Abstract
Background
Children with congenital heart disease are at risk for impaired neurodevelopment (ND). We investigated the association of fetal cerebrovascular resistance with ND in patients with single ventricle lesions.
Methods
In the Single Ventricle Reconstruction (SVR) and Infant Single Ventricle trials, 14-month ND was assessed using the Bayley Scales of Infant Development II. We investigated associations between ND scores and fetal middle cerebral artery pulsatility index (MCA-PI) z-scores, a Doppler-derived estimate of cerebrovascular resistance in a subset of those infants.
Results
Neurodevelopment assessments were performed at age 14.3 ± 1 months in 170 (74%) of 230 Infant Single Ventricle and 321 (58%) of 555 SVR subjects. Fetal echocardiographic data were available in 119 subjects, 72 (61%) of which had ND testing. Mean Psychomotor Development Index (PDI) (76 ± 20) and Mental Development Index (MDI) (89 ± 17) scores were lower than normative means (100 ± 15, P < .001). Mean MCA-PI z-score was −0.95 ± 1.52. Middle cerebral artery pulsatility index z-score correlated negatively with PDI (r = −0.27, P = .02) but was not associated with MDI. When MCA-PI z-score was added to a multivariable model controlling for factors identified in the SVR trial to predict PDI, the percentage of explained variation increased from 23% to 30%, and MCA-PI z-score remained an independent predictor (r = −3.864, P = .03). Middle cerebral artery pulsatility index z-score was not an independent predictor in a model adjusting for site.
Conclusions
Among fetuses with single ventricle anomalies, lower cerebrovascular resistance was associated with higher ND scores. This relationship is opposite to that observed with advanced intrauterine growth retardation and may represent a unique ability of these congenital heart disease fetuses to compensate for diminished cerebral oxygen delivery.
Advances in surgical techniques and perioperative care have improved survival of infants born with hypoplastic left heart syndrome (HLHS) and other single ventricle lesions. However, survivors are at high risk for neurodevelopmental (ND) delay with the percentage affected reported to be as high as 70%.1,2 The spectrum of neurocognitive effects is wide, ranging from learning disability to attention deficit disorder to mental retardation.3 In addition to our inability to predict the type and degree of deficit likely to be encountered, we are currently unable to predict who among our patients is at highest risk.
The healthy fetus maintains adequate cerebral oxygenation through a range of adaptive physiologic responses. This includes vasodilation of the cerebral arteries to increase brain perfusion if the fetus is exposed to acute or chronic hypoxia.4,5 As a result of this cerebral vasodilation, the diastolic flow in the middle cerebral artery (MCA) increases, whereas the MCA pulsatility index (MCA-PI) decreases. With further deterioration, the protective brain sparing reflex may be overwhelmed, and cerebral ischemia and metabolic acidosis may ensue leading to intraventricular hemorrhage and periventricular leukomalacia.
Although decreased cerebrovascular resistance may predict poor neurologic outcomes in fetal disease states such as intrauterine growth retardation (IUGR),6,7 the significance of this finding in the setting of congenital heart disease (CHD) is unclear. In univentricular hearts, the fetal systemic arterial saturation is lower than usual due to intracardiac mixing of oxygenated and deoxygenated blood. In some, this is compounded by abnormal flow patterns related to either right- or left-sided outflow tract obstruction. This chronically lower arterial saturation may explain why some fetuses with CHD, particularly the group with HLHS, demonstrate aberrant cerebrovascular resistance on fetal ultrasound in the absence of placental failure.8,9 Our aim was to investigate associations between fetal cerebrovascular resistance and early ND in patients with univentricular hearts.
