 6 and 7).
Sertoli cells play a key role in the control of germ cell development. Androgens are recognized as a major factor to support male germ cell development, and Sertoli cells express AR and are important targets for androgen actions (for a review, see refs. 8 and 9). Recent studies have also shown that 17β-estradiol, the classic ERs, ESR1 (ERα) and ESR2 (ERβ), and the G-protein coupled estrogen receptor (GPER) are involved with proliferation, maintenance of homeostasis and function of Sertoli cells (for a review, see ref. 10), and germ cells from rodents (for a review, see ref. 11).
Post-testicular sperm maturation is regulated in the epididymis. Androgens are responsible for maintaining epididymal structure and functions (for a review, see ref. 12), and the role of estrogens in epididymal function remains not completely understood, but ESR1, ESR2 and GPER are present in the epididymis (for a review, see ref. 13).
Androgens are involved in every aspect of prostate development, growth, and function, not only in early male embryogenesis, but also in the development of prostatic hyperplasia in aging men and dogs (for a review, see ref. 14) and prostate cancer (for a review, see ref. 15). Several studies point out the important role of ERs in the normal prostate growth,16 and development and progression of prostate cancer (for a review, see refs. Seventeen and 18).
This review highlights the possible roles of the Wnt/β-catenin signaling pathway in the physiological aspects of the testis, epididymis and prostate, the cross-regulation between Wnt/β-catenin and AR and ERs signaling, and the involvement of Wnt/β-catenin pathway in the development of testis and prostate cancer.
Wnt/β-catenin Signaling Pathway
Wnt signaling is currently known to include two major pathways: the canonical (or Wnt/β-catenin pathway), which involves the stabilization of β-catenin protein and its nuclear accumulation, and the noncanonical pathway, which does not involve β-catenin stabilization (for a review, see refs. 2 and 5). The Wnt proteins are secreted cysteine-rich proteins with important roles in the developing embryo and tissue homeostasis in the adult. The effects include cell proliferation, cell polarity, cell fate specification, and cell differentiation (for a review, see ref. 19). Dysregulation of Wnt signals can lead to human birth defects, several types of cancer, including prostate cancer, and other diseases (for a review, see refs. 2 and 5). In mammals, the complexity and the specificity in the signaling of Wnt are in part achieved through the 19 members of the Wnt family (for a review, see ref. 20). The β-catenin-independent noncanonical pathways stimulate the planar cell polarity pathway by activating the small GTPases Rho and Rac. These induce cytoskeletal rearrangements that lead to the development of lateral asymmetry in epithelial sheets and other structures. Wnt can also provoke release of calcium from intracellular stores, probably via frizzled G-protein coupled receptors and heterotrimeric G-proteins.21 A less well understood mechanism involves activation of the Ror and Ryk tyrosine kinase receptors, which controls the activities of the c-Jun N-terminal kinase (JNK) and Src family of tyrosine kinases, respectively (for a review, see refs. 20 and 22).
In some circumstances, noncanonical Wnt pathway can inhibit canonical Wnt signaling. For example, competition for Disheveled (Dvl) protein, which is shared by both pathways,21 and upregulation of Siah2 (siah E3 ubiquitin protein ligase 2), induced by Wnt5a, can stimulate β-catenin degradation.23
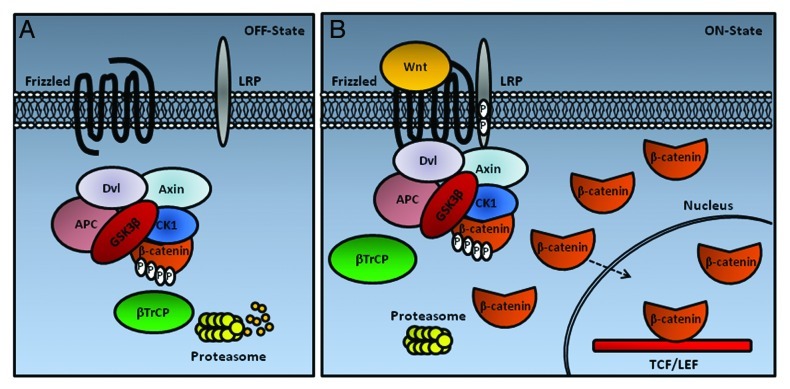
In cells not exposed to Wnt signals (Fig. 1A), phosphorylation and degradation of cytosolic β-catenin is observed by the action of the Axin complex. The scaffolding protein Axin has separate domains that interact with glycogen synthase kinase 3 (GSK3), casein kinase 1 (CK1), and β-catenin, and coordinates sequential phosphorylation of β-catenin at serine 45 by CK1α, and threonine 41, serine 37 and serine 33 by GSK3.24 The phosphorylation of β-catenin at serine 33 and 37 creates a binding site for the β-TrCP, an E3 ubiquitin ligase subunit, which leads to β-catenin ubiquitination and degradation.25 Axin also contains a regulator of G protein signaling domain that interacts with adenomatous polyposis coli tumor suppressor protein (APC). In addition to β-catenin, GSK3 and CK1 phosphorylate Axin and APC, leading to increased association of Axin and APC with β-catenin, and thus enhanced phosphorylation/degradation of β-catenin.24 Degradation of β-catenin prevents β-catenin from reaching the nucleus, and Wnt target genes are thereby repressed by the DNA-bound T cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins. TCF represses gene expression by interacting with the repressor Groucho (TLE1 in human), which promotes histone deacetylation and chromatin compaction (for a review, see refs. 26 and 2).
