Abstract
Background
Black dialysis patients have significantly lower mortality compared to white patients, in contradistinction to the higher mortality seen in blacks in the general population. It is unclear if a similar paradox exists in non–dialysis-dependent CKD, and if it does, what its underlying reasons are.
Study Design
Historical cohort.
Setting & Participants
518,406 white and 52,402 black male US veterans with non-dialysis dependent CKD stages 3–5.
Predictor
Black race.
Outcomes & Measurements
We examined overall and CKD stage-specific all-cause mortality using parametric survival models. The effect of sociodemographic characteristics, comorbidities and laboratory characteristics on the observed differences was explored in multivariable models.
Results
Over a median follow-up of 4.7 years 172,093 patients died (mortality rate, 71.0 [95% CI, 70.6–71.3] per 1000 patient-years). Black race was associated with significantly lower crude mortality (HR, 0.95; 95% CI, 0.94–0.97; p<0.001). The survival advantage was attenuated after adjustment for age (HR, 1.14; 95% CI, 1.12–1.16), but was even magnified after full multivariable adjustment (HR, 0.72; 95% CI, 0.70–0.73; p<0.001). The unadjusted survival advantage of blacks was more prominent in those with more advanced stages of CKD, but CKD stage-specific differences were attenuated by multivariable adjustment.
Limitations
Exclusively male patients.
Conclusions
Black patients with CKD have lower mortality compared to white patients. The survival advantage seen in blacks is accentuated in patients with more advanced stages of CKD, which may be explained by changes in case mix and laboratory characteristics occurring during the course of kidney disease.
Index words: race, mortality, chronic kidney disease
Black patients are significantly over-represented among patients with end stage renal disease (ESRD) on dialysis in the US relative to their proportion in the general population.1;2 Major reasons for this include a higher population prevalence of chronic kidney disease (CKD)3 and a faster rate of CKD progression among blacks,4;5 but also a significant survival advantage associated with black race among dialysis patients.6–14 The latter observation is in stark contrast to the higher mortality rates of black individuals in the general population.15–17 The exact reasons behind this discrepancy remain unclear. A plausible hypothesis is that during the course of CKD a selection process takes place whereby more black patients with characteristics predisposing to better survival (such as younger age and fewer comorbidities) reach ESRD and start dialysis. Based on this hypothesis it would be expected that the survival disadvantage of blacks seen in the general population will at some point during the course of the development and progression of CKD change to a survival advantage, and this advantage will become progressively larger with more advanced stages of CKD. Furthermore, such a gradual selection process should be accompanied by a progressive evolution of race-specific differences in demographic and clinical characteristics responsible for the survival paradox observed in black dialysis patients.
Studies that examined mortality rates in black vs. white patients with non-dialysis dependent CKD have not been uniform in their findings, in that some described a survival advantage among blacks with CKD18–21 but others did not.5;22;23 This may be explained by the predominance of patients in early stages of CKD in some of these studies.21–23 In a study examining patients with CKD stages 3–4 from Southern California 5 Derose et al18 reported higher pre-dialysis mortality associated with black race in patients with estimated GFR 45–59 ml/min/1.73m2 and a reversal of this association in patients with lower estimated GFR. This study also suggested a change in the ratio of race-specific mortality vs end stage renal disease outcomes with longer duration of follow-up, but without exploring the separate role of each outcome. Similar studies have not been performed in nationally representative CKD cohorts and in patients with all stages of CKD, and no studies have compared the characteristics of cohorts in which blacks had higher vs. lower mortality compared to whites.
We compared all-cause mortality in blacks and whites in a large nationally representative cohort of US veterans with CKD stages 3–5 to determine race specific mortality rates in all stages of CKD, and to examine the effect that CKD stage has on them. We also compared sociodemographic and clinical characteristics of patient groups in which blacks experienced higher vs. lower mortality risks compared to whites.
METHODS
Cohort definition
We identified patients with CKD based on a stable estimated GFR (eGFR) and the presence of an elevated spot urine microalbumin/creatinine ratio (for those with eGFR ≥60 ml/min/1.73m2)24 using laboratory data on serum creatinine from the US Department of Veterans Affairs (VA) Decision Support System National Data Extracts Laboratory Results file (a VA-wide database containing select laboratory results obtained in the clinical setting),25 as detailed previously.26 GFR was estimated from serum creatinine measurements and demographic characteristics by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.27 From a total of 4,381,049 6 patients with any available eGFR between October 1, 2004 and September 30, 2006, we identified 657,609 patients with non-dialysis dependent CKD.
Race
Data on cohort participants' race was obtained from combining data from VA sources with those obtained from Medicare sources through the VA-Medicare data merge project.28 In the case of discrepancies we used the race determination from Medicare due to its more accurate nature.29–31 Out of the 657,609 patients with CKD, 569,809 patients (86.6%) were white, 61,238 patients (9.3%) were black, 17,803 patients (2.7%) were of other race and 8,759 patients (1.3%) had no (missing) race determination. Data analysis was restricted to the 631,047 patients of either white or black race. Since the vast majority of the patient population was comprised of men we excluded 16,853 women (2.7%). Finally, we excluded 43,386 (7.1%) patients with CKD stages 1 and 2 in order to minimize selection bias induced by the selective availability of data on urine microalbumin/creatinine ratio in the VA database. The final study population consisted of 570,808 patients (518,406 white and 52,402 black).