Patients and methods
This was an approved ancillary study to the Pediatric Heart Network’s (PHN) Single Ventricle Reconstruction (SVR) and Infant Single Ventricle (ISV) trials. All PHN sites that contributed patients to the SVR or the ISV trial and had fetal echocardiograms available for these patients were invited to participate. Local approval from the institutional review board or its equivalent was obtained at each site; institutional review board approval for the SVR and ISV trials along with written parental informed consent had been obtained previously. Pediatric Heart Network’s studies were supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068270, HL068279, HL068281, HL068285, HL068290, HL068288, HL085057) and the FDA Office of Orphan Products Development. In addition, Dr Williams received support from grant number 1K23HD061601 from the National Institute of Child Health & Human Development of the National Institutes of Health.
Although this study was designed to allow for the prospective collection of data among fetuses likely to be eligible for SVR or ISV enrollment, final determination of PHN study eligibility could not be conducted until after birth. Therefore, although some centers enrolled fetuses and prospectively collected data in a standardized fashion, the majority of fetal data were obtained retrospectively from clinically indicated fetal echocardiograms of patients who were subsequently enrolled in the PHN trials. These retrospective echocardiograms were recorded according to local center practice guidelines.
SVR and ISV trials
Details of the SVR and ISV trials have been published.10,11 In brief, the SVR trial prospectively enrolled infants with HLHS and other complex single right ventricle lesions at 15 centers from May 2005 to July 2008, with the goal of assessing the impact of the modified Blalock-Taussig (subclavian-to-pulmonary artery) versus Sano (right ventricle-to-pulmonary artery) shunt during the Norwood procedure on 12-month transplant-free survival. The ISV trial prospectively enrolled infants at 10 centers with any form of single ventricle lesion from August 2003 to May 2007 to assess the effect of angiotensin-converting enzyme inhibition on growth at 14 months of age. Both trials recorded subject characteristics in a standardized fashion at all hospitalizations and through 14 months of age. Medical and surgical data collected included cardiac anatomical details, surgeries performed, type and length of intraoperative and postoperative cardiac and respiratory support, perioperative and postoperative complications, and length of hospital stay. Demographic factors such as socioeconomic status and maternal education level were also recorded.
Fetal echocardiography
Subjects from either trial with available fetal echocardiograms were eligible for this ancillary study. Fetal MCA Doppler flow patterns were reviewed centrally by 1 of 2 investigators (I.W. and A.S.) blinded to ND scores for tracing quality. The peak systolic, end-diastolic, and the time averaged maximum velocities were remeasured, and the MCA-PI was calculated using a standard formula (see the online Appendix).
To account for differences attributable to gestational age (GA), MCA-PI z-scores were calculated using published norms12 as previously described.9 The MCA-PI is a surrogate measure for resistance within the cerebral vasculature with a lower PI estimating lower resistance. The MCA-PI was selected as the primary predictor instead of the resistance index, another commonly used measure (see the online Appendix), due to the availability of normal data allowing for z-score calculation, which is not available for the resistance index.
Neurodevelopmental testing
Subjects participating in the ISV and/or the SVR trial underwent standardized 14-month ND testing using the Bayley Scales of Infant Development, Second Edition (BSID-II).13 To ensure consistency in testing across PHN sites, all local psychologists underwent centralized training and were certified by a single expert via review of videotaped sessions before study participation. The BSID-II is approved for the assessment of children ages 1 to 42 months and provides 2 summary scores: the Psychomotor Development Index (PDI) and the Mental Development Index (MDI). The normative mean ± SD for both the MDI and the PDI is 100 ± 15.
Statistical analysis
Data are reported as means with SDs or medians with ranges as appropriate. Preliminary associations between fetal cerebrovascular resistance and ND were assessed using the Pearson correlation coefficient between MCA-PI z-score from the initial fetal echocardiogram and MDI and PDI scores. To account for the variable number of fetal echocardiograms per subject and to minimize the likelihood of a type I error while maximizing our sample size, we limited these primary analyses to data recorded at the time of the first fetal echocardiogram. Differences in mean MDI and PDI scores among subjects with an MCA-PI z-score of less than −2 and subjects who always had an MCA-PI z-score of −2 or higher were evaluated using the Student t test. To control for variables that could confound or mediate the relationship between fetal cerebrovascular resistance and ND, we entered MCA-PI z-score into a multivariable regression model that included factors found in the SVR trial to independently predict PDI score. Interrater reliability of the MCA-PI measurement was calculated between the 2 investigators using the intraclass correlation coefficient.
