Abstract
Background
Few studies have examined the association between hypertension and mercury exposure in the general population. We examined cross-sectional associations between blood (mainly methylmercury) or urinary mercury (mainly inorganic mercury) and hypertension in representative U.S. adults and effect modifications by dietary omega-3 fatty acids and serum selenium.
Methods
We examined 6,607 adults aged 20 years or older, using the National Health and Nutrition Examination Survey (NHANES) from 2003/2004 to 2005/2006 (2,201 adults were available for urinary mercury from NHANES 2003–2006; 2,117 available for serum selenium from NHANES 2003–2004 aged 40 years or older). The average of omega-3 fatty acids from two 24-hour recalls was calculated.
Results
The weighted prevalence of hypertension was 32.2%. The geometric means (95% confidence intervals) of blood total and urinary mercury were 1.03 (0.95, 1.11) µg/L and 0.51 (0.47, 0.54) µg/L, respectively. The adjusted odds ratios for a doubling increase in blood mercury and urinary mercury were 0.94 (0.87 to 1.01) and 0.87 (0.78 to 0.99), respectively, after adjusting for potential confounders. The associations remained similar, even after adjusting for either omega-3 fatty acids or selenium or both. No significant effect modification by either omega-3 fatty acids or selenium was observed.
Conclusions
In this cross-sectional study of the U.S. general population, we found no association of hypertension with blood mercury but a suggestive inverse association with urinary mercury. Future prospective studies are warranted to confirm these findings.
Keywords: blood pressure, hypertension, mercury, omega-3 fatty acids, selenium
INTRODUCTION
Cardiovascular disease is the number one cause of mortality and morbidity in the U.S. and the most of the world. Hypertension is one of the most important risk factors for cardiovascular disease (Linne et al., 2004). According to data from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2000, one in three U.S. adults (≥18 years of age) suffers from hypertension (Fields et al., 2004). While obesity, physical inactivity, and cigarette smoking are well-established risk factors for hypertension, potential impacts of environmental pollutants have gained little attention.
Mercury is a cardio-toxic metal. Elevated mercury exposure from fish intake – mainly methylmercury from large or long-lived fish, such as, shark, swordfish, and albacore white tuna (FDA, 2004; Park and Mozaffarian, 2010)– is of concern, although omega-3 fatty acids from oily fish intake is associated with anti-hypertensive effects (Appel et al., 1993; Geleijnse et al., 2002; Morris et al., 1993), which may reduce the burden of hypertension in the general population. Methylmercury is considered to be more toxic than inorganic mercury, because of its ability to cross biological membranes, such as the blood brain barrier and placenta, thus rendering fetuses of pregnant women susceptible to intoxication (Aschner and Aschner, 1990; 2004; Mahaffey, 1999).
Studies concerning mercury exposure and hypertension risk have yielded inconsistent results. A study on Nunavik Inuit found a significant positive association between blood mercury and systolic blood pressure (SBP) (Valera et al., 2009). Another study in the Brazilian Amazon showed a significant positive association between SBP (≥130mmHg) and hair total mercury (odds ratio (OR)=2.91, 95% confidence interval (CI), 1.26–7.28) but not with fish intake (Fillion et al., 2006). In a Japanese study designed to assess the effects of long-term methylmercury exposure on hypertension, the residents of Minamata, as compared to those of a neighboring control area, showed higher prevalence of hypertension (OR=1.6, 95% CI, 1.2–2.1) (Yorifuji et al., 2010). Omega-3 fatty acids may negatively confound or modify the association between methylmercury and hypertension because these fatty acids are derived from fish consumption and are known to have cardio-protective properties (Guallar et al., 2002). For example, Guallar and colleagues showed the increased odds ratios associated with toenail mercury on myocardial infarction after controlling for omega-3 fatty acid and cardiovascular risk factors (Guallar et al., 2002). In a study of women aged 16 to 49 from NHANES 1999–2000 dataset, no association between blood mercury (median 0.9 µg/L) and hypertension was found (Vupputuri et al., 2005). When individuals were stratified according to fish consumption, a significant positive association between SBP and blood mercury was observed among non-fish consumers (median 0.5 µg/L) (β=1.83 mmHg/(1.3 µg Hg/L), 95% CI, 0.36–3.30) but not among fish-consumers (median 1.2 µg/L) (Vupputuri et al., 2005). Another national study based in Korea failed to find an association between blood mercury and the prevalence of hypertension (Lee and Kim, 2011). Selenium, another possible negative confounder found in fish, antagonizes the toxicity of mercury via multiple mechanisms such as formation of inert mercury-selenium compounds and antioxidant selenoenzymes and inhibition of methyl radicals from methylmercury (Ganther et al., 1972; Ikemoto et al., 2004). A recent study documented that reduction in hypertension associated with omega-3 fatty acid was greater among individuals with high selenium and low mercury levels (Xun et al., 2011).
Another source of exposure to mercury in the general population is elemental mercury, a form of inorganic mercury, which comprises about 50% of dental amalgam fillings. Cardiovascular health effects of inorganic mercury are poorly understood, and there is no epidemiologic study that has examined the association between inorganic mercury exposure and hypertension, except for one recent pilot study by us which documented reduced SBP among male dental professionals in association with inorganic mercury exposure (Goodrich, 2012). Such an association is plausible given that several laboratory rodent studies have reported that unlike methylmercury, inorganic mercury exposure causes depressed arterial systolic pressure (Massaroni et al., 1995; Rhee and Choi, 1989; Rossoni et al., 1999).
A recent report about the risk and benefit of fish consumption suggests investigating dietary-based estimates/biomarkers of both omega-3 and mercury exposures together to obtain unconfounded risk and benefit coefficients (Stern and Korn, 2011). In the present study, we examined both dietary omega-3 fatty acids from 24-hour dietary recalls and biomarkers of mercury exposure from blood (indicates both methylmercury and inorganic mercury exposure) and urinary mercury (indicates mainly inorganic mercury exposure) in representative U.S. adults. The purpose of the present study was to examine whether different biomarkers of mercury exposure are associated with hypertension in U.S. adults aged 20 years or older using data from NHANES 2003–2006. In addition, we explored whether dietary omega-3 fatty acids and serum selenium negatively confounded and modified the association between both biomarkers of mercury exposure and hypertension.