Sociodemographic characteristics, comorbidities, and laboratory variables
Data on patient age, gender, marital (married, single, divorced or widowed) and insurance status (presence or absence of any kind of health insurance outside the VA system), geographic location (Veteran Integrated Service Network (VISN) number) and blood pressure was obtained through the VA Corporate Data Warehouse (CDW). Data on baseline comorbidities was collected from the VA Inpatient and Outpatient Medical SAS Datasets32;33 using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic and procedure codes and Current Procedural Terminology codes recorded during the October 1, 2004 to September 30, 2006 time period. Cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina or myocardial infarction, or procedure codes for percutaneous coronary interventions or coronary artery bypass grafting. We calculated the Charlson comorbidity index using the Deyo modification for administrative datasets, without including kidney disease.34 Data on select baseline laboratory variables was collected from the VA Decision Support System National Data Extracts Laboratory Results file.25
Statistical analyses
Descriptive analyses were performed and skewed variables were log-transformed. Baseline characteristics of black and white patients were compared in the overall cohort, and in patients with different stages of CKD. Differences in baseline characteristics were described by calculating the mean differences and 95% confidence intervals (CI) for means (for continuous variables) and proportions (for categorical variables).35;36
The start of the follow-up period was the date of CKD cohort entry (the date when the stable estimated GFR used to establish CKD was recorded). Patients were followed up until death or until the date of the last health care or administrative encounter, as documented in the VA Vital Status Files. The VA Vital Status Files are a registry containing dates of death or last medical/administrative encounter from all available sources in the VA system (the Beneficiary Identification Records Locator Subsystem (BIRLS), the Patient Treatment File (PTF), Medicare and Social Security Administration (SSA)). The sensitivity and specificity of the Vital Status Files using the National Death index as gold standard were shown to be 98.3% and 99.8% respectively.37
As the race-specific hazard of mortality was not proportional with time in the overall cohort, associations were evaluated by using flexible parametric survival models.38 As blacks appeared to have a higher mortality compared to whites at the beginning of the follow-up period, which reversed later, we studied the role of CKD stage and patient characteristics in time-stratified survival models39 by separately examining crude and adjusted 1-year mortality hazard ratios (HR) associated with black vs. white race in the first, second, third, fourth and fifth years of follow-up, conditional on surviving to the beginning of the examined (first, second, etc.) year. All analyses were repeated in subgroups of patients with different stages of CKD24 at baseline. The effect of differences in baseline sociodemographic characteristics, comorbid conditions and various relevant laboratory variables on observed survival differences between blacks and whites was examined in multivariable models with sequential adjustment for such characteristics. Variables recorded repeatedly throughout follow-up (blood pressure and all laboratory variables) were treated as time-dependent.
Statistical analyses were performed using STATA MP version 11 (StataCorp LP, College Station, TX). The study protocol was approved by the Research and Development Committee at the Memphis VA Medical Center.
RESULTS
Study Participants
The mean age of the cohort at baseline was 75.0±8.9 (standard deviation) years and the mean estimated GFR (eGFR) was 47.5±10.0 ml/min/1.73m2. Baseline characteristics of all cohort participants categorized by their race are shown in Table 1 and the mean differences between the baseline characteristics are shown in Table S1 (provided as online supplementary material). Black patients were younger, less likely to be married and more likely to be uninsured and diabetic, and less likely to have cardiovascular disease. Blacks also had a higher Charlson comorbidity index, systolic and diastolic blood pressure, serum cholesterol and alkaline phosphatase, and lower blood hemoglobin and WBC count. The differences in the baseline characteristics of black vs. white patients in the various subgroups divided according to their baseline CKD stage were similar in nature to those seen in the overall cohort, but became gradually more accentuated with more advanced stages of CKD (Table 1).
Table 1.
Baseline characteristics of individuals stratified by race, overall and in subgroups with different CKD stages.