Results
Baseline data
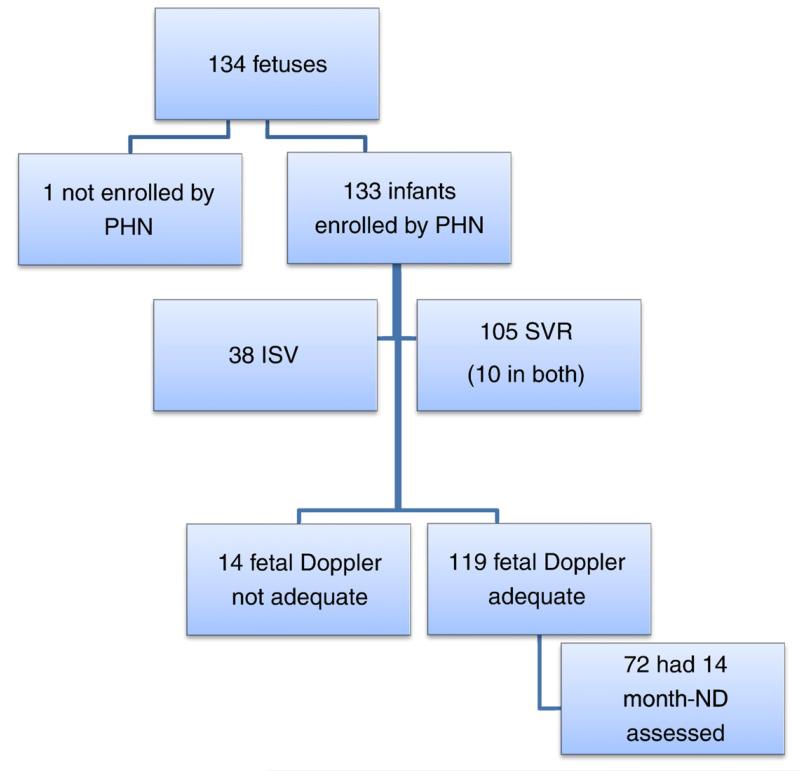
Of the 555 SVR and 230 ISV subjects enrolled by the PHN, fetal data were available in 133. The SVR trial contributed more subjects (n = 105) than the ISV trial (n = 38), and 10 subjects were enrolled in both studies. One fetus was enrolled prospectively in this study but after birth was deemed ineligible for the SVR trial. Therefore, the total number of fetuses enrolled was 134 (Figure 1).
Figure 1.
Study subject flowchart.
Baseline subject characteristics are shown in Table I. Of the 134 subjects, 11 had a single left ventricle, and 122 had a single right ventricle lesion, of which 103 (79%) had HLHS (online Appendix Supplementary Table). Among those with HLHS, 58 (56%) had aortic atresia (AA).
Table I.
