Abstract
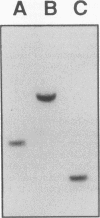
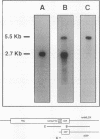
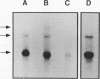
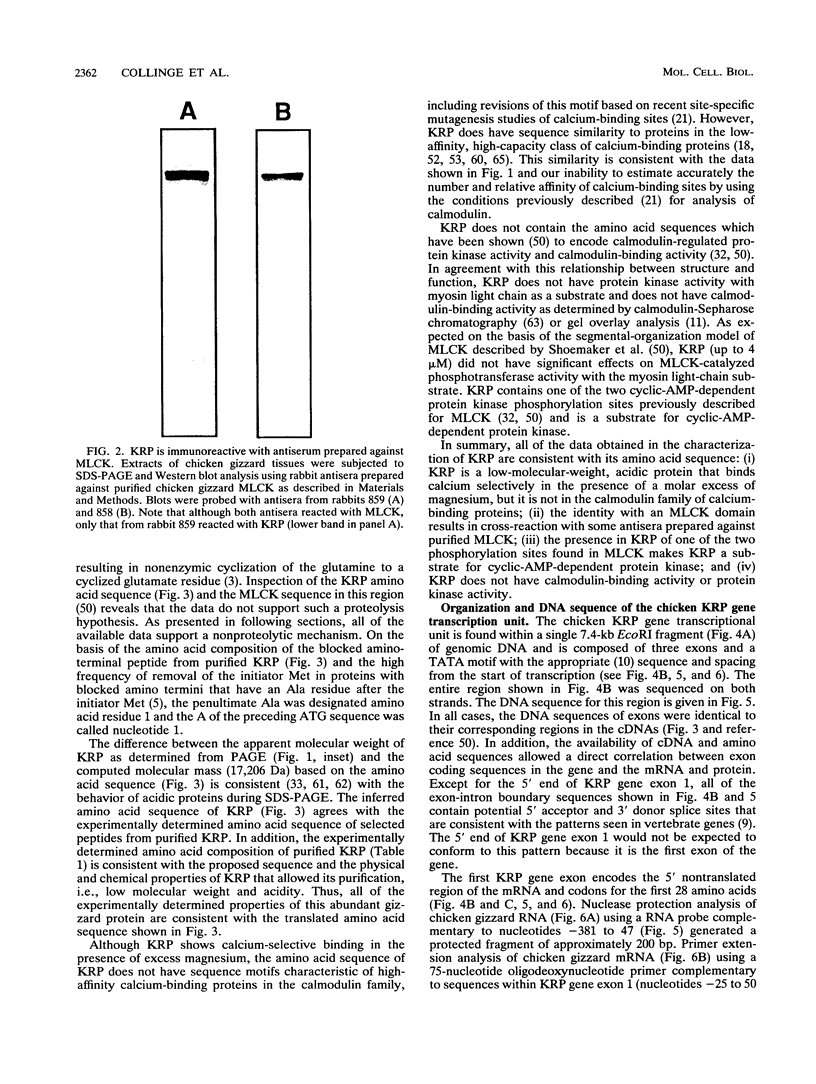
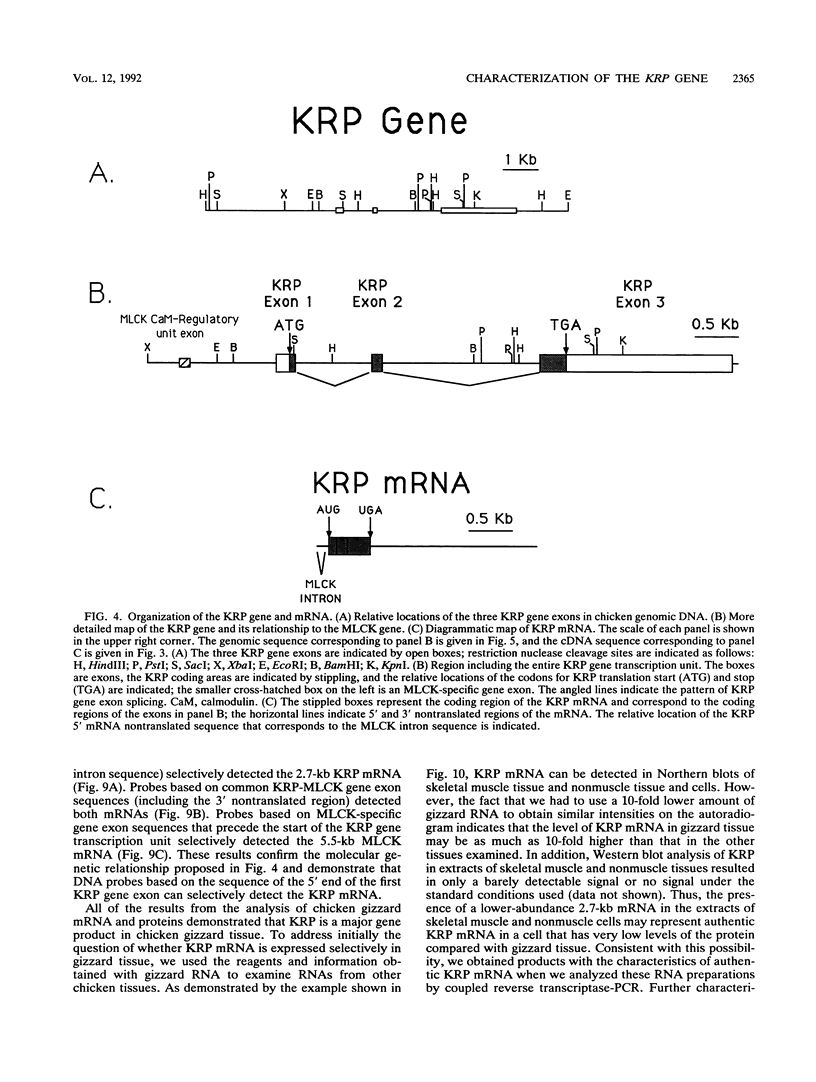
We have determined the first genomic structure and characterized the mRNA and protein products of a novel vertebrate gene that encodes a calcium-binding protein with amino acid sequence identity to a protein kinase domain. The elucidation of the complete DNA sequence of this transcription unit and adjacent genomic DNA, Southern blot and polymerase chain reaction analyses of cellular genomic DNA, and examination of mRNA and protein species revealed that the calcium-binding kinase-related protein (KRP)-encoding gene is contained within the gene for a calmodulin-regulated protein kinase, myosin light-chain kinase (MLCK). The KRP gene transcription unit is composed of three exons and a 5'-flanking sequence containing a canonical TATA box motif. The TATA box, the transcription initiation site, and the first 109 nucleotides of the 5' noncoding region of the KRP mRNA correspond to an MLCK gene intron sequence. Both KRP and MLCK are produced in the same adult chicken tissue in relatively high abundance from a single contiguous stretch of genomic DNA and utilize the same reading frame and common exons to produce distinct mRNAs (2.7 and 5.5 kb, respectively) that encode proteins with dissimilar biochemical functions. There appears to be no precedent in vertebrate molecular biology for such a relationship. This may represent a mechanism whereby functional diversity can be achieved within the same vertebrate tissue by use of common exons to produce shuffled domains with identical amino acid sequences in different molecular contexts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification of smooth muscle myosin light-chain kinase. Methods Enzymol. 1982;85(Pt B):298–308. doi: 10.1016/0076-6879(82)85029-5. [DOI] [PubMed] [Google Scholar]
- Alexandrova E. A., Marekov L. N., Beltchev B. G. Involvement of protein HMG1 in DNA replication. FEBS Lett. 1984 Dec 3;178(1):153–155. doi: 10.1016/0014-5793(84)81260-0. [DOI] [PubMed] [Google Scholar]
- Andresen K., Tom T. D., Strand M. Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J Biol Chem. 1991 Aug 15;266(23):15085–15090. [PubMed] [Google Scholar]