METHODS
Study population
The NHANES utilizes a stratified multistage probability cluster design with oversampling to estimate representative measurements in the general population. For this study, two NHANES survey cycles (2003–2004 and 2005–2006) were combined. Eligible study participants were 10,020 adults aged 20 or older who completed physical examinations including blood pressure measures, questionnaires, 24-hour dietary recall interviews and laboratory analyses for blood mercury and/or urine mercury. We excluded 1130 missing or with only the first measure on blood pressure, 48 missing on hypertension medication, 986 missing blood mercury values, 893 who did not complete two 24-hour dietary recall interviews, and 356 missing other important covariates. These exclusions resulted in the final sample size to 6,607 adults. Urinary mercury was measured in about one-third of the NHANES 2003–2006 participants (NCHS, 2008b). Available urine mercury data were from 2,201 participants. Serum selenium was available in the NHANES 2003–2004 participants aged 40 years or older. Therefore, the analyses considering selenium as either a confounder or an effect modifier were restricted to 2,117 participants. The Institutional Review Board for the National Center for Health Statistics approved the survey protocols of NHANES 2003–2004 and 2005–2006.
Blood pressure measurements
Blood pressure was measured by certified examiners with standardized protocols as a part of physical examination interviews at the mobile examination center (MEC) (Ostchega et al., 2003). After sitting quietly for five minutes, certified examiners measured participant’s blood pressure three (sometimes four) consecutive times. We calculated means of SBP and diastolic blood pressure (DBP) by averaging up to three measures after disregarding the first reading. Hypertension was defined as SBP/DBP ≥ 140/90 mmHg, self-reported physician diagnosis of hypertension, or self-reported use of hypertension medication.
Measurements of blood mercury and urinary mercury
A certified phlebotomist performed venipuncture. All laboratory data were standardized under the NHANES quality control and assurance (QA/QC) protocols (NCHS, 2006; NCHS, 2008a). Some measurements below the detection limit were reported with values calculated to the detection limit divided by the square root of two.
Inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer ELAN 6100, Waltham, MA) was used to measure whole blood mercury in the NHANES 2003–2006 (Guo and Baasner, 1993; NCHS, 2008a). The lower limit of detection (LLOD) for blood mercury was 0.14 µg/L in the NHANES 2003–2006 (Jones, 2004b; Jones, 2004c). The percentages below LLOD were 12.1% (1017/8373) for NHANES 2003–2004 and 25.4% (2140/8407) for NHANES 2005–2006. For these participants, a value equal to the LLOD divided by the square-root of two was replaced.
In one-third of the total survey of participants aged six or older (NCHS, 2008b), urinary mercury was measured by flow injection cold vapor atomic absorption (CVAA) analysis for the NHANES 2003–2004 (Jones, 2004a), and by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectroscopy (ICP-DRC-MS) for the NHANES 2005–2006 (Jones, 2006). The LLOD were 0.11 µg/L in NHANES 2003–2004 and 0.06 µg/L in NHANES 2005–2006 (Jones, 2004a; Jones, 2006). The percentages below LLOD were 19.0% (483/2538) in NHANES 2003–2004 and 7.4% (191/2578) in NHANES 2005–2006. The standard protocol for quality control was conducted.
Omega-3 fatty acids, serum selenium, and fish intake
We examined dietary fish intake from two 24-hour recall. At the MEC, trained dietary interviewers performed an in-person interview using computer-assisted dietary interview software. About 3–10 days after the first interview, the second 24-hour recall interview was conducted by telephone with an automated dietary interview system. The 24-hour recall data were coded utilizing a U.S. Department of Agriculture food code (USDA) Automated Multiple-Pass Method (AMPM) (NCHS, 2007a). For the present study, the total amount of omega-3 fatty acid intake was calculated as the sum of eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3).
Serum selenium was only available in participants aged 40 years or older of the NHANES 2003–2004 (NCHS, 2007b). Serum specimens drawn from the participants were stored at 4oC and shipped to the Trace Elements Laboratory, Wadsworth Center, at the New York State Department of Health. ICP-DRC-MS was used to determine serum selenium concentrations. The between-assay coefficients of variations of selenium ranged from 2.5% to 2.9%. Several quality control schemes were used.
Potential confounders
Demographic, behavioral, and laboratory data were obtained by self-reported questionnaire, computer-assisted personal interview, and physical examination. The following covariates were considered as potential confounding factors: age (years, continuous); gender (male/female); race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other); education (<high school, high school diploma, some college and over); body mass index (BMI, kg/m2, continuous); alcohol usage (yes/no); serum cotinine (µg/L, continuous); urinary creatinine (mg/dL, continuous); and total calorie intake. Isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry was utilized to measure serum cotinine. Urinary creatinine was utilized in order to adjust the urinary dilution of spot urine samples, measured by a Jaffe rate reaction with a Beckman Synchron Analyzer in the NHANES 2003–2006. Creatinine in urine was missing in 3.1% (243/7982) of the 2003–2004 survey and 3.0% (242/8086) of the 2005–2006 survey.
Statistical analysis
NHANES used a complex, stratified, multistage cluster sampling design to obtain a nationally representative sample of the non-institutionalized US civilian population. To account for this design effect, the survey procedures in SAS statistical software (version 9.2; SAS Institute Inc., Cary, NC) were utilized. Two waves of continuous NHANES 2003–2004 and 2005–2006 surveys were combined and four-year sampling weights were calculated. Because dietary recalls were used as inclusion criteria and urinary mercury was measured in a subsample (one third), dietary two-day sample weight (WTDR2D) and the MEC weights of subsample A (WTSA2YR) were used for blood mercury and urine mercury, respectively. Because the distributions of blood mercury and urinary mercury were highly skewed, we computed geometric means and 95% CIs. We constructed multivariate logistic regression models to estimate ORs for the risk of hypertension associated with blood mercury or urinary mercury, after adjusting for the following covariates: model 1 adjusted for age, gender, and race/ethnicity; model 2 additionally adjusted for education, BMI, alcohol usage, and cotinine; model 3 further adjusted for omega-3 fatty acids and total caloric intake. We used serum cotinine levels instead of other smoking variables to control for potential effects of cigarette smoking because of better goodness-of-fit of models. Urinary mercury models included an additional adjustment for urinary creatinine (logtransformed) to account for dilution in spot urine samples. Blood mercury and urinary mercury were log-transformed and fit as a continuous variable in each model in order to better handle outliers (supplemental figure). We also examined these variables in quartiles to capture nonlinear relationships. To examine the association of serum selenium, we conducted multivariate logistic regression models as follow; Model 1 was adjusted for age, gender, race/ethnicity, education, body mass index, alcohol usage, and cotinine (for urinary mercury, log-transformed urinary creatinine added): Model 2 was further adjusted for selenium: Model 3 was additionally adjusted for omega-3 fatty acids, and total caloric intake. We computed generalized variance inflation factors to assess multicollinearity among mercury biomarker, selenium, and omega-3 fatty acids as those variables were assumed to be correlated. We performed a sensitivity analysis by replacing omega-3 fatty acids from 24-hour dietary recalls with total fish intake from food frequency questionnaires (FFQ), but the results were similar, therefore, we only presented the results using omega-3 fatty acids in this paper. Also, we examined if SBP and DBP have the same trend of associations with blood and urinary mercury exposure. Finally, we examined effect modification by omega-3 fatty acids and serum selenium that were categorized into quartiles as well as age (20–39, 40–59, 60+ years) and gender. To evaluate effect modification, multiplicative interaction terms along with the main effects were included in regression models. We also ran separate regression models stratified by each effect modifier.
