Abstract
Objective:
Overweight/obese (OW/OB) African American (AA) adolescents have a more diabetogenic insulin secretion/sensitivity pattern compared with their American white (AW) peers. The present study investigated β-cell lipotoxicity to test whether increased free fatty acid (FFA) levels result in greater β-cell dysfunction in AA vs AW OW/OB adolescents.
Research Design and Methods:
Glucose-stimulated insulin secretion was modeled, from glucose and C-peptide concentrations during a 2-hour hyperglycemic (225 mg/dL) clamp in 22 AA and 24 AW OW/OB adolescents, on 2 occasions after a 12-hour overnight infusion of either normal saline or intralipid (IL) in a random sequence. β-Cell function relative to insulin sensitivity, the disposition index (DI), was examined during normal saline and IL conditions. Substrate oxidation was evaluated with indirect calorimetry and body composition and abdominal adiposity with dual-energy X-ray absorptiometry and magnetic resonance imaging at L4-L5, respectively.
Results:
Age, sex, body mass index, total and sc adiposity were similar between racial groups, but visceral adiposity was significantly lower in AAs. During IL infusion, FFAs and fat oxidation increased and insulin sensitivity decreased similarly in AAs and AWs. β-Cell glucose sensitivity of first- and second-phase insulin secretion did not change significantly during IL infusion in either group, but DI in each phase decreased significantly and similarly in AAs and AWs.
Conclusions:
Overweight/obese AA and AW adolescents respond to an overnight fat infusion with significant declines in insulin sensitivity, DI, and β-cell function relative to insulin sensitivity, suggestive of β-cell lipotoxicity. However, contrary to our hypothesis, there does not seem to be a race differential in β-cell lipotoxicity. Longer durations of FFA elevation may unravel such race-related contrasts.
Traditionally, type 2 diabetes mellitus was considered a disease of adults only. However, with the escalating rates of overweight/obesity, there has been a parallel increase in youth type 2 diabetes, with overrepresentation of minority children. Similar to adults, youth type 2 diabetes is a multifactorial disease marked by a strong genetic predisposition together with obesity and other environmental factors that unmask the disease. The hallmark of type 2 diabetes in adults and youth is impaired β-cell function combined with insulin resistance (1, 2). The earliest metabolic disturbance in the natural history of type 2 diabetes is insulin resistance. Initially this insulin resistance is compensated by increased pancreatic β-cell insulin secretion. Over time, especially with increased weight gain and visceral adiposity, in individuals genetically at risk for type 2 diabetes, insulin resistance intensifies and β-cell failure ensues rendering the islets incapable of further increases in insulin secretion to match the insulin resistance. Ultimately hyperglycemia may develop and the transition from normal to abnormal glucose tolerance and to type 2 diabetes occurs (3–6).
The interaction between free fatty acids (FFAs) and glucose in the control of insulin secretion is complex and is not fully understood. In in vitro and in vivo rat and human islet experiments, the acute stimulatory effect of FFAs on islets enhances glucose-stimulated insulin secretion (GSIS) (7, 8). Based mainly on evidence in the male Zucker diabetic fatty rat, prolonged FFA elevation leads to β-cell accumulation of triglycerides and decreased insulin secretion (9–11), hence the term β-cell lipotoxicity. In humans, obesity, especially abdominal obesity, is associated with elevated plasma FFA levels (12–14) that are not fully suppressed by feeding or the associated hyperinsulinemia (12, 13). These elevated FFA levels may play a lipotoxic role in β-cell impairment in individuals at high risk for type 2 diabetes.
Both overweight/obesity and African American (AA) race incur a heightened risk for type 2 diabetes in youth. African American normal-weight youth compared with their American white (AW) peers exhibit a higher first-phase insulin secretion during a hyperglycemic clamp (15, 16), which correlates positively with basal FFA levels (15). On the other hand, overweight/obese (OW/OB) AA adolescents in contrast to their AW peers fail to increase insulin secretion to compensate for the insulin resistance associated with increased visceral adiposity (17). Therefore, we postulated that although in normal-weight AA youth FFA may have a stimulatory effect, in overweight/obese youth, it may have a lipotoxic effect. Thus, the present investigation aimed to examine β-cell lipotoxicity in OW/OB AA vs AW adolescents, hypothesizing that elevation in FFA levels results in greater impairment in β-cell function in AA vs AW OW/OB adolescents.