| All | CKD stage3a | CKD stage3b | CKD stage4 | CKD stage 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White (N=518,406) |
Black (N=52,402) |
White (n=349,231) |
Black (n=33,496) |
White (n=136,104) |
Black (n=13,711) |
White (n=30,054) |
Black (n=4,133) |
White (n=3,017) |
Black (n=1,062) |
|
| Age (y) | 75.3±8.5 | 71.5±10.9 | 74.2±8.6 | 70.9±10.7 | 77.5±7.9 | 73.3±10.7 | 77.8±8.5 | 72.2±11.5 | 74.3±10.0 | 66.2±11.9 |
| Married | 339,529 (67) | 24,614 (49) | 230,452 (68) | 15,882 (49) | 88,025 (67) | 6,314 (48) | 19,089 (66) | 1,918 (48) | 1,963 (67) | 500 (49) |
| Insured | 369,628 (76) | 32,088 (66) | 251,480 (76) | 20,499 (66) | 96,653 (75) | 8,506 (67) | 19,708 (70) | 2,455 (63) | 1,787 (63) | 628 (64) |
| SBP (mmHg) | 135±18 | 139±19 | 134±18 | 138±19 | 135±19 | 139±20 | 135±20 | 141±21 | 138±21 | 142±23 |
| DBP (mmHg) | 71±11 | 75±12 | 72±11 | 75±12 | 70±11 | 74±12 | 68±11 | 74±13 | 69±11 | 75±13 |
| DM | 200,561 (39) | 25,116 (48) | 126,484 (36) | 15.076 (45) | 57,814 (42) | 7,091 (52) | 14,655 (49) | 2,330 (56) | 1,608 (53) | 619 (68) |
| CVD | 236,188 (46) | 16,030 (31) | 148,047 (42) | 9,433 (28) | 69,682 (51) | 4,742 (35) | 16,944 (56) | 1,542 (37) | 1,515 (50) | 313 (29) |
| Hypertension | 441,436 (85) | 49,141 (94) | 290,339 (83) | 30,964 (92) | 120,745 (89) | 13,145 (96) | 27,547 (92) | 4,015 (97) | 2,805 (93) | 1,017 (96) |
| CHF | 76,905 (15) | 9,255 (18) | 40,227 (12) | 4,783 (14) | 27,125 (20) | 2,917 (21) | 8,790 (29) | 1,307 (32) | 763 (25) | 248 (23) |
| PVD | 19,864 (4) | 2,462 (5) | 11,205 (3) | 1,359 (4) | 6,384 (5) | 785 (7) | 2,086 (7) | 270 (7) | 189 (6) | 48 (5) |
| CBVD | 78,442 (15) | 8,785 (17) | 48,037 (14) | 5,203 (16) | 24,000 (18) | 2,602 (19) | 5,921 (20) | 811 (20) | 484 (16) | 169 (16) |
| Chronic Lung Disease | 122,916 (24) | 10,633 (20) | 80,608 (23) | 6,763 (20) | 33,815 (25) | 2,856 (21) | 7,823 (26) | 859 (21) | 670 (22) | 155 (15) |
| Rheumatologic Disease | 10,304 (2) | 859 (2) | 6,896 (2) | 546 (2) | 2,839 (2) | 235 (2) | 538 (2) | 65 (2) | 31 (1) | 13 (1) |
| Peptic ulcer Disease | 13,759 (3) | 1,591 (3) | 8,946 (3) | 1,018 (3) | 3,825 (3) | 427 (3) | 906(3) | 122(3) | 82 (3) | 24 (2) |
| Liver disease | 3,831 (0.7) | 445 (0.8) | 2418 (0.7) | 271 (0.8) | 1096 (0.8) | 120 (0.9) | 290 (1.0) | 45 (1.1) | 27 (0.9) | 9 (0.9) |
| Malignancy | 91,503 (18) | 11,659 (22) | 59,359 (17) | 7,242 (22) | 25,820 (19) | 3,286 (24) | 5,815 (19) | 955 (23) | 509 (17) | 176 (17) |
| AIDS/HIV | 597 (0.1) | 518 (1) | 459 (0.1) | 313 (1) | 109 (0.1) | 126 (1) | 24 (0.1) | 39 (1) | 5 (0.2) | 40 (4) |
| Charlson index | 3.8±1.8 | 4.2±2.1 | 3.6±1.7 | 4.0±2.0 | 4.0±1.9 | 4.5±2.1 | 4.4±2.1 | 4.8±2.2 | 4.4±2.0 | 4.5±2.1 |
| eGFR (ml/min/1.73m2) | 47.6±9.9 | 46.4±11.4 | 53.3±4.2 | 53.4±4.3 | 38.9±4.1 | 38.8±4.2 | 24.8±3.9 | 24.3±4.1 | 11.0±2.9 | 9.6±3.3 |
| WBC count (10^3/µL) | 7.5±4.2 | 6.6±4.4 | 7.4±4.1 | 6.5±3.7 | 7.6±4.6 | 6.8±5.7 | 7.7±4.3 | 6.9±4.3 | 7.6±2.4 | 6.8±3.4 |
| Cholesterol (mg/dl) | 168±38 | 174±41 | 170±37 | 176±40 | 166±38 | 172±42 | 163±41 | 170±44 | 158±43 | 165±42 |
| Bicarbonate (mEq/L) | 27.3±3.0 | 26.8±3.3 | 27.6±2.8 | 27.4±3.0 | 26.9±3.1 | 26.3±3.3 | 25.7±3.4 | 24.9±3.4 | 24.8±4.4 | 25.7±5.0 |
| Calcium (mg/dl) | 9.3±0.5 | 9.3±0.5 | 9.3±0.5 | 9.3±0.5 | 9.3±0.5 | 9.2±0.5 | 9.1±0.5 | 9.1±0.6 | 9.0±0.7 | 9.0±0.8 |
| Hemoglobin (g/dl) | 13.9±1.7 | 13.0±1.7 | 14.2±1.6 | 13.3±1.7 | 13.5±1.7 | 12.7±1.7 | 12.7±1.7 | 11.9±1.7 | 12.2±1.6 | 12.0±1.8 |
| Albumin (g/dl) | 4.0±0.4 | 3.9±0.5 | 4.0±0.4 | 3.9±0.4 | 3.9±0.4 | 3.8±0.5 | 3.9±0.4 | 3.7±0.5 | 3.7±0.5 | 3.7±0.5 |
| ALP (U/l) | 78±33 | 85±44 | 77±31 | 82±42 | 81±35 | 87±45 | 87±39 | 93±48 | 96±56 | 101±60 |
NOTE: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation. CKD stages 3a, 3b, 4 and 5 include patients with estimated glomerular filtration rates of 45–59.9, 30–44.9, 15–29.9 and <15 ml/min/1.73 m2, respectively.