Baseline characteristics
| Subject characteristics | All subjects, N = 134 |
Subjects with BSID-II scores, n = 82/134 (61%) |
Subjects without BSID-II scores, n = 52/134 (39%) |
P |
|---|---|---|---|---|
| Male | 86/132 (65.2%) | 48/86 (55.8%) | 38/86 (44.2%) | .04 |
| Hispanic | 19/130 (14.6%) | 10/19 (52.6%) | 9/19 (47.4%) | .35 |
| GA at first fetal echocardiogram (wk) | 26.7 ± 5.3 (n = 131) | 26.6 ± 5.1 (n = 82) | 27 ± 5.7 (n = 49) | .67 |
| GA at birth (wk) | 38.4 ± 1.7 (n = 132) | 38.6 ± 1.3 (n = 82) | 38.1 ± 2.2 (n = 50) | .16 |
| Birth weight (g) | 3123 ± 594 (n = 131) | 3200 ± 563 (n = 82) | 2980 ± 623 (n = 49). | 04 |
| BW <2500 g | 19/131 (14.5%) | 8/19 (42.1%) | 11/19 (57.9%) | .05 |
| Birth HC z-score | −0.97 ± 1.5 (n = 122) | −1.1 ± 1.5 (n = 81) | −0.89 ± 1.5 (n = 41) | .42 |
| HLHS | 103/131 (78.6%) | 65/103 (63.1%) | 38/103 (36.9%) | .82 |
| MCA-PI z-score at first fetal echocardiogram | −0.95 ± 1.5 (n = 119) | −0.93 ± 1.5 (n = 72) | −0.98 ± 1.5 (n = 47) | .88 |
| Minimum MCA-PI z-score ever recorded | −1.7 ± 1.5 (n = 134) | −1.7 ± 1.5 (n = 82) | −1.7 ± 1.6 (n = 52) | .92 |
Abbreviation: HC, Head circumference.
Middle cerebral artery pulsatility index
Of 134 fetuses, 119 (89%) had adequate Doppler data for MCA-PI calculation. The mean MCA-PI z-score at the first fetal echocardiogram was −0.95 ± 1.5 (mean GA of 26.9 ± 5.3 weeks, range 18-38 weeks). Of this group, 79% (n = 94/119) had HLHS, and 75% (n = 90/119) had multiple fetal examinations. Across gestation, 22% had an MCA-PI z-score of less than −2 at least once. When we assigned each subject the lowest MCA-PI z-score calculated on any fetal echocardiogram, the mean MCA-PI z-score was −1.7 ± 1.5. Interrater reliability was excellent with an intraclass correlation coefficient of 0.989.
Neurodevelopmental testing
Among subjects enrolled in the PHN trials, 321 (55%) of 555 SVR and 170 (74%) of 230 ISV subjects returned at a mean age of 14.3 ± 1 months for BSID-II testing. Among the 134 fetal subjects, 82 (61%) had ND testing, and of the 119 subjects with MCA-PI z-scores, 72 (61%) had ND testing. There was no difference in mean MCA-PI z-score between subjects who did and did not undergo ND testing (Table I).
Mean PDI score for the group (n = 82) was 76.4 ± 19.8, and mean MDI was 88.5 ± 16.6. These values were significantly lower than normative values (P < .001). Psychomotor Development Index score was more severely affected than MDI score with 62% having a PDI <85 (<1 SD below the mean) and 35% having an MDI <85. Psychomotor Development Index and MDI were highly correlated (r = 0.652, P < .001). The MDI score of the fetal group was lower than that of the ISV trial (P < .001), but otherwise, BSID-II scores did not differ from the overall ISV and SVR cohorts (ISV trial PDI [80 ± 18] and MDI [96 ± 14], SVR trial PDI [74 ± 19] and MDI [89 ± 18]).