Research Design and Methods
Twenty-two AA and 24 AW nondiabetic OW/OB adolescents recruited through community and newspaper advertisements were studied. Participants were 12 to younger than 18 years of age, Tanner stages II-V, and OW/OB [sex and age adjusted body mass index (BMI) ≥ 85th percentile and ≥ 95th percentile]. Subjects were excluded if they had been dieting, experienced significant weight change in the preceding months, were anemic, had elevated glycosylated hemoglobin, or were being treated for genetic dyslipidemia. None were on medications known to affect glucose or lipid metabolism including oral contraceptives. Race was verified by self-identity in 3 generations on both sides of the parents as reported before (15). Family history of type 2 diabetes was obtained in all participants, and a positive history was defined as the presence of known cases of diagnosed diabetes in any of 3 generations as described before (18). All subjects were in good health as assessed by history, a physical examination, and routine hematological and biochemical tests. Pubertal development was assessed according to the criteria of Tanner (19) and was confirmed by measurements of plasma T in males and estradiol in females. Participants were prescribed a weight-maintenance diet containing 55% carbohydrate, 30% fat, and 15% protein throughout the investigations. All studies were approved by the University of Pittsburgh Institutional Review Board, and consents and assents were obtained prior to the investigation.
Hyperglycemic clamps
All evaluations were performed in the Pediatric Clinical and Translational Research Center at Children's Hospital of Pittsburgh. Each subject was admitted twice to the Pediatric Clinical and Translational Research Center, 1–3 weeks apart for a 2-hour hyperglycemic clamp to measure first- and second-phase insulin and C-peptide concentrations as previously described (15). The hyperglycemic clamp experiments were preceded by an overnight fast and 12-hour infusion of either 0.9% normal saline (NS) at 0.02 mL/kg·min, control condition or 20% intralipid [(IL) 20% soybean oil, 1.2% egg yolk phospholipids, and 2.25% glycerol; Kabi Pharmacia, Clayton, North Carolina] at 0.02 mL/kg·min, experimental condition, in random order. These continued through the clamp to assess the effects of an intralipid challenge on basal and glucose-stimulated insulin and C-peptide secretion. During each experiment, 1 iv catheter was inserted in a forearm vein for administration of insulin, glucose, and NS or IL, and a second in a vein of the contralateral heated hand for sampling of arterialized blood. Baseline indirect calorimetry was performed using a ventilated hood system during NS and IL conditions (Deltratrac metabolic monitor; Sensormedics, Anaheim, California). On the morning prior to the first clamp, a 2-hour oral glucose tolerance test was performed, as reported before (4), to assess the glucose tolerance status of the participants.
Biochemical measurements
At each sampling point, blood samples were placed on ice and plasma was immediately separated in a refrigerated centrifuge. Plasma samples were divided into aliquots and stored at −80°C until analysis. Plasma glucose was measured by the glucose oxidase method using a glucose analyzer (Yellow Springs Instruments, Yellow Springs, Ohio). Plasma insulin and C-peptide were determined by RIA (20). Enzymatic colorimetric methods were used to determine plasma FFAs (Wako nonesterified fatty acid C test kit; Wako, Osaka, Japan) and triglyceride concentrations.
Body composition
Body composition was determined by dual-energy x-ray absorptiometry as reported before (21). Subcutaneous and visceral abdominal adipose tissues were quantified at L4-L5 with magnetic resonance imaging at the University of Pittsburgh Magnetic Resonance Research Center as reported before (22).
Calculations
During the hyperglycemic clamp, baseline glucose, insulin, and C-peptide concentrations were calculated as the means of the −30, −15, and 0 minute samples. Baseline fat (FOX) and glucose oxidation (GOX) rates were calculated according to the formulas developed by Frayn (23). First-phase glucose, insulin, and C-peptide concentrations were calculated as the mean of 5 determinations at 2.5, 5, 7.5, 10, and 12.5 minutes of the clamp. The first-phase FFA and triglyceride concentrations were the mean of the 5- and 10-minute determinations. The second-phase glucose concentrations were calculated as the mean of 22 determinations from 15 to 120 minutes of the clamp. Second-phase insulin and C-peptide concentrations were calculated as the mean of 8 determinations from 15 to 120 minutes of the clamp (15). The second-phase FFAs were the mean of the 30-, 60-, 90-, and 120-minute determinations, and the second-phase triglycerides were the mean of the 60- and 120-minute measurements. Insulin sensitivity during the clamp was calculated as the glucose infusion rate over the last 60 minutes of the clamp divided by the steady-state insulin concentration and multiplied by 100 as before (24) and expressed per kilogram of fat-free mass (FFM).