Conversion factors for units: serum total cholesterol in mg/dL to mmol/L, ×0.02586; serum calcium in mg/dl to mmol/L, ×0.2495
ALP, Alkaline phosphatase ; CBVD, Cerebrovascular disease; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; PVD, peripheral vascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; WBC, white blood cell count; HIV, human immunodeficiency virus
Mortality
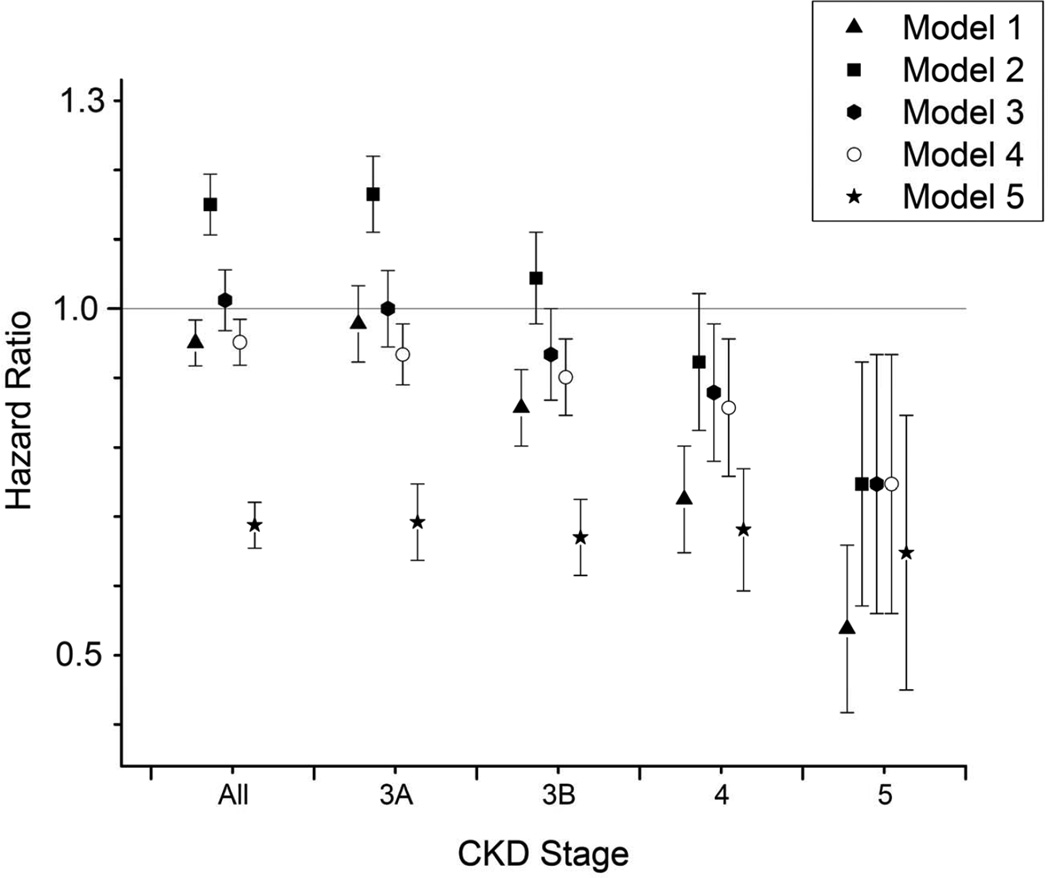
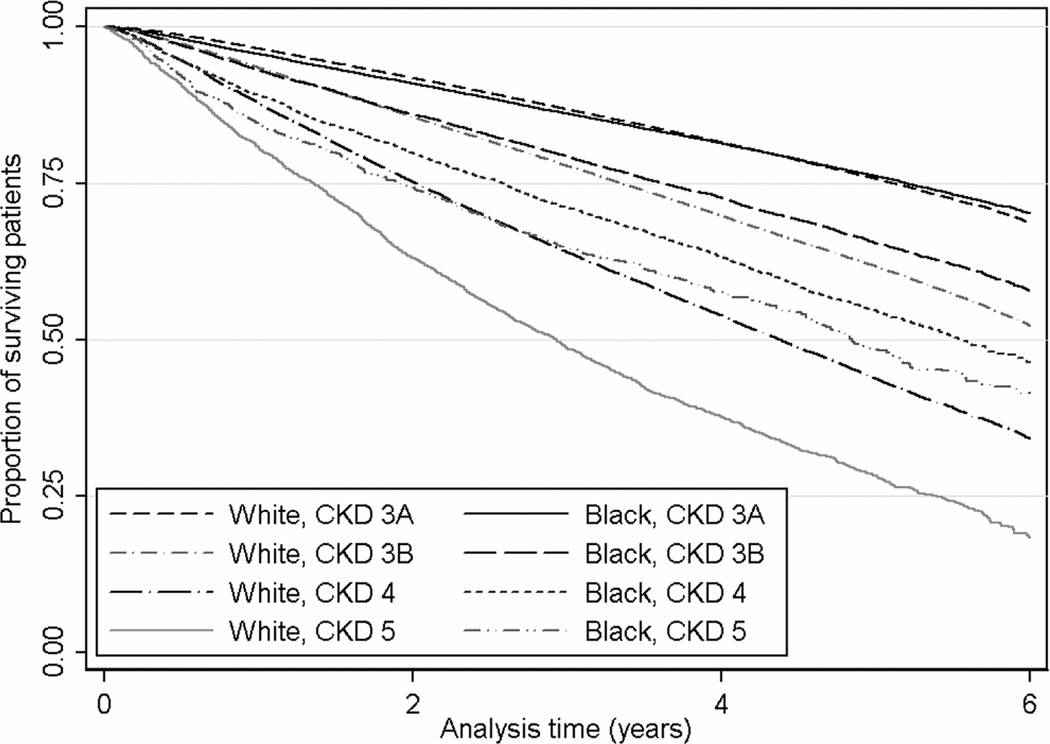
A total of 172,093 patients died (mortality rate, 71.0 [95% CI, 70.6–71.3] per 1,000 patient-years) during a median follow-up of 4.7 years. There were 157,006 deaths in white patients (mortality rate, 71.3 [95% CI, 70.9–71.6] per 1000 patient-years) and 15,087 deaths in black patients (mortality rate, 68.1 [95% CI, 67.0–69.2] per 1000 patient-years). Compared to whites, black patients had an overall lower mortality, with a crude mortality HR of 0.95 (95% CI, 