Figure 1. A new Wnt/β-catenin signaling model based on the study from Li et al.27 (A) In the absence of Wnt protein (Off State), the destruction complex (Axin, GSK3, CK1, APC and Dvl) resides in the cytoplasm, where it binds, phosphorylates, and ubiquitinates β-catenin by β-TrCP. The proteasome recycles the complex by degrading β-catenin. (B) In the presence of Wnt (On State), this protein induces the association of the intact complex with phosphorylated LRP. After binding to LRP, the destruction complex stills captures and phosphorylates β-catenin, but ubiquitination by β-TrCP is blocked. Newly synthesized β-catenin accumulates (Adapted from Clevers and Nusse2).
In a simplified model of the canonical Wnt/β-catenin signaling pathway (Fig. 1B), Wnt proteins bind to Frizzled seven transmembrane receptors (Fz1-Fz10), and these receptors cooperate with low-density lipoprotein receptor-related proteins 5 and 6 (LRP-5 and LRP-6). The signaling by dimeric Wnt receptors includes a ligand-induced conformational change of the receptors followed by phosphorylation of key target proteins. A crucial step in signaling is the binding of Axin to the cytoplasmic tail of LRP6, after phosphorylation by GSK3 and CK1γ.25 The cytoplasmic part of Fz interacts with the cytosolic protein disheveled homolog (Dvl-1-Dvl-3), facilitating interaction between the LRP tail and Axin (for a review, see ref. 2). Recent data show that Wnt-mediated relocation of Axin to LRP leads to inhibition of β-catenin ubiquitination that normally occurs within the complex. The complex becomes saturated by the phosphorylated form of β-catenin. Subsequently, newly synthesized β-catenin accumulates in a free cytosolic form and translocates to the nucleus to activate target genes.27 Stabilized β-catenin associates with TCF/LEF-1 in the nucleus, and, together with co-activators such as B-cell lymphoma 9 protein (Bcl-9), pygopus homologs 1 and 2 and cyclic AMP response element-binding (CREB) protein-binding protein (CPB), activates transcription of genes that contain TCF/LEF-1 binding sites, such as proto-oncogene MYC, matrix metallopeptidase 7 (MMP7) and vascular endothelial growth factor (VEGF) (for a review, see ref. 2).
In addition to its function in the Wnt signaling pathway, β-catenin also binds tightly to the cytoplasmic domain of type I cadherins, and plays an essential role in the structural organization and function of cadherins, by linking cadherins to the actin cytoskeleton through α-catenin. Another catenin, p120, binds to the membrane proximal domain of cadherin, and regulates the structural integrity and function of the cadherin complex28 (for a review, see ref. 29). Although the function of β-catenin in Wnt signaling involves a dynamic cytoplasmic pool of the protein that is responsive to Wnt signals, its adhesion function is mediated by a relatively stable pool at cell membrane. However, disruption of cadherin-mediated cell adhesion can lead to β-catenin release and activation of the Wnt signaling (for a review, see ref. 30). Furthermore, transcription factors such as Twist-related proteins 1 and 2 and zinc finger proteins SNAI1, which inhibit E-cadherin gene expression, are target genes of Wnt/β-catenin (for a review, see refs. 29 and 31).
Wnt antagonists and agonists
Wnt/β-catenin signaling is regulated at many levels. The secreted Frizzled-related proteins (Sfrps) and Wnt inhibitory protein (WIF) bind to Wnts and inhibit the interaction between Wnt and Wnt receptors.32 Other Wnt inhibitors include proteins of the Dickkopf (DKK)33 and the WISE/SOST families, which antagonize signaling by binding LRP5/6.34,35 Two types of proteins, Norrin and R-spondins, are unrelated to Wnts but act through the Fz/LRP complex as Wnt agonists (for a review, see refs. 26 and 2).
Cross-regulation between Wnt/β-catenin and Androgen Receptor (AR) and Estrogen Receptors (ERs) Signaling Pathways
Wnt and AR signaling pathways
A crosstalk between Wnt and AR signaling pathways seems to be involved in the development of normal prostate, and in development, growth and progression of prostate cancer (for a review, see refs. 6 and 36). β-catenin regulates AR function or vice versa, and these mechanisms occur in several levels: (a) β-catenin binds directly to the complex ligand-AR as a co-activator of AR transcription; (b) GSK-3β phosphorylates AR and modulates its ability to activate transcription; (c) the transcription of AR gene is upregulated by TCF transcription factors in a process that is activated through the canonical Wnt signaling pathway; (d) the expression of AR protein is downregulated through a serine/threonine protein kinase (Akt)- and p53 E3 ubiquitin protein ligase homolog (mouse) (MDM2)-mediated degradation process that is activated by Wnt signaling; (e) The AR competes with TCF/LEF for β-catenin (for a review, see refs. 6, 36 and 5). Furthermore, AR plays a role in the regulation of Wnt expression, for example Wnt11.37 The interactions between Wnt/β-catenin signaling and the AR are complex and highly dependent on the cellular context.