Modeling of glucose and C-peptide was performed as previously described (25). During the hyperglycemic clamp, insulin secretion is described as the sum of the following 3 components: 1) basal (postabsorptive) secretion rates; 2) insulin secretion in response to the rate of increase in plasma glucose [dynamic secretion component (26, 27), known as first-phase secretion]; and 3) insulin secretion in response to the actual glucose levels above the postabsorptive glucose concentration [static or proportional secretion component (28), known as second phase secretion]. The proportional component is further boosted by a gain factor, which comes into play in the last part of the clamp and is proportional to the integral of the hyperglycemic stimulus (28, 29). Parameters were estimated by implementing this minimal model of C-peptide secretion in the SAAM-II 1.2 software (SAAM Institute, Seattle, Washington). Numerical values of the unknown parameters were estimated by using nonlinear least squares. Weights were chosen optimally, ie, equal to the inverse of the variance of the measurement errors, which were assumed to be additive, uncorrelated, with zero mean, and a constant coefficient of variation (CV) of 13%.
The main outputs of this model are basal insulin secretion rate (picomoles per minute per kilogram FFM); glucose sensitivity of first-phase insulin secretion (derivative or dynamic secretion component), expressed as the amount of insulin secreted in response to a rate of increase in glucose concentration of 1 mmol/L between time 0 and 1 minute of the study (picomoles per kilogram FFM per millimole per liter per minute) [the CV of glucose sensitivity of first phase secretion, as estimated by the model was 7.2% (5.4–10.2); median (interquartile range)]; and glucose sensitivity of second-phase insulin secretion (proportional or static secretion component), expressed as the steady-state insulin secretion rate in response to a step increase in glucose concentration of 1 mmol/L (picomoles per minute per kilogram FFM per millimole per liter) [the CV of glucose sensitivity of second-phase secretion, as estimated by the model, was 14.4% (8.6–23.3); median (interquartile range)]. β-Cell function relative to insulin sensitivity, the disposition index (DI), was calculated as the product of insulin sensitivity expressed per FFM times the first-phase or second-phase insulin secretion expressed per FFM, respectively (30).
Statistical analysis
Comparison of continuous variables between AAs and AWs was performed using independent t tests. Categorical variables were compared using χ2 analysis. Nonparametric statistics were used when appropriate. A 2 × 2 repeated-measures ANOVA (within subject factor of condition, ie, NS vs IL, between subject factor of race; and condition × race interaction) was used for all analyses comparing triglycerides, FFAs, glucose, insulin, C-peptide, FOX, GOX, basal insulin secretion rate, β-cell glucose sensitivities of first- and second-phase insulin secretion, insulin sensitivity, and DI. Adjustments for visceral fat and a family history of diabetes were made in the primary outcome variables of basal insulin secretion rate, β-cell glucose sensitivity of first- and second-phase insulin secretion, insulin sensitivity, and DI. Paired t tests were used to determine individual differences between NS and IL for each race. A P ≤ .05 was considered statistically significant. All values are reported as means ± SE. Analyses were performed using SPSS software (SPSS, Chicago, Illinois).
Results
Descriptive and hormonal parameters (Table 1)
Table 1.
Descriptive and Hormonal Characteristics of the Subjects
| African American | American White | P Value | |
|---|---|---|---|
| n | 22 | 24 | |
| Age, y | 14.3 ± 0.3 | 13.8 ± 0.4 | NS |
| Males/females | 11/11 | 13/11 | NS |
| Tanner stage | NS | ||
| II–III | 4 | 7 | |
| IV–V | 18 | 17 | |
| Family history of T2DM (+/−) | 20/2 | 14/10 | .01 |
| OW/OB classification | NS | ||
| Overweight (85th to < 95th percentile), % | 27 | 33 | |
| Obese (≥ 95th percentile), % | 73 | 67 | |
| BMI, kg/m2 | 31.6 ± 1.1 | 30.9 ± 1.4 | NS |
| BMI percentile | 96.1 ± 1.0 | 95.7 ± 1.0 | NS |
| FFM, kg | 48.6 ± 2.0 | 48.4 ± 2.9 | NS |
| Fat mass, kg | 33.5 ± 2.5 | 35.0 ± 2.8 | NS |
| Body fat, % | 39.5 ± 2.0 | 40.0 ± 1.2 | NS |
| Visceral fat, cm2 | 38.8 ± 3.7 | 61.5 ± 4.8 | .001 |
| Subcutaneous fat, cm2 | 361.6 ± 34.8 | 380.4 ± 38.0 | NS |
| T, nmol/L | 327.1 ± 71.1 | 207.5 ± 43.8 | NS |
| Estradiol, pmol/L | 162.3 ± 51.4 | 91.1 ± 18.7 | NS |
| OGTT status, n | |||
| NFG/IFG | 21/1 | 18/6 | NS |
| NGT/IGT | 22/0 | 21/3 | NS |
| HbA1C, % | 5.3 ± 0.1 | 5.3 ± 0.1 | NS |
Abbreviations: HbA1C, glycosylated hemoglobin; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NFG, normal fasting glucose; NGT, normal glucose tolerance; NS, not significant (P > .05); OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus. Data are mean ± SEM.