0.94–0.97; p<0.001; Figures 1 and 2). Adjustment for age reversed the survival advantage of blacks (HR, 1.14; 95% CI, 1.12–1.16; p<0.001), but further adjustment for other differences in sociodemographic characteristics, comorbidities and laboratory findings resulted in a significant reduction in mortality risk associated with black race (HR, 0.72; 95% CI, 0.70–0.73). The crude survival advantage of black patients was least present in patients with CKD stage 3a (eGFR, 45–59 ml/min/1.73m2) and increased linearly with CKD stages 3b through 5 (Figures 2 and 3). Adjustment for age and other case-mix and laboratory differences had a similar effect on the black-white survival difference in patients with various stages of CKD as it did in the overall population, and resulted in a relatively uniform multivariable-adjusted survival advantage of blacks in all CKD stages (Figure 2).
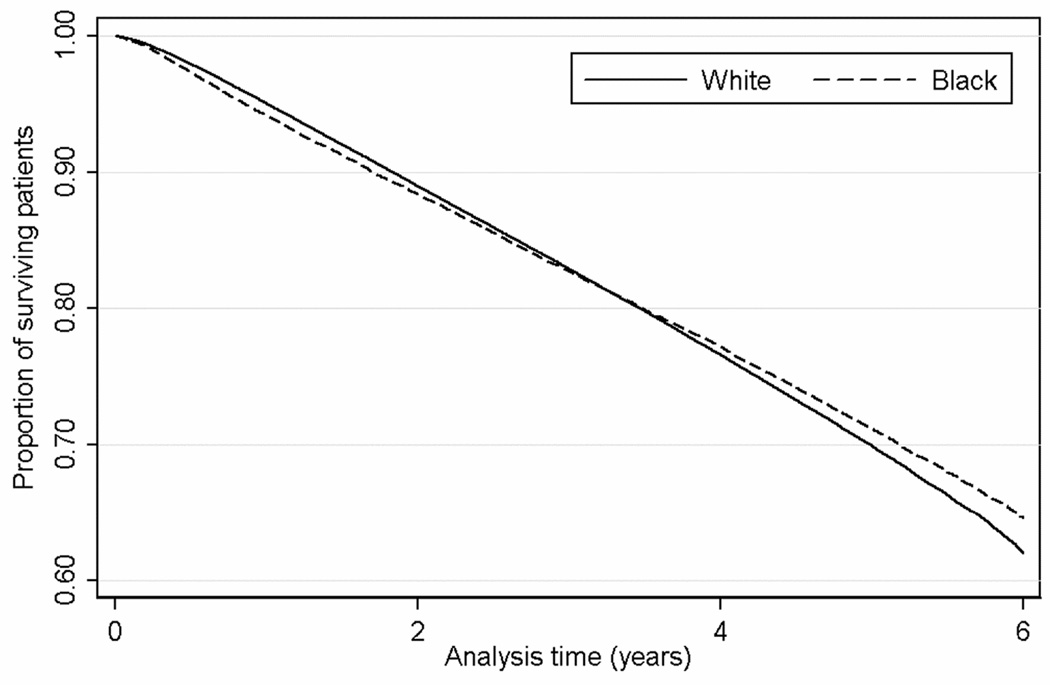
Figure 1.