- Arfin S. M., Bradshaw R. A. Cotranslational processing and protein turnover in eukaryotic cells. Biochemistry. 1988 Oct 18;27(21):7979–7984. doi: 10.1021/bi00421a001. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bourbon H. M., Lapeyre B., Amalric F. Structure of the mouse nucleolin gene. The complete sequence reveals that each RNA binding domain is encoded by two independent exons. J Mol Biol. 1988 Apr 20;200(4):627–638. doi: 10.1016/0022-2836(88)90476-7. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Watterson D. M., Van Eldik L. J. Identification of calmodulin-binding proteins in chicken embryo fibroblasts. J Cell Biol. 1984 Aug;99(2):550–557. doi: 10.1083/jcb.99.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M., Puigdomènech P., Tancredi T., Palau J. Interaction between domains in chromosomal protein HMG-1. EMBO J. 1984 Jun;3(6):1255–1261. doi: 10.1002/j.1460-2075.1984.tb01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gallagher P. J., Herring B. P. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem. 1991 Dec 15;266(35):23945–23952. [PMC free article] [PubMed] [Google Scholar]
- Haiech J., Kilhoffer M. C., Lukas T. J., Craig T. A., Roberts D. M., Watterson D. M. Restoration of the calcium binding activity of mutant calmodulins toward normal by the presence of a calmodulin binding structure. J Biol Chem. 1991 Feb 25;266(6):3427–3431. [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Huang B., Mengersen A., Lee V. D. Molecular cloning of cDNA for caltractin, a basal body-associated Ca2+-binding protein: homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J Cell Biol. 1988 Jul;107(1):133–140. doi: 10.1083/jcb.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Watterson D. M., Lee V. D., Schibler M. J. Purification and characterization of a basal body-associated Ca2+-binding protein. J Cell Biol. 1988 Jul;107(1):121–131. doi: 10.1083/jcb.107.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Dabrowska R., Guerriero V., Jr, Hartshorne D. J. Identification in turkey gizzard of an acidic protein related to the C-terminal portion of smooth muscle myosin light chain kinase. J Biol Chem. 1989 Aug 25;264(24):13971–13974. [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Kaplan D. J., Duncan C. H. Full length cDNA sequence for bovine high mobility group 1 (HMG1) protein. Nucleic Acids Res. 1988 Nov 11;16(21):10375–10375. doi: 10.1093/nar/16.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas T. J., Watterson D. M. Purification of calmodulin and preparation of immobilized calmodulin. Methods Enzymol. 1988;157:328–339. doi: 10.1016/0076-6879(88)57088-x. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Wiggins M. E., Watterson D. M. Amino Acid sequence of a novel calmodulin from the unicellular alga chlamydomonas. Plant Physiol. 1985 Jul;78(3):477–483. doi: 10.1104/pp.78.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercik M. H., Bourguignon L. Y. Insulin receptor capping and its correlation with calmodulin-dependent myosin light chain kinase. J Cell Physiol. 1985 Sep;124(3):403–410. doi: 10.1002/jcp.1041240308. [DOI] [PubMed] [Google Scholar]