There were no significant differences in age, sex, Tanner stage, pubertal hormonal profile, BMI, BMI percentile, FFM, fat mass, percent body fat, sc abdominal adipose tissue, glycosylated hemoglobin, and glucose tolerance status between AAs and AWs. Consistent with previous observations (17, 22), a family history of type 2 diabetes was more common and visceral adiposity was lower in AAs vs AWs.
Baseline and hyperglycemic clamp hormone and substrate profiles (Table 2)
Table 2.
Hyperglycemic Clamp Hormone and Substrate Profile During Normal Saline vs Intralipid Infusion
| African American |
American White |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Normal Saline | Intralipid | Normal Saline | Intralipid | Condition | Race | Interaction | |
| Baseline fasting | |||||||
| TG, mg/dL | 68.2 ± 9.6 | 1275.6 ± 183.1 | 117.0 ± 9.0 | 1409.2 ± 171.3 | <.001 | NS | NS |
| FFA, mmol/L | 0.3 ± 0.04 | 2.3 ± 0.2 | 0.3 ± 0.03 | 2.0 ± 0.2 | <.001 | NS | NS |
| Glucose, mg/dL | 93.3 ± 1.1 | 97.3 ± 1.4 | 95.9 ± 1.1 | 99.4 ± 1.3 | <.001 | NS | NS |
| Insulin, μU/mL | 24.2 ± 2.7 | 37.3 ± 6.8 | 23.6 ± 2.6 | 41.7 ± 6.5 | <.001 | NS | NS |
| C-peptide, ng/mL | 2.6 ± 0.2 | 3.9 ± 0.6 | 3.0 ± 0.2 | 5.0 ± 0.5 | <.001 | NS | NS |
| FOX, mg/kg · min | 0.7 ± 0.06 | 1.1 ± 0.07 | 0.8 ± 0.06 | 1.2 ± 0.07 | <.001 | NS | NS |
| GOX, mg/kg · min | 1.7 ± 0.2 | 1.2 ± 0.1 | 1.7 ± 0.2 | 1.3 ± 0.1 | .001 | NS | NS |
| First phase | |||||||
| TG, mg/dL | 68.3 ± 9.3 | 1232.9 ± 191.1 | 111.0 ± 8.7 | 1387.0 ± 178.8 | <.001 | NS | NS |
| FFA, mmol/L | 0.3 ± 0.03 | 2.3 ± 0.2 | 0.3 ± 0.03 | 2.0 ± 0.2 | <.001 | NS | NS |
| Glucose, mg/dL | 225.1 ± 1.9 | 223.5 ± 2.2 | 223.5 ± 1.8 | 226.9 ± 2.1 | NS | NS | NS |
| Insulin, μU/mL | 264.1 ± 29.9 | 277.6 ± 30.6 | 173.6 ± 28.6 | 186.4 ± 29.3 | NS | .03 | NS |
| C-peptide, ng/mL | 9.6 ± 0.8 | 10.7 ± 0.9 | 8.1 ± 0.7 | 9.8 ± 0.9 | <.001 | NS | NS |
| Second phase | |||||||
| TG, mg/dL | 64.3 ± 9.8 | 1163.9 ± 176.5 | 110.2 ± 9.2 | 1175.9 ± 165.1 | <.001 | NS | NS |
| FFA, mmol/L | 0.13 ± 0.02 | 2.1 ± 0.2 | 0.11 ± 0.02 | 1.9 ± 0.2 | <.001 | NS | NS |
| Glucose, mg/dL | 225.2 ± 0.7 | 226.9 ± 0.4 | 224.6 ± 0.6 | 226.6 ± 0.4 | .001 | NS | NS |
| Insulin, μU/mL | 256.8 ± 32.8 | 304.7 ± 42.4 | 220.4 ± 32.1 | 255.9 ± 41.5 | .004 | NS | NS |
| C-peptide, ng/mL | 11.5 ± 0.9 | 11.9 ± 1.0 | 11.8 ± 0.8 | 12.9 ± 1.0 | .02 | NS | NS |
Abbreviation: NS, not significant (P > .05). Data are means ± SEM.