RESULTS
General characteristics of the study population are presented in Table 1. The average age of the study participants was 46.6 (SE=0.5) years. The weighted prevalence of hypertension was 32.2%, with 2,447 subjects classified as hypertensive among 6,607 study participants. As compared to those without hypertension, subjects with hypertension were older and had higher BMI, but they currently drank less alcohol and smoked less. As for the mercury exposure levels, blood mercury levels were not different between participants with and without hypertension. Although urinary mercury was lower in subjects with hypertension, this difference disappeared after urinary creatinine was considered. The geometric means (95% CI) of blood mercury and urinary mercury in participants with hypertension were 1.05 (0.94, 1.16) µg/L and 0.44 (0.39, 0.48) µg/L, respectively, as compared to 1.02 µg/L and 0.55 µg/L in those without hypertension. No differences in fish intake frequencies, estimated levels of omega-3 fatty acids, and serum selenium levels were observed between those adults with and without hypertension.
Table 1.
Characteristics of the study population from the NHANES) 2003–2006 (n=6,607)
| Hypertension | |||||
|---|---|---|---|---|---|
| Total (n=6,607) |
No (n=4,160) (67.8%) |
Yes (n=2,447) (32.2%) |
p-value | ||
| Age (years) a | 46.6±0.50 | 40.7±0.45 | 58.4±0.62 | <0.0001 | |
| Female (%) | 51.4 | 51.3 | 51.8 | 0.7536 | |
| Race/ethnicity (%) | |||||
| Non-Hispanic White | 75.2 | 74.2 | 77.2 | ||
| Non-Hispanic Black | 10.3 | 9.2 | 12.5 | <0.0001 | |
| Mexican American | 7.5 | 9.1 | 4.4 | ||
| Other Hispanic | 2.6 | 3.0 | 1.7 | ||
| Others | 4.5 | 4.6 | 4.2 | ||
| Education (%) | |||||
| Less than high school | 15.7 | 13.5 | 20.0 | <0.0001 | |
| High school | 25.1 | 23.8 | 27.5 | ||
| Greater than high school | 59.3 | 62.6 | 52.5 | ||
| Body mass index (kg/m2) a | 28.3±0.19 | 27.3±0.17 | 30.3±0.24 | <0.0001 | |
| Alcohol usage (%) | 72.0 | 76.3 | 63.4 | <0.0001 | |
| Smoking (%) | |||||
| Never | 50.2 | 52.1 | 46.2 | ||
| Former | 26.2 | 21.5 | 35.6 | <0.0001 | |
| Current | 23.6 | 26.4 | 18.2 | ||
| Cotinine (ng/ mL) b | 0.47 (0.35, 0.62) |
0.56 (0.40, 0.78) |
0.32 (0.24, 0.44) |
0.0004 | |
| Blood mercury (µg/L) b | 1.03 (0.95, 1.11) |
1.02 (0.94, 1.10) |
1.05 (0.94, 1.16) |
0.5060 | |
| Urinary creatinine (mg/dL)bc | 97.5 (93.6, 101) |
102 (96.6, 107) |
89.3 (84.0, 95.0) |
0.0021 | |
| Urinary mercury (ng/ mL) b c | 0.51 (0.47, 0.54) |
0.55 (0.50, 0.59) |
0.44 (0.39, 0.48) |
0.0014 | |
| Creatinine-corrected urinary mercury (ng/mg) b c | 0.52 (0.49, 0.55) |
0.54 (0.50, 0.57) |
0.49 (0.44, 0.54) |
0.0793 | |
| Systolic blood pressure (mmHg) a | 123.2±0.41 | 115.6±0.23 | 138.2±0.59 | <0.0001 | |
| Diastolic blood pressure (mmHg) a | 71.2±0.25 | 69.0±0.23 | 75.7±0.49 | <0.0001 | |
| Hypertensive medication (%) | 24.3 | - | 72.9 | - | |
| Fish intake frequency (servings per week) | |||||
| <1 | 62.1 | 61.8 | 62.6 | 0.6985 | |
| 1–2 | 20.1 | 20.5 | 19.2 | ||
| ≥2 | 17.8 | 17.7 | 18.1 | ||
| Omega-3 fatty acids (gm/day) | |||||
| (quartile) | 0 (0–0.0155) | 27.0 | 27.7 | 25.6 | |
| 1 (0.0155–0.0410) | 23.8 | 23.5 | 24.3 | 0.1660 | |
| 2 (0.0410–0.1075) | 24.4 | 25.0 | 23.3 | ||
| 3 (0.1075–4.7775) | 24.8 | 23.8 | 26.8 | ||
| Serum selenium (µg/L) a d | 137.2±1.39 | 136.3±1.4 | 138.1±1.6 | 0.1139 | |
Weighted mean±standard error
Geometric mean (95% confidence limit)
Urinary mercury data are available in one-third of the survey population (n=2,201) where urinary creatinine and creatinine-corrected urinary mercury were calculated.
p-value from the Rao-Scott log-likelihood ratio test (continuous variables) or the Rao-Scott Chi-square test (categorical variables)
Serum selenium data are only available from the NHANES 2003–2004 survey, including 2,117 participants aged 40 years or older
Hypertension was defined as those individuals who have taken anti-hypertensive medication, have a systolic blood pressure of ≥140 mmHg, or have a diastolic blood pressure of ≥ 90 mmHg
Table 2 lists blood and urinary mercury levels according to different study population characteristics. Participants aged 40 to 59 years had significantly higher levels of blood and urinary mercury than younger or older participants. Men had significantly higher blood mercury levels than women, but there was no gender-related difference in urinary mercury levels. Blood mercury but not urinary mercury exhibited differences according to race/ethnic groups: ‘others (including multi-racial individuals)’ and ‘other Hispanic’ had higher levels of blood mercury than any other ethnic groups. Participants with higher educational levels demonstrated higher levels of blood mercury. While urine mercury levels did not differ among BMI categories, blood mercury was lower in persons with BMI greater than 30 kg/m2. Those who consume alcohol had higher levels of blood total and urinary mercury than non-drinkers. Participants with higher cotinine levels demonstrated lower levels of both biomarkers of blood and urinary mercury. Blood mercury was highly significantly associated with omega-3 fatty acid level, while the relationship with urine mercury was borderline. Blood and urine mercury levels did not differ among quartiles of blood selenium levels.