Kaplan-Meier survival curves of black and white patients in the entire cohort of 570,808 patients.
Figure 2.
Overall and CKD stage-specific hazard ratios (95% confidence intervals) of allcause mortality associated with black race in unadjusted survival models (Model 1) and after adjustment for age (Model 2), age + gender + marital and insurance status + geographic region (Model 3), model 3 variables + blood pressure + comorbidities (diabetes mellitus, cardiovascular disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, Charlson comorbidity index; Model 4), and model 4 variables + laboratory variables (eGFR, serum albumin, cholesterol, hemoglobin, white blood cell count and serum alkaline phosphatase; Model 5).
Figure 3.
Kaplan-Meier survival curves of black and white patients in subgroups of patients with CKD stages 3 through 5 at baseline. CKD stages 3a, 3b, 4 and 5 include patients with estimated glomerular filtration rates of 45–59.9, 30–44.9, 15–29.9 and <15 ml/min/1.73m2 respectively.
Mortality appeared to be higher in black patients than white patients at the beginning of the follow-up, with a reversal of this trend later on (Figure 1). Crude 1-year mortality hazard ratios associated with black race were higher during the first year of follow-up (HR, 1.19; 95% CI, 1.14–1.23; p<0.001) and became progressively lower for each subsequent year of follow-up (Table 2). Adjustment of the time-stratified models for case-mix and laboratory characteristics resulted in an attenuation of the differences seen with length of follow-up, with black race showing a significant association with survival advantage throughout the entire follow-up period. The same trends were apparent when examining 1-year mortality rates in patients stratified by their baseline CKD stage (Table 2).
Table 2.
One-year unadjusted and multivariable adjusted mortality hazard ratios associated with black vs. white race
| 1 y | 2 y | 3 y | 4 y | 5 y | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| unadjusted | adjusted | unadjusted | adjusted | unadjusted | adjusted | unadjusted | adjusted | unadjusted | adjusted | |
| Overall | 1.19 (1.14–1.23) | 0.77(0.73–0.81) | 0.97 (0.94–1.01) | 0.70 (0.67–0.73) | 0.93 (0.90–0.97) | 0.71 (0.68–0.74) | 0.93 (0.89–0.97) | 0.74 (0.70–0.77) | 0.99 (0.93–1.05) | 0.88 (0.82–0.94) |
| CKD stage | ||||||||||
| 3a | 1.25 (1.19–1.32) | 0.81 (0.75–0.88) | 1.01 (0.96–1.07) | 0.73 (0.68–0.78) | 0.98 (0.93–1.03) | 0.71 (0.67–0.76) | 0.93 (0.88–0.98) | 0.72 (0.68–0.77) | 1.03 (0.95–1.11) | 0.88 (0.81–0.97) |
| 3b | 1.06 (0.99–1.13) | 0.72 (0.65–0.79) | 0.91 (0.86–0.98) | 0.68 (0.62–0.74) | 0.85 (0.79–0.91) | 0.69 (0.63–0.75) | 0.90 (0.83–0.97) | 0.74 (0.68–0.81) | 0.86 (0.77–0.96) | 0.81 (0.71–0.92) |
| 4 | 0.90 (0.81–0.99) | 0.76 (0.66–0.87) | 0.73 (0.66–0.81) | 0.68 (0.59–0.78) | 0.71 (0.63–0.79) | 0.66 (0.58–0.76) | 0.80 (0.70–0.91) | 0.79 (0.69–0.92) | 0.96 (0.79–1.17) | 1.01 (0.81–1.27) |
| 5 | 0.79 (0.66–0.94) | 0.78 (0.6–1.02) | 0.51 (0.42–0.63) | 0.63 (0.47–0.83) | 0.55 (0.55–0.68) | 0.78 (0.59–1.03) | 0.47 (0.36–0.62) | 0.52 (0.36–0.75) | 0.70 (0.48–1.03) | 0.83 (0.48–1.44) |
NOTE: Values shown in parentheses are 95% confidence intervals. Associations in the first, second, third, fourth and fifth year of follow-up, conditional on surviving to the beginning of the examined year, overall and in subgroups of patients with different stages of CKD at baseline. CKD stages 3a, 3b, 4 and 5 include patients with estimated glomerular filtration rates of 45–59.9, 30–44.9, 15–29.9, and <15 ml/min/1.73 m2, respectively.
CKD, chronic kidney disease.