Baseline triglycerides (TGs), FFAs, glucose, insulin, C-peptide, and FOX increased, and GOX decreased significantly with IL infusion, with no difference between AAs and AWs (Table 2). During NS infusion only, basal and first- and second-phase TGs were lower in AAs vs AWs (t test P < .01), consistent with previous reports in the adult and pediatric literature (17, 31). During IL infusion, first- and second-phase C-peptide and second-phase insulin concentrations were significantly higher in AAs and AWs with no change in first-phase insulin (Table 2). Consistent with prior findings (15, 16), first-phase insulin was significantly higher in AAs vs AWs under NS conditions.
Model-derived parameters of insulin secretion (Figure 1, A–C)
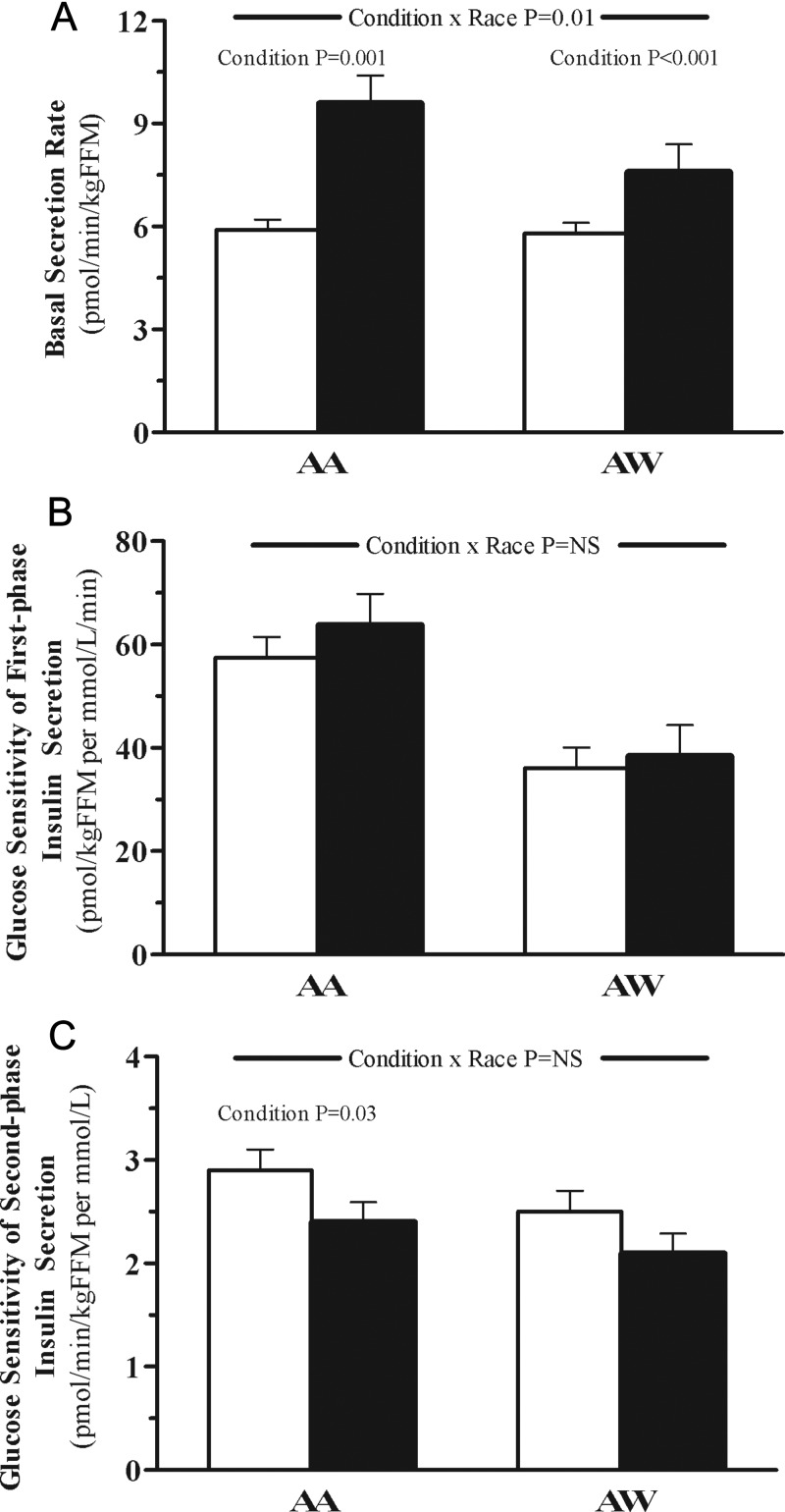
Figure 1.