Table 2.
Mercury exposures according to different characteristics of U.S. adults from the NHANES 2003–2006 (n=6,607)
| Blood mercury (µg/L) (n=6,607) |
Urinary mercury (µg/L) (n=2,201) |
||||||
|---|---|---|---|---|---|---|---|
| WP (%) |
Geometric means | p | WP (%) |
Geometric means | p | ||
| Age (years) | |||||||
| 20–39 | 38.0 | 0.88 (0.79, 0.99) | <0.0001 | 36.0 | 0.52 (0.46, 0.58) | <0.0001 | |
| 40–59 | 38.1 | 1.15 (1.06, 1.24) | 41.5 | 0.57 (0.51, 0.64) | |||
| 60+ | 23.8 | 1.09 (0.98, 1.21) | 22.5 | 0.39 (0.35, 0.43) | |||
| Gender | |||||||
| Male | 48.6 | 1.07 (0.97, 1.16) | 0.0349 | 51.7 | 0.51 (0.48, 0.55) | 0.7324 | |
| Female | 51.4 | 0.99 (0.91, 1.07) | 48.3 | 0.50 (0.45, 0.56) | |||
| Race/ethnicity | |||||||
| Mexican American | 7.5 | 0.69 (0.60, 0.79) | 7.4 | 0.47 (0.39, 0.57) | |||
| Non-Hispanic Black | 10.3 | 1.13 (1.01, 1.27) | 6.7 | 0.54 (0.46, 0.63) | |||
| Non-Hispanic White | 75.2 | 1.02 (0.93, 1.13) | <0.0001 | 76.2 | 0.51 (0.46, 0.56) | 0.5906 | |
| Other Hispanic | 2.6 | 1.23 (0.97, 1.57) | 3.3 | 0.57 (0.39, 0.84) | |||
| Others | 4.5 | 1.52 (1.19, 1.93) | 3.4 | 0.46 (0.37, 0.57) | |||
| Education | |||||||
| Less than high school | 15.7 | 0.77 (0.68, 0.87) | 15.9 | 0.38 (0.33, 0.44) | |||
| High school | 25.1 | 0.82 (0.74, 0.91) | <0.0001 | 26.0 | 0.45 (0.39, 0.52) | <0.0001 | |
| Greater than high school | 59.3 | 1.22 (1.13, 1.31) | 58.1 | 0.58 (0.52, 0.63) | |||
| Body mass index (kg/m2) | |||||||
| <25 | 32.5 | 1.07 (0.97, 1.18) | 31.6 | 0.50 (0.45, 0.57) | |||
| 25–29.9 | 34.7 | 1.10 (1.002, 1.21) | <0.0001 | 36.7 | 0.49 (0.44, 0.55) | 0.7012 | |
| 30+ | 32.8 | 0.91 (0.83, 1.002) | 31.7 | 0.53 (0.47, 0.58) | |||
| Alcohol usage | |||||||
| No | 28.0 | 0.83 (0.76, 0.92) | <0.0001 | 29.1 | 0.42 (0.38, 0.46) | 0.0009 | |
| Yes | 72.0 | 1.11 (1.03, 1.20) | 70.9 | 0.55 (0.50, 0.60) | |||
| Cotinine | |||||||
| Quartile 0 | 24.3 | 1.15 (1.04, 1.28) | 22.1 | 0.56 (0.51, 0.63) | |||
| 1 | 24.5 | 1.23 (1.11, 1.36) | <0.0001 | 25.4 | 0.58 (0.52, 0.63) | 0.0126 | |
| 2 | 23.6 | 0.99 (0.88, 1.11) | 24.9 | 0.46 (0.40, 0.52) | |||
| 3 | 27.6 | 0.81 (0.73, 0.90) | 27.6 | 0.45 (0.40, 0.51) | |||
| Omega-3 fatty acids | |||||||
| Quartile 0 | 27.0 | 0.73 (0.65, 0.80) | 26.3 | 0.48 (0.43, 0.53) | |||
| 1 | 23.8 | 0.90 (0.81, 0.99) | <0.0001 | 24.4 | 0.49 (0.43, 0.56) | 0.0523 | |
| 2 | 24.4 | 1.04 (0.95, 1.14) | 24.1 | 0.49 (0.43, 0.55) | |||
| 3 | 24.8 | 1.68 (1.53, 1.85) | 25.2 | 0.58 (0.52, 0.64) | |||
| Serum selenium (µg/L) | |||||||
| Quartile 0 | 24.7 | 0.90 (0.72, 1.1) | 24.0 | 0.47 (0.38, 0.58) | |||
| 1 | 25.0 | 1.19 (0.95, 1.49) | 0.1959 | 26.6 | 0.50 (0.38, 0.66) | 0.7180 | |
| 2 | 25.1 | 1.03 (0.85, 1.23) | 25.1 | 0.50 (0.42, 0.59) | |||
| 3 | 25.2 | 1.14 (0.93, 1.40) | 24.3 | 0.44 (0.35, 0.54) | |||
WP, Weighted Prevalence (%), Weighted mean±standard error, Geometric mean (95% confidence limit), P, p-value for trend test (log-transformed)
Urinary mercury data are available in one-third of the survey population where urinary creatinine and creatinine-corrected urinary mercury were calculated.
Serum selenium data are only available from the NHANES 2003–2004 survey, whose 2,309 participants were 40 years or older.
Hypertension was defined as those individuals who have taken anti-hypertensive medication, have a systolic blood pressure of ≥140 mmHg, or have a diastolic blood pressure of ≥ 90 mmHg
Table 3 presents the estimated ORs of hypertension associated with blood mercury and urinary mercury. Lack of association between blood mercury and the risk for hypertension was observed, whereas there were inverse associations between hypertension and urinary mercury. The ORs for hypertension were 0.94 (95% CI 0.87–1.01) and 0.87 (0.78, 0.99) associated with a doubling increase in blood mercury and urinary mercury, respectively, after adjusting for age, gender, race/ethnicity, education, BMI, alcohol usage, cotinine, omega-3 fatty acids, and total caloric intake (for urinary mercury, urinary creatinine was additionally included) (model 3). Quartile levels of blood total and urinary mercury were also evaluated to account for potential non-linear relationships. In general, there were no differences in the odds of hypertension among upper three quartiles compared with the first quartile of total blood mercury. U- or J-shape associations were found with urinary mercury and the second quartile had significantly lower ORs compared with the first quartile in all models. Additionally, linear regression coefficients of SBP and DBP in association with mercury exposure are presented in Table 4. SBP was inversely associated with blood and urinary mercury exposure, whereas DBP showed no associations with blood and urinary mercury. We also further adjusted for serum selenium using a subpopulation (participants from NHANES 2003–2004 who were ages 40 or older (n=2,117)), but the results remained similar as in Table 3 (data not shown). There was no evidence of multicollinearity among mercury biomarker, omega-3 fatty acids and selenium (generalized variance inflation factors <2, data not shown).