Wnt and ER interaction
During the early development of the rat prostate the neonatal treatment with estrogen upregulates Wnt5a protein.38 Nevertheless this estrogen action may be indirect because estrogen response elements are not detected in the promoter region of the Wnt5a gene.39
Dehydroepiandrosterone (DHEA), which can be metabolized to androgens and estrogens in humans, induces β-Catenin/T-cell factor signaling (β-CTS) in DU145 cells via increasing association of ESR2 with Dvl2, mediated by Gαq-subunits. In PC-3 cells DHEA does not induce an effect because these cells have low expression of Gαq. However, overexpression of Gαq in PC-3 cells increases the associations of Gαq/Dvl2 and ESR2/Dvl2, β-CTS, and c-Myc and Cyclin D1 protein expression.40
The collaboration between Wnt/β-Catenin signaling and estrogen receptors in prostate is emerging and its possible significance to prostate cancer remains to be elucidated.
Wnt/β-catenin Signaling in Testis
The expression of several Wnts, including Wnt1,41 Wnt3,42 Wnt4,43 Wnt5a,44 and Wnt7a,45 has been reported in the developing testis or in the testis of adult rodents and human. Several other components of the canonical Wnt signaling pathway, such as Fz9,46 β-catenin, and Nkd1, an antagonist of this signaling pathway,47 have also been detected in the testis.
β-catenin is highly expressed in fetal Sertoli cells and germ cells of mice. It has been shown that perturbation of β-catenin signaling in embryonic Sertoli cells results in testicular degeneration, testicular cord disruption, and Mullerian duct regression.48,49 Similarly, aberrant activation of β-catenin leads to impaired development of primordial germ cells.50
The role of Wnt/β-catenin signaling in the postnatal testis has not been so well studied, but it has been suggested that it affects normal spermatogenesis. The expression of β-catenin persists in Sertoli and germ cells in the testis of the adult rodents.51,52 β-catenin is found in the ectoplasmic specialization (ES), a testis-specific adherens junction formed between Sertoli cells at the basal compartment (basal ES), site of the blood-testis barrier, as well as between Sertoli and germ cells at the adluminal compartment (apical ES) of the seminiferous epithelium (for a review, see ref. 53).
Spermatid-specific deletion of β-catenin in mice results in an increase of germ cell apoptosis, acrosomal defects, abnormal chromatin compaction, and loss of Sertoli cell-germ cell adhesion at the apical ectoplasmic specialization, leading to impaired fertility.54 These defects are likely due to alteration of several genes involved in Sertoli cell-germ cell adhesion and germ cell differentiation.43 Wnt3a, which activates β-catenin signaling, stimulates proliferation of a spermatogonial cell line in vitro.55 Wnt1, a potent activator of Wnt/β-catenin pathway, is secreted by spermatids,56 which represent 70% of the cells in the seminiferous epithelium. Wnt5a has been shown to promote spermatogonial stem cells activity through β-catenin-independent mechanisms. This effect was abolished by inhibiting the JNK pathway.44
The constitutive activation of Wnt/β-catenin in mice Sertoli cells induces testicular atrophy associated with degeneration of the seminiferous epithelium, starting by 5 weeks of age and resulting in a complete loss of germ cells before 4 mo.57 Furthermore, this constitutive activation maintains Sertoli cells in postnatal testis in an immature state, with overexpression of glial cell-derived neurotrophic factor (GDNF), leading to disruption of the germ cell microenvironment and, subsequently, infertility.52 A recent study has also shown that mutant mice with overexpression and sustained activation of β-catenin in Sertoli cells have reduced spermatogonial stem cell activity. Sertoli cells from these animals present a granulosa cell phenotype and start to express markers of female sex differentiation, including Wnt4. This same study also showed that in vitro treatment of cells with recombinant Wnt4 reduces spermatogonial stem cell activity, which would suggest that Wnt4 secreted by Sertoli cells is the downstream factor responsible for germ cell loss.58 Taken together, these results suggest that Wnt4 and GDNF are involved in germ cells apoptosis. However, since gene expression of Wnt4 is undetectable in normal testis58 and mutant male mice for Wnt4 are fertile and have normal spermatogenesis, the Wnt4/β-catenin pathway is probably not a physiological regulator of spermatogenesis.58
In the Nkd1−/− mice, in which the Nkd1 protein lacks the EF-hand motif essential for inhibition of the canonical Wnt/β-catenin pathway, the testis has lower numbers of haploid spermatids.47 Thus, the role of each Wnt and their mechanisms involved in opposite biological effects in germ cells must be elucidated.