Associations between fetal MCA-PI and ND testing
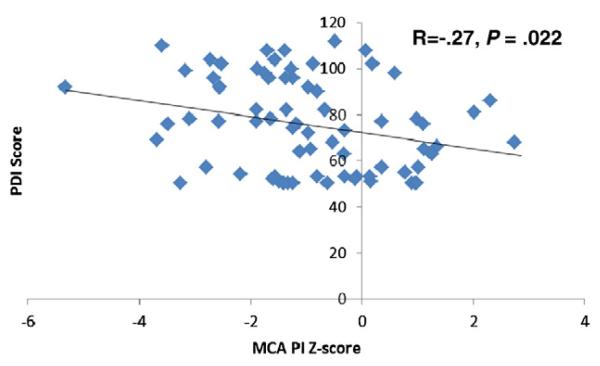
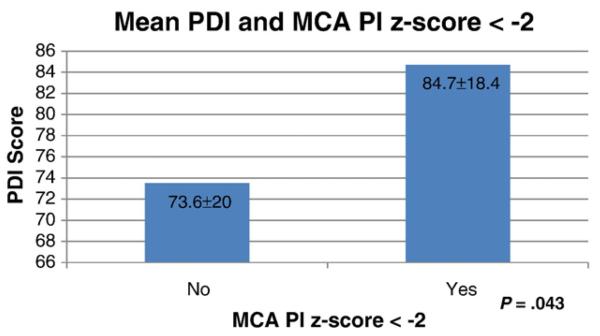
Middle cerebral artery pulsatility index z-score correlated negatively with PDI (r = −0.269, P = .022) but did not correlate with MDI score (r = −0.038, P = .75) (Figure 2). Subjects who had an MCA-PI z-score of less than −2 at any point scored an average of 11 points higher on the PDI compared with those who did not (84.7 ± 18.4 vs 73.6 ± 20.0, P = .04) (Figure 3). Although MDI mean score was 6 points higher among subjects with an MCA-PI z-score of less than −2, this association was not statistically significant (92.3 ± 17.4 vs 86.8 ± 16.8, P = .24).
Figure 2.
Correlation of MCA-PI z-score and 14-month PDI score.
Figure 3.
Difference in mean PDI score among subjects who had an MCA-PI z-score of less than −2 at any point in gestation versus subjects who always had an MCA-PI z-score of −2 or higher.
Effect of GA
To assess if there was a possible effect of GA on the relationship between fetal cerebrovascular resistance and ND score, we conducted 2 subgroup analyses. First, we tested the correlation between MCA-PI z-score and PDI score for subjects whose first fetal echocardiogram was performed < 25 weeks GA (n = 31). Second, we tested the correlation for subjects whose first fetal echocardiogram was performed >28 weeks GA (n = 22). In both cases, although the direction and the magnitude of the MCA-PI z-score correlation with PDI score were similar to that seen with the entire cohort, it was no longer statistically significant (r = −0.328, P = .1 for <25 weeks GA, r = −0.276, P = .21 for >28 weeks GA).
Effect of the presence of AA on MCA-PI
In an effort to understand whether anatomical subtype was important, mean MCA-PI z-scores and the mean minimum MCA-PI z-score ever recorded among subjects with HLHS with AA (n = 58/103) were compared with scores of HLHS subjects without AA (non-AA, n = 45/103). There was no difference in mean MCA-PI z-scores between groups (−0.87 ± 1.5 AA vs −1.1 ± 1.5 non-AA, P = .48 at first echocardiogram, −1.7 ± 1.3 AA vs −1.9 ± 1.7 non-AA, P = .54 for lowest MCA-PI z-score at any time). There was also no association between presence of AA or a diagnosis of HLHS and an MCA-PI z-score of less than−2 at any time.
Effect of site on MCA-PI z-score and BSID-II score
In total, 6 of the 15 SVR and 10 ISV sites contributed fetal data for this study. The number of subjects contributed varied from 5 to 64. Middle cerebral artery pulsatility index z-score varied by site (Table II); however, PDI and MDI score did not.
Table II.