Table 3.
Odds Ratios (95% confidence intervals) of hypertension in relation to mercury exposure.
| Doubling of Mercury (log-transformed) |
Quartile | Test for Trend |
||||
|---|---|---|---|---|---|---|
| 1 | 2 OR (95%CI) |
3 OR (95%CI) |
4 OR (95%CI) |
|||
| Blood mercury (n=6,607) | ||||||
| 0.10–0.49 | 0.50–0.95 | 0.96–1.83 | 1.84–32.8 | |||
| Crude | 1.02 (0.96, 1.09) |
1.0 (ref) |
1.26 (1.09, 1.46) |
1.26 (1.04, 1.53) |
1.16 (0.90, 1.49) |
0.3760 |
| Model 1 | 0.92 (0.87, 0.98) |
1.0 (ref) |
1.13 (0.99, 1.30) |
0.98 (0.76, 1.26) |
0.78 (0.62, 0.98) |
0.0189 |
| Model 2 | 0.95 (0.89, 1.02) |
1.0 (ref) |
1.14 (0.96, 1.35) |
1.003 (0.77, 1.31) |
0.88 (0.68, 1.13) |
0.1852 |
| Model 3 | 0.94 (0.87, 1.01) |
1.0 (ref) |
1.13 (0.95, 1.33) |
0.98 (0.75, 1.27) |
0.83 (0.63, 1.09) |
0.1116 |
|
Urinary mercury (n=2,201) |
||||||
| 0.06–0.21 | 0.22–0.45 | 0.46–1.02 | 1.03–50.2 | |||
| Crude | 0.91 (0.84, 0.99) |
1.0 (ref) |
0.71 (0.52, 0.98) |
0.85 (0.61, 1.19) |
0.79 (0.53, 1.17) |
0.1820 |
| Model 1 | 0.86 (0.78, 0.96) |
1.0 (ref) |
0.59 (0.41, 0.84) |
0.75 (0.49, 1.16) |
0.70 (0.42, 1.14) |
0.3131 |
| Model 2 | 0.88 (0.78, 0.99) |
1.0 (ref) |
0.60 (0.42, 0.85) |
0.74 (0.45, 1.21) |
0.76 (0.44, 1.28) |
0.5376 |
| Model 3 | 0.87 (0.78, 0.99) |
1.0 (ref) |
0.60 (0.42, 0.85) |
0.75 (0.46, 1.21) |
0.75 (0.44, 1.28) |
0.5154 |
Model 1: age, gender, and race/ethnicity (for urinary mercury, urinary creatinine added).
Model 2: Additionally adjusted for education, body mass index, alcohol usage, and cotinine (log-transformed).
Model 3: Further adjusted for omega-3 fatty acids and total caloric intake.
Hypertension was defined as those individuals who have taken anti-hypertensive medication, have a systolic blood pressure of ≥140 mmHg, or have a diastolic blood pressure of ≥ 90 mmHg.
Table 4.
Linear regression coefficients (mm Hg) of systolic and diastolic blood pressure associated with mercury exposure.
| Quartile | ||||||
|---|---|---|---|---|---|---|
| Doubling of Mercury (log-transformed) |
1 Ref (95%CI) |
2 coefficient (95%CI) |
3 coefficient (95%CI) |
4 coefficient (95%CI) |
Test for Trend |
|
| Blood total mercury (n=6,607) | ||||||
| Systolic blood pressure | ||||||
| Crude | 0.09 (−0.38, 0.57) |
Ref 0.00 |
1.72 (−0.57, 4.02) |
1.08 (−0.65, 2.81) |
0.57 (−1.46, 2.60) |
0.8407 |
| Model 1 | −0.53 (−0.90, −0.17) |
Ref 0.00 |
0.14 (−1.88, 2.17) |
−1.02 (−2.63, 0.60) |
−2.13 (−3.67, −0.58) |
0.0056 |
| Model 2 | −0.43 (−0.82, −0.04) |
Ref 0.00 |
0.12 (−1.91, 2.15) |
−0.96 (−2.64, 0.73) |
−1.77 (−3.40, −0.14) |
0.0264 |
| Model 3 | −0.48 (−0.86, −0.10) |
Ref 0.00 |
0.06 (−1.98, 2.11) |
−1.04 (−2.71, 0.64) |
−1.97 (−3.48, −0.46) |
0.0116 |
| Diastolic blood pressure | ||||||
| Crude | 0.44 (0.11, 0.77) |
Ref 0.00 |
1.16 (0.07, 2.26) |
1.59 (0.19, 3.001) |
1.62 (0.305, 2.94) |
0.0226 |
| Model 1 | 0.35 (0.01, 0.70) |
Ref 0.00 |
0.95 (−0.11, 2.01) |
1.44 (0.04, 2.84) |
1.24 (−0.18, 2.66) |
0.0925 |
| Model 2 | 0.26 (−0.12, 0.64) |
Ref 0.00 |
0.55 (−0.51, 1.60) |
0.92 (−0.45, 2.30) |
0.81 (−0.75, 2.36) |
0.3003 |
| Model 3 | 0.20 (−0.17, 0.57) |
Ref 0.00 |
0.48 (−0.56, 1.51) |
0.83 (−0.53, 2.18) |
0.56 (−0.94, 2.06) |
0.4404 |
| Urinary mercury (n=2,201) | ||||||
| Systolic blood pressure | ||||||
| Crude | −1.25 (−1.88, −0.63) |
Ref 0.00 |
−0.93 (−3.90, 2.05) |
−2.58 (−5.86, 0.69) |
−4.06 (−6.77, −1.36) |
0.0024 |
| Model 1 | −1.20 (−1.71, −0.70) |
Ref 0.00 |
−0.84 (−3.02, 1.34) |
−2.53 (−5.50, 0.44) |
−3.91 (−6.28, −1.54) |
0.0019 |
| Model 2 | −1.05 (−1.56, −0.54) |
Ref 0.00 |
−0.49 (−2.76, 1.78) |
−2.23 (−5.28, 0.82) |
−3.18 (−5.73, −0.63) |
0.0110 |
| Model 3 | −1.06 (−1.58, −0.54) |
Ref 0.00 |
−0.49 (−2.77, 1.79) |
−2.23 (−5.29, 0.82) |
−3.18 (−5.72, −0.64) |
0.0108 |
| Diastolic blood pressure | ||||||
| Crude | −0.01 (−0.47, 0.45) |
Ref 0.00 |
0.84 (−1.20, 2.88) |
1.43 (−0.92, 3.78) |
0.64 (−1.76, 3.03) |
0.5569 |
| Model 1 | 0.23 (−0.20, 0.67) |
Ref 0.00 |
1.08 (−0.84, 3.01) |
2.04 (−0.17, 4.26) |
1.56 (−0.79, 3.90) |
0.1518 |
| Model 2 | 0.20 (−0.23, 0.63) |
Ref 0.00 |
0.85 (−1.15, 2.85) |
1.66 (−0.59, 3.90) |
1.34 (−1.01, 3.68) |
0.2176 |
| Model 3 | 0.18 (−0.24, 0.60) |
Ref 0.00 |
0.86 (−1.12, 2.85) |
1.67 (−0.54, 3.88) |
1.27 (−1.003, 3.53) |
0.2202 |
Model 1: Age, gender, race/ethnicity, and hypertension medication (for urinary mercury, urinary creatinine was added).