Wnt/β-catenin signaling also plays a role in Sertoli cells. The activation of Wnt/β-catenin signaling in cultured adult human Sertoli cells by GSK-3β inhibitors, SB216763 and lithium chloride, induces an increase in c-Myc expression and cell proliferation.59 Mutant mice that express constitutively active form of β-catenin specifically in Sertoli cells develop testicular Sertoli cell tumor at 8 mo of age.3 Furthermore, concomitant dysregulation of Wnt/β-catenin signaling and phosphatidylinositol 3-kinase (PI3K)/Akt pathway in Sertoli cells also lead to testicular tumors with histological and ultrastructural characteristics of granulosa cell tumors.4 It is important to emphasize that Akt can activate Wnt/β-catenin signaling by inactivating GSK3β and thereby cause the hypophosphorylation, stabilization and accumulation of β-catenin, which translocates to the nucleus and modulates the transcriptional activity of specific target genes, including Cyclin D2.60
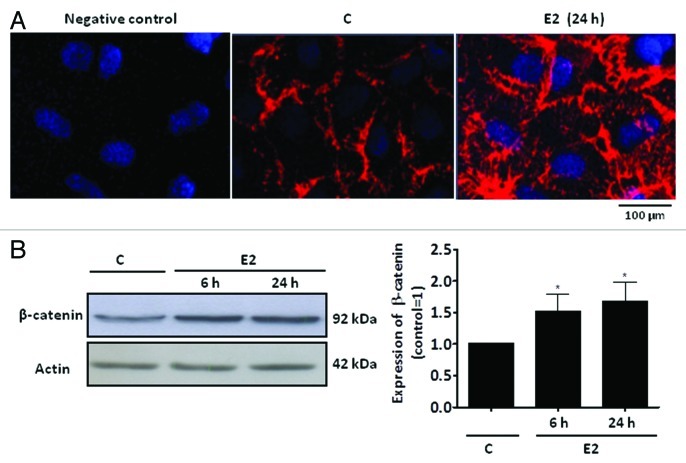
Sertoli cells are constantly exposed to the modulators of Wnt/β-catenin signaling, such as androgen (for review, see refs. 8 and 9) and estrogen (for review see ref. 10). Furthermore, 17β-estradiol-ESR1, through activation of epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase 3/1 (MAPK3/1) and PIK3/Akt pathways, upregulates Cyclin D1.61 Akt can promote Wnt/β-catenin signaling by inactivating GSK3β in different cells.60 We now report that 17β-estradiol induces an increase in β-catenin expression in Sertoli cells from 20-d-old rats. Intense β-catenin immunostaining is found near the plasma membrane and in the cytoplasm of Sertoli cells (Fig. 2A). Western Blot assays confirmed this result (Fig. 2B). In Sertoli cells, the regulation of β-catenin by 17β-estradiol may play a role in Sertoli cell-germ cell adhesion at the apical ectoplasmic specialization and/or in the stabilization and accumulation of β-catenin in the cytosol, which translocates to the nucleus and modulates the transcriptional activity of specific target genes. Considering the fundamental role of Sertoli cell for spermatogenesis, it is now important to elucidate the interplay of the several signaling networks activated by steroid hormones and paracrine factors in these cells, and components of the Wnt-β catenin pathway certainly play an important role mediating the interaction between somatic and germ cells.
Figure 2. Expression of β-catenin in a primary culture of Sertoli cells from 20-d-old rats. (A) Detection of β-catenin in Sertoli cells by immunofluorescence. Specific immunostaining for β-catenin using rabbit polyclonal antibody generated against the amino acid sequence 680–781 from the C-terminal of human β-catenin (red) under basal conditions (C, control) and after incubation with 17β-estradiol (E2, 0.1 nM) for 24 h. Negative control was performed using normal rabbit serum at the same dilution of the antibody. Nuclei were stained with 4’, 6-diamidino-2-phenylindole (blue). Bar = 100 µm. The data shown are representative of three independent experiments. (B) Detection of β-catenin in Sertoli cells by western blot. Cells were incubated in the absence (C, control) and presence of E2 (0.1 nM) for 6 and 24 h. Total cell lysates (40 µg protein/lane) were resolved on 7.5% SDS-PAGE. Immunoblotting using the anti-β-catenin antibody revealed specific bands (top panel) or with antibody that recognizes actin (bottom panel). The data shown are representative of six independent experiments. Bars represent the densitometric analysis of the western blot assays. Results were normalized to actin expression in each sample and plotted (mean ± SEM) in relation to control (C = 1). * β-catenin expression significantly different from control (p < 0.05, Student t-test).