Mean MCA-PI z-score at the first fetal echocardiogram, MDI and PDI score per site/hospital center where the subject was seen
| Site | MCA-PI z-score, mean ± SD (n) |
MDI score, mean ± SD (n) |
PDI score, mean ± SD (n) |
|---|---|---|---|
| 1 | −2.1 ± 1.0 (4) | 87.2 ± 21.4 (5) | 86.8 ± 23.0 (5) |
| 2 | −0.7 ± 0.6 (4) | 88.4 ± 16.2 (9) | 74.4 ± 24.4 (9) |
| 3 | −1.8 ± 1.0 (27) | 86.8 ± 16.1 (17) | 78.3 ± 17.5 (17) |
| 4 | −0.4 ± 1.5 (64) | 86.3 ± 16.5 (41) | 72.5 ± 19.2 (41) |
| 5 | −2.2 ± 1.9 (5) | 90.5 ± 12.0 (2) | 92 ± 0 (2) |
| 6 | −0.9 ± 1.6 (14) | 103.6 ± 13.2 (8) | 86 ± 19.1 (8) |
| Total | −0.95 ± 1.5 (118) | 88.48 ± 16.6 (82) | 76.4 ± 19.8 (82) |
Multivariable regression model predicting PDI score
To investigate how fetal cerebrovascular resistance relates to PDI in the context of other factors known to be associated with ND, MCA-PI z-score was added to a multivariable model containing the following factors found in the SVR trial to predict PDI score: site, birth weight <2.5 kg versus ≥2.5 kg, number of complications post-Norwood, and log transformation of the length of Norwood hospital stay.14 We selected the SVR model rather than the ISV model due to the higher number of subjects in our study who were enrolled in the SVR trial. In our cohort of 82 subjects, site was not statistically related to PDI score, and therefore, site was not included in our model. Once MCA-PI z score was added to the model, the percentage of explained variance increased from 23% to 30%, and MCA-PI z-score was an independent predictor of PDI score (β = −3.864, P = .03, n = 55). However, because site was highly correlated with PDI in the original SVR trial, we also constructed a model that adjusted for sites that contributed subjects to this smaller fetal cohort. In this model, MCA-PI z-score was no longer an independent predictor of PDI score (β = −0.95, P = .65).
Discussion
In this ancillary study of the PHN ISV and SVR trials, we demonstrated that the MCA-PI, a surrogate measure of fetal cerebrovascular resistance, was associated with 14-month ND outcome. This is the first large study to link abnormalities in fetal cerebral Doppler flow patterns to ND in patients with single ventricle lesions.
In a pilot study of 16 fetuses with CHD, Williams et al demonstrated that decreased cerebrovascular resistance was associated with a lower 18-month cognitive score.15 This earlier report differs from the current study in many respects: the sample size was smaller; the subject population was a mix of HLHS, d-transposition of the great arteries, and tetralogy of Fallot; fetal data were collected prospectively and assessed earlier in pregnancy (mean GA 23.2 ± 2.8 vs 26.9 ± 5.3 weeks); and ND was measured at 18 months with the Bayley Scales of Infant Development, Third Edition, which provides 3 scores: a cognitive, motor, and a language index. Therefore, the findings of both studies, rather than being contradictory, are best viewed as being different and supportive of an overarching hypothesis that a true association between fetal cerebrovascular resistance and ND exists. Multiple questions remain, including the impact of different cardiac lesions and the timing of assessment. Perhaps low cerebrovascular resistance early in pregnancy is associated with poor outcomes, whereas low resistance later in pregnancy is adaptive, or maybe low resistance is associated with better outcomes in fetuses with left-sided obstructive lesions, such as HLHS, but is associated with worse outcomes in fetuses with right-sided obstructive lesions, such as tetralogy of Fallot. These questions would best be addressed by a large, prospective, multicenter investigation that allows sequential observations throughout pregnancy.