Model 2: Further adjusted for education, body mass index, alcohol drink, and cotinine (log-transformed).
Model 3: Additionally adjusted for omega-3 fatty acids and total caloric intake.
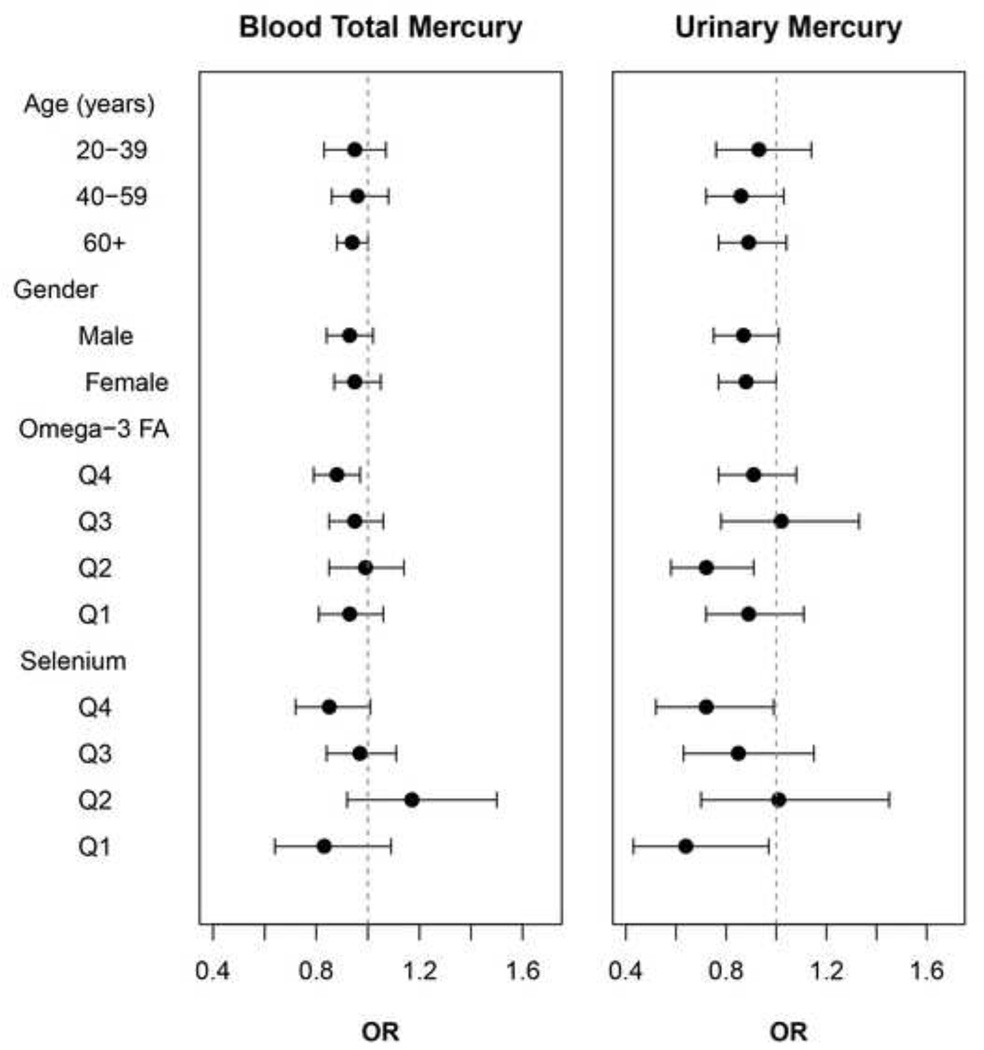
Before we test effect modification by omega-3 fatty acids or selenium, we examined their main effects on hypertension and blood pressure. There were no significant associations of omega-3 fatty acids, whereas serum selenium was significantly positively associated with the odds of hypertension (p for trend=0.05) and marginally significantly associated with DBP (p for trend=0.06) (see supplemental table). Figure 1 depicts the modifying effects by the quartiles of omega-3 fatty acids or selenium and age and gender on the associations between mercury markers and hypertension. No significant effect modification by either omega-3 fatty acids or selenium was observed. There was no effect modification by age or gender.
Figure 1.
Adjusted odds ratios (95% confidence intervals) of hypertension for a doubling in blood mercury or urinary mercury by age, gender, and quartiles of dietary omega-3 fatty acids and serum selenium. All models were adjusted for age, gender, race/ethnicity, education, body mass index, alcohol usage, cotinine, omega-3 fatty acids, and total caloric intake (for urinary mercury, log-transformed urinary creatinine added).
DISCUSSION
In this cross-sectional study of a representative sample of 6,607 US adults who participated in NHANES 2003/04 and 2005/06, we found no association of hypertension with blood mercury but a suggestive inverse association with urinary mercury, even after adjusting for age, gender, race/ethnicity, education, BMI, alcohol usage, cotinine, omega-3 fatty acids, and serum selenium. No significant effect modification by either omega-3 fatty acids or selenium was observed with either biomarker of mercury.