Wnt/β-catenin Signaling in Epididymis
In the epididymis of the adult rat, immunostaining for three catenins (α, β and p120) has been detected along the lateral plasma membrane between adjacent epithelial cells, suggesting their role as part of the adhering junctions.62 Although β-catenin is present in initial segment, caput, corpus and cauda of the epididymis, the highest expression is observed in corpus and cauda regions. Orchidectomy of adult rats decreases β-catenin expression in the lateral plasma membrane, with a concomitant increase in cytoplasmic expression in each epididymal region. Testosterone replacement blocks the effect of orchidectomy, suggesting that expression and localization of the β-catenin are regulated by androgens. Modulation of β-catenin expression is confirmed during postnatal development, and the maximal expression of β-catenin at the lateral plasma membrane occurs in 42-d-old rats,62 when the androgen levels increase. Furthermore, all three catenins interact with E-cadherin and form part of the adhering junctions in the epididymis.62
Although the epididymis is an androgen-dependent tissue,63 testosterone replacement following rete testis ligation or castration restores epithelial morphology in most, but not in all epididymal regions.64 Furthermore, differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation.65 Thus, other factors such as estrogens have been suspected to play a role in epididymal function.66,67 Recent studies have shown that estrogen receptors ESR1 and ESR2 are present in all regions of the rat epididymis.13 Treatment with the estrogen receptor antagonist ICI 182,780 (fulvestrant) induces downregulation of Wnt4 expression in the cauda of the epididymis from bonnet monkey, and reduces Wnt4 mRNA levels in the caput of the epididymis from rats, indicating that estrogen may regulate this protein.68
The formation of the blood-epididymal barrier involves both adhering junctions, which are necessary for cell adhesion and intercellular signaling, and tight junctions, which form the seal between adjacent epithelial cells. This barrier creates a microenvironment essential for sperm maturation and for protection of the sperm from the immune system (for a review, see ref. 69). Therefore, there is a need to better understand the expression and regulation of several components of these barriers, including the β-catenin signaling. Research is also being conducted in our laboratory to further understand the cross-regulation between Wnt/β-catenin and ER signaling pathways.
Wnt/β-catenin Signaling in Prostate
Wnt and prostate development
Prostate gland development is an androgen-dependent process regulated through AR in mesenchymal cells (for a review, see refs. 70 and 71). Other steroid receptors, including ERs and retinoid receptors (RARs and RXRs), are expressed in a cell specific manner during early development, and contribute to prostate morphogenesis and differentiation.72,73 Wnt signaling may also be involved in these processes, regulating prostatic epithelial branching morphogenesis, luminal epithelial cell differentiation and proliferation of prostate epithelial progenitor cells (for a review, see ref. 22).
Several Wnts, Fz and Dvl are expressed in the developing rat ventral prostate. The Wnt signaling components, which include two canonical Wnts (Wnt2, Wnt2b), three non-canonical Wnts (Wnt4, Wnt5a and Wnt11), Fz2 and 4 and Dvl, are highly expressed during early development, and expression declines during and after the completion of morphogenesis. Only Wnt7b presents opposite profile, with low levels at birth and an increase of expression upon functional cytodifferentiation.71
The Sfrp1 acts by binding Wnt ligands and/or Frizzled receptors to modulate signaling. Sfrp1 is relatively high during prostatic development and low in the adult prostate from mice.74 Prostate of Sfrp1 null mutant mice exhibits reduced branching morphogenesis, delayed proliferation and an increase in the expression of genes encoding prostate-specific secretory proteins, while overexpression of Sfrp1 in the adult prostates of transgenic mice yields opposite effects, including prolonged epithelial proliferation and decreased expression of genes encoding secretory proteins. Furthermore, Sfrp1 acts through the non-canonical Wnt/JNK pathway in the prostate.75
Organ cultures of ventral prostates from 2-d-old rat treated with Wnt3a, a canonical Wnt, present enlarged ductal tips and reduced number of tertiary ducts at 7th day. On the other hand, prostates treated with the Wnt signaling inhibitor DKK1 show a decrease in size, fewer epithelial branches and lack of enlarged ductal tips. Wnt3a treatment enhances cell proliferation and reduces luminal epithelial cell differentiation, whereas DKK1 treatment reduces cell proliferation and enhances cell differentiation.76 Furthermore, immunohistochemical analysis of rat prostate organ cultures using basal (p63) and luminal (CK8) cell markers, show that modulation of Wnt signaling can influence differentiation of progenitor cells into luminal cells, suggesting that Wnt signaling regulates the terminal differentiation of basal cells into luminal cells by controlling the proliferation and/or maintenance of epithelial progenitor cells.76
The noncanonical Wnt5a is essential for normal prostate development. This protein is involved in initial bud positioning, regulation of ductal outgrowth along the proximal-distal axis, branchpoint formation, luminal cell polarity and lumen formation within the prostatic ducts. Wnt5a may interact with other morphoregulatory genes to control branching morphogenesis and glandular maturation.38 In rat prostate during the early development, the expression of Wnt5a is downregulated by testosterone and neonatal estrogen treatment upregulates this protein.38
The role of Wnt/β-catenin signaling in the normal adult prostate is poorly known. The knowledge about Wnt signaling involvement in the regulatory mechanisms controlling prostate gland development and normal adult prostate homeostasis is important to understand the genesis of abnormal growth processes associated with aging and cancer.