In this study, the direction of the relationship between fetal cerebrovascular resistance and neurologic outcome is opposite to that observed in other fetal disease states. In fetuses with advanced IUGR, lower cerebrovascular resistance is associated with ischemic brain damage. Conversely, in our cohort, lower cerebrovascular resistance was associated with improved neurocognitive outcome. One possible explanation is the difference in underlying pathophysiology. In IUGR, the primary problem is a diseased placenta, leading to inadequate gas and substrate exchange. In the setting of univentricular CHD however, the presumed mechanism would be the hemodynamic consequences of intracardiac mixing with or without outflow tract obstruction leading to the delivery of relatively deoxygenated blood to the brain. It is possible that systemic oxygen desaturation in single ventricle fetuses is never as severe as can occur with IUGR, and therefore, the autoregulatory response of cerebral vasodilation may be sufficient and even adaptive. Szwast et al recently reported that single ventricle fetuses with aortic obstruction are more likely to demonstrate lower MCA-PI z-scores compared with single ventricle fetuses with obstruction to pulmonary flow, which supports this hypothesis.16 Future studies using animal models or human subjects undergoing clinically indicated fetal intervention may prove instructive in this regard. Putting the data together, one could suggest that because lower cerebral resistance in IUGR is a marker of significant disease, those with the lowest resistance are at the highest risk for a poor outcome. In contrast, lower cerebral resistance in our population may be a marker of the best adaptive response, and therefore, those with the lowest resistance may be the patients at lowest risk for significant ND delays.
Our findings provide further documentation that neurologic impairment or the potential for neurologic impairment is present in the patient with CHD preoperatively. Imaging and autopsy studies of the brains of newborns with CHD have revealed multiple anomalies. In 2002, Mahle et al17 reported that brain abnormalities were present in CHD infants both preoperatively and postoperatively on magnetic resonance imaging (MRI). In addition to white matter injury, Miller et al18 reported abnormalities on magnetic resonance spectroscopy and diffusion tensor imaging preoperatively in CHD newborns that were suggestive of abnormal prenatal brain development. Using 3-dimensional volumetric MRI, Limperopoulos et al19 showed altered fetal brain growth in CHD fetuses compared with controls. Whether the altered fetal cerebrovascular resistance seen in our study contributes directly to abnormal brain development or whether changes in fetal cerebral blood flow are merely markers of neurologic outcomes cannot be determined at this point.
We found a significant association with the PDI but not with the MDI score. This is not an altogether unexpected finding. There is a known tendency for the PDI to be more severely affected than the MDI among CHD patients.1,20,21 In both the PHN ISV and the SVR trials, the models developed to predict PDI differed from the models for the MDI score. Although PDI and MDI correlate modestly with one another, factors influencing them may be distinct and separate. Finally, it is possible that fetal cerebrovascular resistance is also associated with MDI, but we lacked statistical power to demonstrate this.
The initial finding that MCA-PI z-score is an independent predictor of PDI even when controlling for birth weight, length of Norwood hospital stay, and number of complications post-Norwood suggests that fetal cerebrovascular resistance may explain a unique portion of the variation in ND that is not accounted for by postnatal factors. It is important to note, however, that when site was included in the model, MCA-PI z-score no longer remained significant. This is likely due to the high correlation between MCA-PI z-score and site found in this analysis. Reasons for this association are speculative. As all fetal Doppler waveforms were retraced centrally by investigators who demonstrated high interrater agreement, measurement bias is unlikely. Furthermore, ND was assessed in a standardized fashion by psychologists who underwent centralized training and certification. It may very well be that “site” is a surrogate for a host of additional factors that contribute to outcome that are either unmeasurable or were not measured by the SVR trial. A larger, prospective, multicenter fetal trial may be illuminating in this regard.
Limitations
This study must be viewed in light of its limitations. Although originally designed to be prospective, most fetal data were obtained retrospectively from available clinical fetal echocardiograms. Thus, our ability to evaluate the influence of GA on fetal Doppler findings and their correlates is limited. Trajectory analysis of MCA-PI z-scores across gestation in individual subjects and the relationship of these trajectories to outcomes were beyond the scope of this initiative; many subjects had only 1 available fetal echocardiogram. In addition, there is theoretical value in being able to obtain information from the initial fetal cardiology encounter alone because some centers only perform 1 fetal echocardiogram and there is no consensus for follow-up evaluation prenatally. As an ancillary study to existing PHN trials, our ability to explore the relationship of fetal data and postnatal outcomes was restricted to those variables collected by the PHN. Although there was considerable overlap in the types of data collected by the ISV and the SVR trials, the variables recorded were not always the same. Because of our sample size, power to detect subgroup associations, including the potential effects of AA and other anatomical variations, was inadequate. In addition, in our cohort, 79% of subjects had HLHS. Therefore, our results may not be generalizable to fetuses with other single ventricle lesions. Finally, although we were able to demonstrate the association between fetal cerebrovascular resistance and ND, we cannot infer causality.