Inverse associations of urinary mercury, mainly reflecting inorganic mercury, with hypertension were observed. To the best of our knowledge, only one study has examined this association, which also observed an inverse association between urinary mercury and SBP but in a convenience sample of male dental professions (Goodrich, 2012). This inverse association is striking but not unrealistic since several laboratory studies have shown that inorganic mercury can decrease blood pressure (Massaroni et al., 1995; Rhee and Choi, 1989; Rossoni et al., 1999). An experimental study with 12 anesthetized rats found a decreased left ventricular systolic pressure (99mmHg to 85mmHg, 80min after HgCI2 administration 5mg/kg) due to an increased resistance in pulmonary vessels (Rossoni et al., 1999). In another experimental study with 10 rabbits, Rhee and colleagues observed reductions in SBP and DBP and heart rate after inorganic mercury injection (HgCI2, 2 mg/kg) (Rhee and Choi, 1989). Because these animal studies employed unrealistic exposure scenarios (single injections of relatively high levels of inorganic mercury), extrapolations to human populations that experience continuous, low-level inhalation exposures need to be carefully considered. Though, it is important to realize that the directionality of response is consistent between the animal studies and ours. The underlying mechanisms are not fully understood, though this inverse association with urinary mercury may be also attributed to the diuretic effect of inorganic mercury, in that the kidney may decrease its function of tubular reabsorption as a result of being a primary target organ of mercury toxicity (Dale and Sanderson, 1954). Given the weak association observed, and the limited understanding of potential mechanisms, it would be premature to make any health recommendations based on the present findings.
Previous studies on the association between methylmercury and hypertension have been inconsistent. This may be due to varying exposure levels and methylmercury sources among the different study populations. In the current study the mercury exposure levels are representative of the general U.S. population unlike previous studies largely focused on highly-exposed groups. A study in Faroese whaling men who eat pilot whale meat as their main diet showed a significant positive association between blood pressure and mercury concentrations in toenails and hair (Choi et al., 2009). A study conducted with a fish-eating population in the Brazilian Amazon also showed a significant positive association between hair mercury and elevated blood pressure (SBP OR=2.91, 95%CI 1.26–7.28, DBP OR=2.29, 95%CI 0.95–6.06) (Fillion et al., 2006). In contrast, a recent large study using two U.S. prospective cohorts found no adverse effects of the fifth quintile of toenail mercury on coronary heart disease (RR 0.88, 0.69–1.04), stroke (0.84, 0.62–1.14), or total cardiovascular disease (0.85, 0.72–1.01), as compared to the first quintile (Mozaffarian et al., 2011). Given that a significant association between mercury exposure and high blood pressure was only found in populations with relatively high methylmercury levels but not among populations with low to moderate fish consumption (more reflective of background populations), we may speculate that the dose-response relationship between methylmercury and hypertension (and probably other cardiovascular diseases) is not linear and there is a threshold. Methylmercury exposure levels found in the general U.S. population may not be high enough to cause hypertension. We expected the estimated associations of this study to become larger after adjusting for omega-3 fatty acids or selenium, because omega-3 fatty acids and selenium may play roles as negative confounders. Selenium is known to antagonize the toxicity of mercury under experimental conditions (Wang et al., 2001). However, ORs remained unchanged after controlling for these variables. The present study also showed a very weak correlation between serum selenium and omega-3 fatty acids (spearman correlation coefficient= 0.0027), suggesting fish may not be a main source of selenium in the U.S. population. Although omega-3 fatty acids have been suggested to be protective against hypertension, they were not associated with hypertension in the present study (data not shown). The 24-hour recall was utilized to estimate omega-3 fatty acids. Although two days of 24-hour recall may have measurement errors, this national survey may minimize this error due to the large sample. Further studies are needed to confirm this result. In sensitivity analyses, we examined not only two 24-hour recalls but also both FFQ and supplementation data to measure omega-3 fatty acids. No association was found with FFQ as well as supplementation data. Due to the availability of FFQ data, we restricted our analyses to the NHANES 2003–2006. We also checked whether demographic characteristics differed depending on individuals missing a portion of FFQ and confirmed no differences.
Residual or unmeasured confounding may also underestimate the true association. For example, participants who identified their race/ethnicity as ‘others’, who had higher education, who had lower BMI, or who had lower prevalence of hypertension, showed higher blood mercury or urinary mercury levels. Although the present study made an effort to adjust for as many potential confounders as possible, bias due to unmeasured confounding may still remain.
Detection limits may also impact the observed associations. In the present study, the number of samples below LLOD differed across the survey cycles and between different mercury biomarkers. The percentages below LLOD were 12.1% for blood and 19.0% for urine in NHANES 2003–2004 and 25.4% for blood and 7.4% for urine in NHANES 2005–2006. Improvements in analytic precision and detection limits may explain the substantial difference in the proportion below LLOD for urinary mercury, but it is unclear for blood mercury as the analytic method for blood mercury did not change from NHANES 2003–2004 to 2005–2006. Despite its flaw, the ad hoc method used in the present study (substitution of LLOD/√2) has been suggested to work fairly well in the case where the proportion below LLOD is less than 50% (Cole et al., 2009). A more sophisticated method, such as maximum likelihood estimate (Cole et al., 2009), may reduce potential bias.
The strength of this study using NHANES 2003–2006 was to evaluate the hypertensive risk associated with mercury exposure in the U.S. general population. Another strength of this study was that we examined two biomarkers of mercury exposure. Mercury levels in blood are generally reflective of exposure to organic mercury sources (mainly via fish consumption), whereas mercury levels in urine are generally reflective of exposure to inorganic mercury sources (mainly dental amalgams) (Mergler et al., 2007). By studying biomarkers for the two main forms of mercury, we were able to tease apart potential associations with blood pressure. Although NHANES speciated mercury in blood to measure the inorganic form of mercury in blood, most of the samples were below detection limits (77% in 2003–2004; 75% in 2005–2006) and therefore, a speciated analysis could not be performed. Future studies should better speciate mercury biomarkers to increase our understanding how inorganic and organic forms influence blood pressure. This study also has limitations. This study is a cross-sectional study, which may raise an issue of validity of causal inference between mercury and hypertension. Furthermore, the biomarkers used in this study (blood and urine mercury) only reflect recent mercury exposure (Clarkson et al., 2003), while hypertension is a chronic condition that may develop over many years.
In conclusion, in this cross-sectional study of U.S. adults with low to moderate mercury exposure, we found no association between blood mercury (mostly methylmercury) and hypertension, and a significant inverse association between urinary mercury (inorganic mercury) with hypertension. The present study should not be interpreted to imply that methylmercury has no impact on blood pressure control or cardiovascular health. Rather, this finding may reflect low power to detect an adverse effect of methylmercury exposure because of few subjects with high exposure to methylmercury. Further prospective studies with a wider range of mercury exposure from fish consumption as well as dental amalgam fillings are warranted to better investigate the association between mercury exposure and hypertension.