Basal insulin secretion rate (A), β-cell glucose sensitivity of first-phase insulin secretion (B), and β-cell glucose sensitivity of second-phase insulin secretion (C) in AA vs AW adolescents after an overnight infusion of NS (empty bars) vs IL (solid bars). The P value for condition × race interaction was adjusted for a family history of diabetes and visceral fat. The P value for condition is shown using a paired t test when significant.
The basal insulin secretion rate increased significantly with IL infusion in the AA and AW groups, with a significant condition × race interaction after adjusting for visceral fat and a family history of diabetes (Figure 1A). During the IL infusion, glucose sensitivity of first- and second-phase insulin secretion did not change significantly in AAs or AWs (Figure 1, B and C).
Insulin sensitivity and β-cell function relative to insulin sensitivity, DI (Figure 2, A–C)
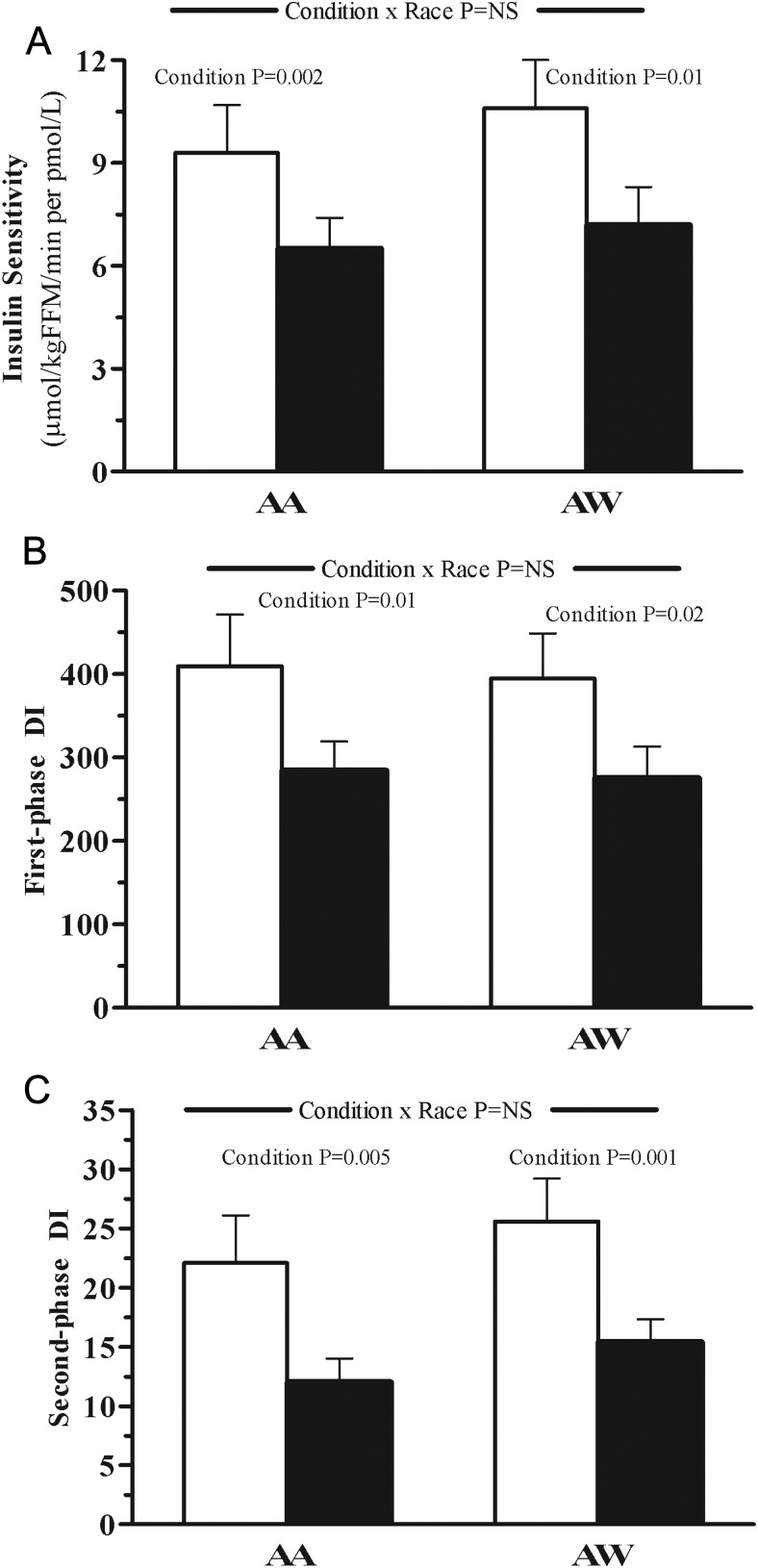
Figure 2.