DISCUSSION
We examined the all-cause mortality of black vs. white patients with non-dialysis dependent CKD in a large, contemporary and nationally representative cohort of male US veterans. Similar to earlier reports in patients on maintenance dialysis6–13 we found that overall black patients had significantly lower all-cause mortality compared to white patients. The mortality-difference was affected by the length of follow-up and the severity of kidney disease, as blacks tended to have higher mortality in those with earlier stages of CKD and in the first year of follow-up. These differences appeared to be mediated by differences in baseline sociodemographic, comorbidity, and laboratory measures between blacks and whites, as adjustments for these differences resulted in a uniform survival advantage of blacks irrespective of baseline CKD stage or length of follow-up. That differences in baseline characteristics between blacks and whites were gradually magnified in patients with more advanced stages of CKD also suggests that the evolution of race-associated survival differences over the course of CKD is influenced by changes in patient characteristics. A major factor affecting survival differences was age, in that black patients were significantly younger than white patients, which could have explained a survival advantage in blacks. Also portending lower mortality risk in blacks were their lower cardiovascular disease prevalence and lower WBC count. Other characteristics of black patients, however, portended worse survival (such as their lower prevalence of health insurance, higher prevalence of diabetes mellitus, higher Charlson comorbidity score and several biochemical characteristics). The observed differences in race-related patient characteristics and their evolution during the course of advancing CKD may be related to complex social and biologic differences between blacks and whites, and indicate ample opportunities for further research and for interventions aimed at alleviating racial discrepancies in this patient population. Nevertheless, it is interesting that while the marked differences in a variety of patient characteristics was able to explain variability in black-race survival difference associated with CKD stage and length of follow-up, they did not explain the overall survival advantage seen in blacks, which remained significant even after full multivariable adjustment for various traditional and novel mortality risk factors. It is possible that race-dependent differences in the etiology of CKD (such as the recently described mutations in the APOL1 (apolipoprotein L1) gene in blacks40–43) results in a different predisposition to mortality in black and white patients with CKD that is independent of most known traditional and CKDassociated novel risk factors. More research in this area is needed to determine the validity of this hypothesis.
Mortality differences in black vs. white patients with non-dialysis dependent CKD have been examined in a number of previous studies. A lower mortality rate in blacks with CKD was described in some of these studies,18–21 but others failed to describe similar findings.5;22;23 The reason for this discrepancy may have been the predominance of patients with mild CKD in some of the latter studies.22;23 Another study by Choi et al using national VA data reported higher age-adjusted mortality in blacks of all stages of CKD,5 but without describing unadjusted hazard ratios associated with black race, hence it is unclear if our study supports or refutes these results. Some of the hypotheses tested by us (that race-associated differences in outcomes vary by CKD stage and duration of follow-up) were also examined in a study by Derose et al which examined end stage renal disease incidence and pre-dialysis mortality in patients with CKD stages 3 and 4 enrolled in a managed care organization in Southern California.18 This study also described a reversal in the risk of mortality associated with black race depending on baseline kidney function, with higher pre-dialysis mortality in black patients with CKD stage 3a and incrementally lower mortality in those with lower kidney function, and reported a relative change in the ESRD-death ratio before dialysis depending on length of follow-up, but without analyzing the effect of longer follow-up on mortality alone and without exploring the role of patient characteristics in the observed mortality-differences. Our study extends these findings by analyzing a US-wide cohort, by examining mortality hazards unaffected by the competing end point of end stage renal disease, and by examining characteristics that could potentially explain the selection of a black cohort with better survival.
Our study is notable for its very large sample size and event numbers, and for it being nationally representative of veterans in the entire geographic United States. Furthermore, in the VA system, enrollment is not hindered by socioeconomic disadvantages and hence it is less likely that observed racial differences in survival are due to such factors. Our study also has limitations that need to be mentioned. Our study population consisted of male patients; hence the results may not apply to females. We used estimated GFR based on the CKD-EPI creatinine equation to define CKD, which uses a correction factor for African American race. This correction factor was suggested to be too high in patients with normal kidney function (GFR >60 ml/min/1.73 m2) and hence this formula may underestimate the true prevalence of CKD in the African American population.44 We used information obtained during the course of clinical practice to define and to characterize our cohort, hence selection bias is possible. However, the key laboratory variable used for cohort definition (serum creatinine) is part of routine panels that are measured in most patients receiving healthcare, hence it is unlikely that a significant proportion of actively enrolled veterans would have been excluded.
We describe significantly lower mortality associated with black race in a large cohort of US veterans with non-dialysis dependent CKD. Racial differences in crude mortality rates were affected by the severity of kidney disease and length of follow-up, largely due to differences in demographic, comorbidity and laboratory characteristics between blacks and whites linked to these factors. Adjustment for the many significant differences between black and white patients did not attenuate racial mortality differences, but rather amplified those, suggesting that other factors lie at the core of the African-American survival paradox in CKD and ESRD. Future studies examining the general population, or studies with more detailed information on other relevant patient characteristics (such as race-specific genetic polymorphisms associated with CKD in blacks40–43) may be able to further expand our knowledge in this field.
Supplementary Material
Acknowledgements
CPK and EHL are employees of the US Department of Veterans Affairs (VA). The views expressed in this work are those of the authors and do not necessarily represent the views of the VA.
Support: This study was supported by grant 1R01DK078106-01 from the National Institute of Diabetes and Digestive and Kidney Diseases to CPK and KKZ, and by resources from the VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Parts of this study were presented at the American Society of Nephrology Renal Week 2011, November 8–13, 2011, Philadelphia, PA.