Supplementary Material
HIGHLIGHTS.
We examined the association between mercury and hypertension in US adults.
Two main biomarkers of mercury exposure, methyl (blood) and inorganic (urine), were examined.
Blood mercury was not associated with the risk of hypertension.
There was a suggestive inverse or U-shaped association with urinary mercury.
No significant effect modification by omega-3 fatty acids or selenium was found.
Acknowledgments
Funding Sources:
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) grant K01-ES016587. The contents of this paper are solely the responsibility of the author(s).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human Subjects Research:
NHANES is a publicly available data set and all participants in NHANES provide written informed consent, consistent with approval by the National Center for Health Statistics Institutional Review Board.
REFERENCE
- Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Int Med. 1993;153:1429–1438. [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990;14:169–176. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen TP, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. New Eng J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol. 2009;38:1674–1680. doi: 10.1093/ije/dyp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RA, Sanderson PH. The mode of action of a mercurial diuretic in man. J Clin Invest. 1954;33:1008–1014. doi: 10.1172/JCI102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. What You Need to Know About Mercury in Fish and Shellfish. U.S. Food and Drug Administration. 2004 [Google Scholar]
- Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- Fillion M, Mergler D, Sousa Passos CJ, Larribe F, Lemire M, Guimaraes JR. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ Health. 2006;5:29. doi: 10.1186/1476-069X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. Int J Hyg Environ Health. 2012 doi: 10.1016/j.ijheh.2012.03.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Guo T, Baasner J. On-line microwave sample pretreatment for the determination of mercury in blood by flow injection cold vapor atomic absorption spectrometry. Talanta. 1993;40:1927–1936. doi: 10.1016/0039-9140(93)80117-a. [DOI] [PubMed] [Google Scholar]
- Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S. Detoxification mechanism of heavy metals in marine mammals and seabirds: interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Arch Environ Contam Toxicol. 2004;47:402–413. doi: 10.1007/s00244-004-3188-9. [DOI] [PubMed] [Google Scholar]
- Jones RL. Laboratory Procedure Manual: Mercury (Urine) National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) 2004a [Google Scholar]
- Jones RL. Laboratory Procedure Manual: Total Mercury. National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) 2004b [Google Scholar]
- Jones RL, Sampson EJ. Laboratory Procedure Manual: Lead Cadmium Mercury. National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) 2004c [Google Scholar]
- Jones RL, Sampson EJ. Laboratory Procedure Manual: Iodine & Mercury (Urine) National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) 2006 [Google Scholar]
- Lee B-K, Kim Y. Relationship between blood manganese and blood pressure in the Korean general population according to KNHANES 2008. Env Res. 2011;111:797–803. doi: 10.1016/j.envres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Linne Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004;12:1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR. Methylmercury: a new look at the risks. Public Health Reports. 1999;114:396–413. [PMC free article] [PubMed] [Google Scholar]
- Massaroni L, Rossoni LV, Amaral SM, Stefanon I, Oliveira EM, Vassallo DV. Haemodynamic and electrophysiological acute toxic effects of mercury in anaesthetized rats and in langendorff perfused rat hearts. Pharmacol Res. 1995;32:27–36. doi: 10.1016/s1043-6618(95)80005-0. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, et al. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. New Eng J Med. 2011;364:1116–1125. doi: 10.1056/NEJMoa1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS. National Health and Nutrition Examination Survey: Blood Lead, Cadmium and Mercury (L06BMT_C) National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) 2006 [Google Scholar]
- NCHS. National Health and Nutrition Examination Survey: Dietary Interview - Total Nutrients, First Day (DR1TOT_C) National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) 2007a [Google Scholar]
- NCHS. National Health and Nutrition Examination Survey: Erythrocyte Protoporphyrin and Selenium (L39EPP_C) National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) 2007b [Google Scholar]
- NCHS. National Health and Nutrition Examination Survey: Blood Total Mercury and Blood Inorganic Mercury (THGIHG_D) National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) 2008a [Google Scholar]
- NCHS. National Health and Nutrition Examination Survey: Urinary Mercury (UHG_D) National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) 2008b [Google Scholar]
- Ostchega Y, Prineas RJ, Paulose-Ram R, Grim CM, Willard G, Collins D. National Health and Nutrition Examination Survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J Clin Epidemiol. 2003;56:768–774. doi: 10.1016/s0895-4356(03)00085-4. [DOI] [PubMed] [Google Scholar]
- Park K, Mozaffarian D. Omega-3 fatty acids, mercury, and selenium in fish and the risk of cardiovascular diseases. Curr Atheroscler Rep. 2010;12:414–422. doi: 10.1007/s11883-010-0138-z. [DOI] [PubMed] [Google Scholar]
- Rhee HM, Choi BH. Hemodynamic and electrophysiological effects of mercury in intact anesthetized rabbits and in isolated perfused hearts. Exp Mol Pathol. 1989;50:281–290. doi: 10.1016/0014-4800(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Rossoni LV, Amaral SM, Vassallo PF, Franca A, Oliveira EM, Varner KJ, et al. Effects of mercury on the arterial blood pressure of anesthetized rats. Braz J Med Biol Res. 1999;32:989–997. doi: 10.1590/s0100-879x1999000800009. [DOI] [PubMed] [Google Scholar]
- Stern AH, Korn LR. An approach for quantitatively balancing methylmercury risk and omega-3 benefit in fish consumption advisories. Environ Health Perspect. 2011;119:1043–1046. doi: 10.1289/ehp.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54:981–986. doi: 10.1161/HYPERTENSIONAHA.109.135046. [DOI] [PubMed] [Google Scholar]
- Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res. 2005;97:195–200. doi: 10.1016/j.envres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wang A, Barber D, Pfeiffer CJ. Protective effects of selenium against mercury toxicity in cultured Atlantic spotted dolphin (Stenella plagiodon) renal cells. Arch Environ Contam Toxicol. 2001;41:403–409. doi: 10.1007/s002440010266. [DOI] [PubMed] [Google Scholar]
- Xun P, Hou N, Daviglus M, Liu K, Morris JS, Shikany JM, et al. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J Intern Med. 2011;270:175–186. doi: 10.1111/j.1365-2796.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Tsuda T, Kashima S, Takao S, Harada M. Long-term exposure to methylmercury and its effects on hypertension in Minamata. Environ Res. 2010;110:40–46. doi: 10.1016/j.envres.2009.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.