Wnt and prostate cancer
Aberrant expression and localization of β-catenin in prostate cancer are more common than predicted by Wnt pathway mutation (for a review, see refs. 22 and 5). In prostate cancer, mutation in exon 3 of β-catenin gene is reported in 5% of tumors and it is thought to prevent degradation of β-catenin.77 Abnormal β-catenin expression was found in 23% of tumor samples from radical prostatectomy, and in 38% of castration resistant prostate cancer (CRPC) samples, and correlates with high Gleason score.78 The localization and the level of β-catenin in the nuclei of prostate cancer cells and its clinical relevance are inconsistent in different studies (for a review, see ref. 5). However, the detection of nuclear β-catenin in hyperplasia and in advanced tumors suggests that activation of Wnt/β-catenin signaling has a role in the premalignant stages of the disease and in the progression to CRPC.
Alteration in the expression of Wnt proteins and endogenous Wnt/β-catenin signaling may occur. Several Wnt proteins, such as Wnt1, Wnt5a, Wnt7b and Wnt11, are upregulated in prostate cancer cell lines, relative to benign prostate epithelial cells37,79 (for a review, see ref. 5). High levels of Wnt1 and β-catenin are detected in patients with late-stage tumors and in human prostate carcinoma cell lines (DU-145, LNCaP and PC-3), whereas low levels of Wnt1 and β-catenin are present in normal human prostate cells (PrEC). The high levels of Wnt1 and β-catenin expression were associated with advanced, metastatic, and hormone-refractory prostate carcinoma.80
There are conflicting data in the literature between studies conducted with human tissues and human cell lines.81,82 In 503 patients with localized prostate cancer, significantly higher Wnt5a expression was detected in tumor compared with benign cores from the same patients, and predicts a favorable outcome after surgery.81 Furthermore, treatment with recombinant Wnt5a (rWnt5a) decreases the invasive behavior of the prostate cancer cell lines 22Rv1 and DU145. Neither the LNCaP nor the PC3 cells respond to rWnt5a with a change in their invasive behavior. However, when Wnt5a expression in LNCaP cells was knockdown using siRNAs their invasiveness was significantly increased.81 In contrast, association between high levels of Wnt5a and prostate cancer relapse after prostatectomy has been shown. Knockdown of Wnt5a in human prostate cancer cell lines reduces their invasiveness, whereas overexpression stimulates their invasion activities.82
The increase of Wnt11 level in prostate cancer has been demonstrated to contribute to tumor progression by promoting neuroendocrine-like differentiation, tumor cell survival and cell migration/invasion.83 Moreover, an autocrine regulatory loop involving transcriptional upregulation of Wnt11 by the estrogen-related receptor α (ERRα) and β-catenin seems to influence the migratory capacity of prostate cancer cells.84
In summary, although the expression of Wnt proteins is increased in prostate cancer, their relevance to the activation of Wnt/β-catenin signaling is not clear.
Concluding Remarks and Future Perspectives
Our understanding on the role of Wnt/β-Catenin signaling in male reproductive tissues is still evolving. Table 1 summarizes the possible roles of the Wnt/β-Catenin signaling in testis epididymis and prostate, and several of these possible functions result from interactions with steroid hormone receptors signaling. Although the androgens are clearly involved in the normal development and function of Sertoli and germ cells, epididymis and prostate, and also in the progression of prostate cancer, the estrogens have been recently pointed out as potential agents in the development and function of these tissues. The interaction of AR, ERs and Wnt/β-Catenin signaling pathways is probably complex and multifactorial, incorporating more than one of the mechanisms already described. Elucidation of these important interactions may help clarify mechanisms that lead to male infertility and/or to cancer.
Table 1. Possible roles of Wnt-β catenin signaling in the male reproductive system.
| Tissue | Possible roles | Refs. |
|---|---|---|
|
Testis |
Fetal: - Differentiation of Mullerian duct, organization of testicular cords, development of primordial germ cells Postnatal: - Blood testis barrier (basal ES) and adherens junctions between Sertoli and germ cells (apical ES) - Germ cell differentiation - Proliferation of spermatogonia - Germ cell apoptosis - Sertoli cell proliferation, testicular tumors |
48–50 53 54 44, 55 57,58 3,4,59 |
|
Epididymis |
- Adherens juctions and formation of blood epididymal barrier |
62 |
| Prostate | - Epithelial branching morphogenesis - Luminal epithelial cell differentiation - Proliferation and epithelial progenitor cells - Cancer |
22,75,38 22,76,38 22,76 5,22,77–84 |
Acknowledgments
Research in the authors’ laboratory is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant numbers 2008/56564–1 and 2010/52306–8 to C.S.P.). Research fellowship (C.S.P. and M.F.M.L.) and doctoral fellowship (C.R.) were supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, CNPq. Postdoctoral fellowship (T.F.G.L.) and Master fellowship (R.P., F.N.C.) were supported by FAPESP. Master fellowship (A.P.G.L.) was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES. We thank Espedita M.J. Silva Santos for technical assistance.