Supplementary Material
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery
Hyperlink: Supplementary Table S1 (PDF)
About: Mean differences in baseline characteristics of black vs white patients.
References
- 1.Easterling RE. Racial factors in the incidence and causation of end-stage renal disease (ESRD) Trans Am Soc Artif Intern Organs. 1977;23:28–33. doi: 10.1097/00002480-197700230-00008. [DOI] [PubMed] [Google Scholar]
- 2.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982;306(21):1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 3.Krop JS, Coresh J, Chambless LE, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159(15):1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 5.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleyer AJ, Tell GS, Evans GW, Ettinger WH, Jr, Burkart JM. Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis. 1996;28(1):72–81. doi: 10.1016/s0272-6386(96)90133-x. [DOI] [PubMed] [Google Scholar]
- 8.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol. 1994;5(5):1231–1242. doi: 10.1681/ASN.V551231. [DOI] [PubMed] [Google Scholar]
- 9.Cowie CC, Port FK, Rust KF, Harris MI. Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care. 1994;17(7):681–687. doi: 10.2337/diacare.17.7.681. [DOI] [PubMed] [Google Scholar]
- 10.Morris D, Samore MH, Pappas LM, Ramkumar N, Beddhu S. Nutrition and racial differences in cardiovascular events and survival in elderly dialysis patients. Am J Med. 2005;118(6):671–675. doi: 10.1016/j.amjmed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Owen WF, Jr, Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998;280(20):1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 12.Pugh JA, Tuley MR, Basu S. Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: the emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis. 1994;23(6):803–807. doi: 10.1016/s0272-6386(12)80133-8. [DOI] [PubMed] [Google Scholar]
- 13.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006;17(10):2910–2918. doi: 10.1681/ASN.2005101078. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(9):493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 15.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 16.Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine RS, Foster JE, Fullilove RE, et al. Black-white inequalities in mortality and life expectancy: 1933–1999: implications for healthy people 2010. Public Health Rep. 2001;116(5):474–483. doi: 10.1093/phr/116.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derose SF, Rutkowski MP, Levin NW, et al. Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int. 2009;76(6):629–637. doi: 10.1038/ki.2009.209. [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(5):973–978. doi: 10.2215/CJN.06031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG. Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol. 2006;1(5):993–999. doi: 10.2215/CJN.01251005. [DOI] [PubMed] [Google Scholar]
- 21.Smith GL, Shlipak MG, Havranek EP, et al. Race and renal impairment in heart failure: mortality in blacks versus whites. Circulation. 2005;111(10):1270–1277. doi: 10.1161/01.CIR.0000158131.78881.D5. [DOI] [PubMed] [Google Scholar]
- 22.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 25.VIReC Research User Guide. Veterans Health Administration Decision Support System Clinical National Data Extracts 2nd Edition. Hines, IL: U. S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2009. Sep 1, [Google Scholar]
- 26.Kovesdy CP, Lott EH, Lu JL, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125(5):677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Veterans Affairs VA Information Resource Center Data Quality Update: Race. 2009. [Google Scholar]
- 29.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21(4):107–116. [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn MW, Zhang H, Arnold N, et al. Transition to the new race/ethnicity data collection standards in the Department of Veterans Affairs. Popul Health Metr. 2006;4:7. doi: 10.1186/1478-7954-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBean AM. Improving Medicare's data on race and ethnicity. Medicare Brief. 2006;(15):1–7. [PubMed] [Google Scholar]
- 32.VIReC Research User Guide. VHA Medical SAS Inpatient Datasets FY2006. 2007. Hines, IL: U.S. Department of Veterans Affairs. VA Information Resource Center; 2007. Sep, [Google Scholar]
- 33.VIReC Research User Guide. VHA Medical SAS Outpatient Datasets FY2006. 2007. Hines, IL: U.S. Department of Veterans Affairs. VA Information Resource Center; 2007. Sep, [Google Scholar]
- 34.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 35.Armitage P, Berry G. Statistical methods in medical research. 3rd ed. London: Blackwell; 1994. Comparison of two means; pp. 102–111. [Google Scholar]
- 36.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Arnold N, Sohn M, Maynard C, Hynes DM. Edward Hines, Jr. VA Hospital. Hines, IL: VA Information ResourceCenter; 2006. Sep 4, VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. [Google Scholar]
- 38.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 39.Dekker FW, de MR, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: timedependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 40.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostrom MA, Lu L, Chou J, et al. Candidate genes for non-diabetic ESRD in African Americans: a genome-wide association study using pooled DNA. Hum Genet. 2010;128(2):195–204. doi: 10.1007/s00439-010-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman BI, Parekh RS, Kao WH. Genetic basis of nondiabetic end-stage renal disease. Semin Nephrol. 2010;30(2):101–110. doi: 10.1016/j.semnephrol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delanaye P, Mariat C, Maillard N, Krzesinski JM, Cavalier E. Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol. 2011;6(4):906–912. doi: 10.2215/CJN.10931210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.