Conclusion
Lower cerebrovascular resistance, as measured by the MCA-PI, in fetuses with single ventricle lesions correlated with a higher 14-month ND score. Fetuses with more normal MCA-PI may be at highest risk for abnormal ND because of diminished compensatory change in cerebrovascular resistance. Future directions include the need to determine whether fetal cerebrovascular resistance predicts neurologic outcomes later in childhood and whether these fetal blood flow alterations are directly responsible for the observed postnatal deficits.
Supplementary Material
Acknowledgments
I.A. Williams received support from grant number 1K23HD061601 from the National Institute of Child Health & Human Development of the National Institutes of Health. Pediatric Heart Network studies were supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068270, HL068279, HL068281, HL068285, HL068290, HL068288, HL085057) and the FDA Office of Orphan Products Development. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institute of Child Health & Human Development.
Footnotes
Trial registration ClinicalTrials.gov number, NCT00115934.
Disclosures
Financial disclosure/conflicts of interest: The authors have no financial relationships or conflicts of interest to disclose.
References
- 1.Tabbutt S, Nord AS, Jarvik GP, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatr. 2008;121(3):476–83. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg CS, Schwartz EM, Brunberg JA, et al. Neurodevelopmental outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137(5):646–52. doi: 10.1067/mpd.2000.108952. [DOI] [PubMed] [Google Scholar]
- 3.Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Op Cardiol. 2005;20(2):94–9. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- 4.Peeters LL, Sheldon RE, Jones MD, Jr, et al. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135(5):637–46. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- 5.Dubiel M, Gunnarsson GO, Gudmundsson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet Gynecol. 2002;20(2):117–21. doi: 10.1046/j.1469-0705.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 6.Dubiel M, Breborowicz GH, Marsal K, et al. Fetal adrenal and middle cerebral artery Doppler velocimetry in high-risk pregnancy. Ultrasound Obstet Gynecol. 2000;16(5):414–8. doi: 10.1046/j.1469-0705.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 7.Bahado-Singh RO, Kovanci E, Jeffres A, et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999;180:750–6. doi: 10.1016/s0002-9378(99)70283-8. [DOI] [PubMed] [Google Scholar]
- 8.Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–43. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaltman JR, Di H, Tian Z, et al. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25(1):32–6. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 10.Ohye RG, Gaynor JW, Ghanayem NS, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136(4):968–75. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arduini D, Rizzo G. Normal values of pulsatility index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med. 1990;18(3):165–72. doi: 10.1515/jpme.1990.18.3.165. [DOI] [PubMed] [Google Scholar]
- 13.Bayley N. Bayley Scales of Infant Development. 2nd ed. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 14.Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the Single Ventricle Reconstruction Trial. Circulation. 2012;125(17):2081–91. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams IA, Tarullo AR, Grieve PG, et al. Fetal Cerebrovascular Resistance and Neonatal EEG Predict 18-month Neurodevelopmental Outcome in Infants with Congenital Heart Disease. Ultrasound Obstet Gynecol. 2012;40(3):304–9. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szwast A, Tian Z, McCann M, et al. Comparative analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet Gynecol. 2012;40(1):62–7. doi: 10.1002/uog.11147. [DOI] [PubMed] [Google Scholar]
- 17.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–14. [PubMed] [Google Scholar]
- 18.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 19.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcome for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133(4):880–7. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Visconti KJ, Rimmer D, Gauvreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82(6):2207–13. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.