Disclosure of Potential Conflicts of Interest
The authors have nothing to declare.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/23181
References
- 1.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Chang H, Guillou F, Taketo MM, Behringer RR. Overactive beta-catenin signaling causes testicular sertoli cell tumor development in the mouse. Biol Reprod. 2009;81:842–9. doi: 10.1095/biolreprod.109.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer A, Paquet M, Laguë MN, Hermo L, Boerboom D. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumor of the testis. Carcinogenesis. 2009;30:869–78. doi: 10.1093/carcin/bgp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kypta RM, Waxman J. Wnt/β-catenin signaling in prostate cancer. Nat Rev Urol. 2012;9:418–28. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 6.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 7.Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res. 2010;316:1763–72. doi: 10.1016/j.yexcr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. Androgens and spermatogenesis: lessons from transgenic mouse models. Philos Trans R Soc Lond B Biol Sci. 2010;365:1537–56. doi: 10.1098/rstb.2009.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–20. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas TF, Pimenta MT, Pisolato R, Lazari MF, Porto CS. 17β-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis. 2011;1:318–24. doi: 10.4161/spmg.1.4.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreau S, Bouraima-Lelong H, Delalande C. Estrogens in male germ cells. Spermatogenesis. 2011;1:90–4. doi: 10.4161/spmg.1.2.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robaire B, Hamzeh M. Androgen action in the epididymis. J Androl. 2011;32:592–9. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- 13.Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl. 2011;32:600–13. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JD. The critical role of androgens in prostate development. Endocrinol Metab Clin North Am. 2011;40:577–90, ix. doi: 10.1016/j.ecl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–7. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–44. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6:437–51. doi: 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman J, Ström A, Gustafsson JA. Current concepts and significance of estrogen receptor β in prostate cancer. Steroids. 2012;77:1262–6. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 20.Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr Pharm Biotechnol. 2011;12:160–70. doi: 10.2174/138920111794295710. [DOI] [PubMed] [Google Scholar]
- 21.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 22.Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J. Wnt signaling in prostate development and carcinogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:11–8. doi: 10.5507/bp.2011.016. [DOI] [PubMed] [Google Scholar]
- 23.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–91. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 25.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–56. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin N, Vincan E. The Wnt signaling pathways and cell adhesion. Front Biosci. 2012;17:784–804. doi: 10.2741/3957. [DOI] [PubMed] [Google Scholar]
- 31.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 33.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 34.Ellwanger K, Saito H, Clément-Lacroix P, Maltry N, Niedermeyer J, Lee WK, et al. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28:4875–82. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semënov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem. 2008;283:21427–32. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99:402–10. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Mazor M, Kawano Y, Walker MM, Leung HY, Armstrong K, et al. Analysis of Wnt gene expression in prostate cancer: mutual inhibition by WNT11 and the androgen receptor. Cancer Res. 2004;64:7918–26. doi: 10.1158/0008-5472.CAN-04-2704. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo D, Yamaguchi T, et al. The role of Wnt5a in prostate gland development. Dev Biol. 2009;328:188–99. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, et al. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–27. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Arnold JT, Blackman MR. Dehydroepiandrosterone administration or Galphaq overexpression induces beta-catenin/T-Cell factor signaling and growth via increasing association of estrogen receptor-beta/Dishevelled2 in androgen-independent prostate cancer cells. Endocrinology. 2010;151:1428–40. doi: 10.1210/en.2009-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson RP, Lai LW, Grimes J. Creating a conditional mutation of Wnt-1 by antisense transgenesis provides evidence that Wnt-1 is not essential for spermatogenesis. Dev Genet. 1993;14:274–81. doi: 10.1002/dvg.1020140405. [DOI] [PubMed] [Google Scholar]
- 42.Katoh M. Molecular cloning and characterization of human WNT3. Int J Oncol. 2001;19:977–82. doi: 10.3892/ijo.19.5.977. [DOI] [PubMed] [Google Scholar]
- 43.Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–40. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 44.Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124:2357–66. doi: 10.1242/jcs.080903. [DOI] [PubMed] [Google Scholar]
- 45.Ikegawa S, Kumano Y, Okui K, Fujiwara T, Takahashi E, Nakamura Y. Isolation, characterization and chromosomal assignment of the human WNT7A gene. Cytogenet Cell Genet. 1996;74:149–52. doi: 10.1159/000134404. [DOI] [PubMed] [Google Scholar]
- 46.Wang YK, Spörle R, Paperna T, Schughart K, Francke U. Characterization and expression pattern of the frizzled gene Fzd9, the mouse homolog of FZD9 which is deleted in Williams-Beuren syndrome. Genomics. 1999;57:235–48. doi: 10.1006/geno.1999.5773. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Ishikawa TO, Miyoshi H, Oshima M, Taketo MM. A targeted mutation of Nkd1 impairs mouse spermatogenesis. J Biol Chem. 2005;280:2831–9. doi: 10.1074/jbc.M405680200. [DOI] [PubMed] [Google Scholar]