- Majercik M. H., Bourguignon L. Y. Insulin-induced myosin light-chain phosphorylation during receptor capping in IM-9 human B-lymphoblasts. Biochem J. 1988 Jun 15;252(3):815–823. doi: 10.1042/bj2520815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Cruzalegui F., LeMagueresse B., Needleman D. S., Slaughter G. R., Ono T. A novel Ca2+/calmodulin-dependent protein kinase and a male germ cell-specific calmodulin-binding protein are derived from the same gene. Mol Cell Biol. 1991 Aug;11(8):3960–3971. doi: 10.1128/mcb.11.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Ostvold A. C., Walass S. I., Lund T., Laland S. G. High-mobility-group proteins P1, I and Y as substrates of the M-phase-specific p34cdc2/cyclincdc13 kinase. Eur J Biochem. 1991 Mar 28;196(3):557–567. doi: 10.1111/j.1432-1033.1991.tb15850.x. [DOI] [PubMed] [Google Scholar]
- Ohmstede C. A., Bland M. M., Merrill B. M., Sahyoun N. Relationship of genes encoding Ca2+/calmodulin-dependent protein kinase Gr and calspermin: a gene within a gene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5784–5788. doi: 10.1073/pnas.88.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Langan T. A., Nissen M. S. Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1671–1675. doi: 10.1073/pnas.88.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H., Tempel B. L. Voltage-gated K+ channels of the mammalian brain. FASEB J. 1991 Feb;5(2):164–170. doi: 10.1096/fasebj.5.2.2004663. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. H., Lukas T. J., Blair I. A., Schultz J. E., Watterson D. M. Amino acid sequence of a novel calmodulin from Paramecium tetraurelia that contains dimethyllysine in the first domain. J Biol Chem. 1987 Jan 25;262(3):1025–1029. [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D. The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J Biol Chem. 1984 Jun 25;259(12):7740–7746. [PubMed] [Google Scholar]
- Shoemaker M. O., Lau W., Shattuck R. L., Kwiatkowski A. P., Matrisian P. E., Guerra-Santos L., Wilson E., Lukas T. J., Van Eldik L. J., Watterson D. M. Use of DNA sequence and mutant analyses and antisense oligodeoxynucleotides to examine the molecular basis of nonmuscle myosin light chain kinase autoinhibition, calmodulin recognition, and activity. J Cell Biol. 1990 Sep;111(3):1107–1125. doi: 10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Isolation and identification of partial cDNA clones for endoplasmin, the major glycoprotein of mammalian endoplasmic reticulum. J Mol Biol. 1987 Mar 20;194(2):345–347. doi: 10.1016/0022-2836(87)90381-0. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J. 1989 Dec 1;8(12):3581–3586. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M., Bernués J., Querol E. Calcium modulates the binding of high-mobility-group protein 1 to DNA. Biochem Int. 1990 Aug;21(5):891–899. [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Piperno G., Watterson D. M. Similarities and dissimilarities between calmodulin and a Chlamydomonas flagellar protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4779–4783. doi: 10.1073/pnas.77.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Reproducible production of antiserum against vertebrate calmodulin and determination of the immunoreactive site. J Biol Chem. 1981 May 10;256(9):4205–4210. [PubMed] [Google Scholar]
- Van Eldik L. J., Wolchok S. R. Conditions for reproducible detection of calmodulin and S100 beta in immunoblots. Biochem Biophys Res Commun. 1984 Nov 14;124(3):752–759. doi: 10.1016/0006-291x(84)91022-2. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Zendegui J. G., Marshak D. R., Watterson D. M. Calcium-binding proteins and the molecular basis of calcium action. Int Rev Cytol. 1982;77:1–61. doi: 10.1016/s0074-7696(08)62463-8. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Spinach calmodulin: isolation, characterization, and comparison with vertebrate calmodulins. Biochemistry. 1980 Dec 9;19(25):5762–5768. doi: 10.1021/bi00566a015. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Vanaman T. C. Affinity chromatography purification of a cyclic nucleotide phosphodiesterase using immobilized modulator protein, a troponin C-like protein from brain. Biochem Biophys Res Commun. 1976 Nov 8;73(1):40–46. doi: 10.1016/0006-291x(76)90494-0. [DOI] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Johnson B. H., Reeck G. R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarain-Herzberg A., Fliegel L., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle calsequestrin gene. J Biol Chem. 1988 Apr 5;263(10):4807–4812. [PubMed] [Google Scholar]
- Zimmer W. E., Schloss J. A., Silflow C. D., Youngblom J., Watterson D. M. Structural organization, DNA sequence, and expression of the calmodulin gene. J Biol Chem. 1988 Dec 25;263(36):19370–19383. [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R. S., Feramisco J. R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]