Insulin sensitivity (A), DI from first-phase insulin secretion (B), and DI from second-phase insulin secretion (C) in AA vs AW adolescents after an overnight infusion of NS (empty bars) vs IL (solid bars). The P value for condition × race interaction was adjusted for a family history of diabetes and visceral fat. The P value for condition is shown using a paired t test when significant.
Insulin sensitivity decreased during IL infusion significantly and similarly in the 2 racial groups (Figure 2A). Disposition indices, calculated from modeled first-phase and second-phase insulin, decreased significantly and similarly in AAs and AWs during IL infusion (Figure 2, B and C).
Visceral adiposity and intralipid-induced β-cell dysfunction and insulin resistance
To assess whether the amount of visceral fat per se, irrespective of race, could predict lipid-induced β-cell dysfunction and insulin resistance, we analyzed the whole cohort with AAs and AWs combined. Bivariate relationships revealed that visceral adipose tissue (VAT) correlated with an IL-induced change in basal insulin secretion (r = 0.54, P < .001) but not first-phase (r = 0.03, P = .85) and second-phase insulin secretion (r = 0.14, P = .37), insulin sensitivity (r = 0.23, P = .12), first-phase (r = −0.09, P = .55) and second-phase DI (r = −0.12, P = .44). Additionally, we performed multiple regression analyses with VAT, age, race, sex, and a family history of diabetes as independent variables and the change in each of the modeled parameters as dependent variables. VAT contributed 29% (P < .001) and race 11% (P = .01) to the variance in IL-induced change in basal insulin secretion, with no significant contribution to IL-induced change in first- and second-phase insulin secretion, insulin sensitivity, and first- and second-phase DI.
Family history of diabetes and intralipid-induced β-cell dysfunction and insulin resistance
To assess whether family history of diabetes per se, irrespective of race, could play a role in lipid-induced β-cell dysfunction and insulin resistance, we analyzed the whole cohort with AAs and AWs combined. Comparing the 34 participants with positive vs the 12 participants with a negative family history of diabetes revealed no significant differences in IL-induced change in basal insulin secretion (P = .62), first-phase (P = .43) and second-phase (P = .77) insulin secretion, insulin sensitivity (P = .31), and first-phase (P = .85) and second-phase DI (P = .79). Additionally, we performed multiple regression analyses with family history of diabetes, age, race, and sex as independent variables and the change in each of the modeled parameters as dependent variables. Family history of diabetes did not significantly contribute to the variance in IL-induced change in any of the dependent variables.
When all the above analyses were performed while expressing the data for whole body (milligrams per minute) instead of per kilogram FFM but with adjustment for FFM, the results did not change except for the basal insulin secretion rate, in which the condition × race interaction P value changed from .01 to .10, likely due to inadequate power for the additional variable adjustment of FFM (data not shown). Expressing the data per kilogram body weight did not change the results either.
Discussion
Using C-peptide modeling, the present study demonstrates that an increase in plasma FFA concentrations with overnight IL infusion in overweight/obese adolescents results in β-cell decompensation, such that despite a significant decline in insulin sensitivity, glucose sensitivity of first- and second-phase insulin secretion did not increase, suggestive of a lipotoxic phenomenon. Contrary to our hypothesis, however, there was no race differential in intralipid-induced β-cell decompensation, anticipated to be worse in AA youth.