- 48.Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–85. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, et al. β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development. 2011;138:1967–75. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Nakamura T, Murayama K, Umehara H, Yamano N, Watanabe S, et al. The stabilization of beta-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev Biol. 2006;300:545–53. doi: 10.1016/j.ydbio.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 51.Lee NP, Mruk D, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 52.Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–32. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang YF, Lee-Chang JS, Harris KY, Sinha-Hikim AP, Rao MK. Role of β-catenin in post-meiotic male germ cell differentiation. PLoS One. 2011;6:e28039. doi: 10.1371/journal.pone.0028039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golestaneh N, Beauchamp E, Fallen S, Kokkinaki M, Uren A, Dym M. Wnt signaling promotes proliferation and stemness regulation of spermatogonial stem/progenitor cells. Reproduction. 2009;138:151–62. doi: 10.1530/REP-08-0510. [DOI] [PubMed] [Google Scholar]
- 56.Shackleford GM, Varmus HE. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987;50:89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- 57.Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D. Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biol Reprod. 2008;79:475–85. doi: 10.1095/biolreprod.108.068627. [DOI] [PubMed] [Google Scholar]
- 58.Boyer A, Yeh JR, Zhang X, Paquet M, Gaudin A, Nagano MC, et al. CTNNB1 signaling in sertoli cells downregulates spermatogonial stem cell activity via WNT4. PLoS One. 2012;7:e29764. doi: 10.1371/journal.pone.0029764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Gao Q, Yin G, Ding X, Hao J. WNT/β-catenin-signaling pathway stimulates the proliferation of cultured adult human Sertoli cells via upregulation of C-myc expression. Reprod Sci. 2012;19:1232–40. doi: 10.1177/1933719112447126. [DOI] [PubMed] [Google Scholar]
- 60.Huang W, Chang HY, Fei T, Wu H, Chen YG. GSK3 beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene. 2007;26:2471–82. doi: 10.1038/sj.onc.1210033. [DOI] [PubMed] [Google Scholar]
- 61.Royer C, Lucas TF, Lazari MF, Porto CS. 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod. 2012;86:108. doi: 10.1095/biolreprod.111.096891. [DOI] [PubMed] [Google Scholar]
- 62.DeBellefeuille S, Hermo L, Gregory M, Dufresne J, Cyr DG. Catenins in the rat epididymis: their expression and regulation in adulthood and during postnatal development. Endocrinology. 2003;144:5040–9. doi: 10.1210/en.2002-0139. [DOI] [PubMed] [Google Scholar]
- 63.Robaire B, Seenundun S, Hamzeh M, Lamour SA. Androgenic regulation of novel genes in the epididymis. Asian J Androl. 2007;9:545–53. doi: 10.1111/j.1745-7262.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 64.Ezer N, Robaire B. Gene expression is differentially regulated in the epididymis after orchidectomy. Endocrinology. 2003;144:975–88. doi: 10.1210/en.2002-220705. [DOI] [PubMed] [Google Scholar]
- 65.Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation. Biol Reprod. 2007;77:165–71. doi: 10.1095/biolreprod.106.059493. [DOI] [PubMed] [Google Scholar]
- 66.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snyder EM, Small CL, Li Y, Griswold MD. Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod. 2009;81:707–16. doi: 10.1095/biolreprod.109.079053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deshpande SN, Vijayakumar G, Rao AJ. Oestrogenic regulation and differential expression of WNT4 in the bonnet monkey and rodent epididymis. Reprod Biomed Online. 2009;18:555–61. doi: 10.1016/S1472-6483(10)60134-4. [DOI] [PubMed] [Google Scholar]
- 69.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221–36. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–59. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–9. doi: 10.1210/en.138.5.1801. [DOI] [PubMed] [Google Scholar]
- 73.Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143:3628–40. doi: 10.1210/en.2002-220184. [DOI] [PubMed] [Google Scholar]
- 74.Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, et al. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65:10423–30. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- 75.Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, et al. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev Biol. 2008;317:161–73. doi: 10.1016/j.ydbio.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang BE, Wang XD, Ernst JA, Polakis P, Gao WQ. Regulation of epithelial branching morphogenesis and cancer cell growth of the prostate by Wnt signaling. PLoS One. 2008;3:e2186. doi: 10.1371/journal.pone.0002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45:323–34. doi: 10.1002/1097-0045(20001201)45:4<323::AID-PROS7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 78.de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9:1801–7. [PubMed] [Google Scholar]
- 79.Thiele S, Rauner M, Goettsch C, Rachner TD, Benad P, Fuessel S, et al. Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J Cell Biochem. 2011;112:1593–600. doi: 10.1002/jcb.23070. [DOI] [PubMed] [Google Scholar]
- 80.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 81.Syed Khaja AS, Helczynski L, Edsjö A, Ehrnström R, Lindgren A, Ulmert D, et al. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One. 2011;6:e26539. doi: 10.1371/journal.pone.0026539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, et al. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–46. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 83.Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, et al. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer. 2010;9:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dwyer MA, Joseph JD, Wade HE, Eaton ML, Kunder RS, Kazmin D, et al. WNT11 expression is induced by estrogen-related receptor alpha and beta-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010;70:9298–308. doi: 10.1158/0008-5472.CAN-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]