There is a paucity of data examining the effects of FFAs on β-cell function in children at high risk for type 2 diabetes. Although adult studies of acute plasma FFA elevations increased GSIS (32–34), the effects of long-term elevations in overweight nondiabetic adults remain controversial (35). In a study of 12 normal and overweight nondiabetic adults, a 6-hour IL infusion followed by an iv glucose tolerance test significantly stimulated the acute insulin response, whereas a 24-hour lipid infusion was associated with inhibited GSIS (36). In contrast to the latter findings, Boden et al (37) demonstrated in 12 normal-weight volunteers who underwent a 48-hour intralipid and heparin infusion that insulin secretion rates increased 46% with a decline in IS over the first 24 hours only. On the other hand, a 48-hour intralipid and heparin infusion followed by a stepped iv glucose infusion in 7 overweight and obese at-risk adults for type 2 diabetes (38) was associated with a 21% decrease in GSIS (P = .0008). In the same study, 7 patients with type 2 diabetes had a slight increase in GSIS (P = .027), but they did not assess the change in IS. Kashyap et al (39) examined β-cell lipotoxicity in adults with vs without a family history of diabetes. They demonstrated that in 13 family history-positive subjects, elevations in plasma FFA concentration (96 hours) markedly impaired both first- and second-phase insulin secretion by 60% and 35%, respectively, compared with 8 family history-negative subjects (39).
In our study, despite uniform recruitment between OW/OB AA and AW adolescents, a family history of type 2 diabetes was more frequent in AAs. This is not surprising because the AA race is a group with higher national rates of type 2 diabetes both in adults and youth (40). However, recent abstract data indicate that in adult offspring of parents with type 2 diabetes, prediabetes incidence does not seem to differ between African American and European Americans (41). Analysis of our total cohort by the presence vs absence of a family history of diabetes revealed no significant between group differences in the outcome variables, suggesting that family history may not have played a role in lipid-induced insulin resistance and β-cell dysfunction. However, our study was not powered to address this question. Furthermore, comparing individuals with vs without a family history within each race may provide a clearer picture with respect to the role of family history and intralipid-induced metabolic alterations conducive to the risk of type 2 diabetes in each racial group. Adjusting for a family history of diabetes (in addition to visceral fat, which is lower in AAs) revealed a significant condition × race interaction in basal insulin secretion, with a greater IL-induced basal insulin secretion in AAs vs AWs but no race-related differences in first- and second-phase insulin secretion.
The clinical significance of this up-regulated basal insulin secretion during a 12-hour IL infusion in AAs is unclear, and the mechanism(s) responsible for it could not be evaluated in the current investigation. A likely possibility is that IL infusion imposes a greater reduction in hepatic insulin sensitivity in AAs, resulting in a greater compensatory basal insulin secretion, whereas in AWs this may not be the case. On the other hand, such an observation could also be interpreted that AWs may be more susceptible to impairment in β-cell function under normoglycemic conditions when combined with elevated FFAs, whereas AAs manifest the β-cell impairment under hyperglycemic conditions only. It is also possible that the contrast in VAT between AAs and AWs may have confounded the results. Based on our analysis of the entire cohort, VAT explained 29% of the variance in IL-induced change in basal insulin secretion. Thus, the higher VAT in AW youth may increase their susceptibility to lipid-induced β-cell dysfunction and insulin secretion. However, when the insulin secretion data were expressed for the whole body with statistical adjustment for FFM, the condition by race interaction P value for basal insulin secretion changed from .01 to .10. Future studies assessing changes in hepatic insulin sensitivity with IL infusion in AAs vs AWs and the impact of VAT might shed further light.
The decline in insulin sensitivity in the present study is in line with our prior investigation using the hyperinsulinemic-euglycemic clamp, in which a shorter duration of IL infusion resulted in a significant and comparable decline in insulin sensitivity in AA vs AW adolescents (21). In the current investigation, expressing first- and second-phase insulin secretion relative to insulin sensitivity during IL infusion reveals significant impairment in DI ranging from 15% to 21% in AA and 15% to 26% in AW OW/OB at-risk youth, consistent with a β-cell lipotoxic phenomenon in both groups. The observation that DI under NS conditions was not lower in AA youth, based on the expectation of their higher risk for type 2 diabetes, is consistent with our previous findings showing comparable DI in obese AA vs AW adolescents (17). However, in that study, we demonstrated that contrary to AWs, AA obese adolescents with high vs low visceral fat did not demonstrate a compensatory increase in first-phase insulin secretion resulting in a lower DI, thus possibly pointing to an enhanced risk of type 2 diabetes with increasing visceral fat.
Strengths of our study include the use of C-peptide modeling and the selection of a study population of OW/OB at-risk youth. Our relatively larger sample size compared with the reported numbers in the literature and the simultaneous assessment of insulin sensitivity together with modeled insulin secretion enables the evaluation of DI, insulin secretion relative to insulin sensitivity, which is a better measure of β-cell function than insulin secretion alone. Another unique aspect of our study is the assessment of the differences in intralipid-induced β-cell response between AA and AW youth because there are no published race-specific studies in these populations, in adults or youth. Similar results were obtained when Caucasians were compared to Oji-Cree adults, a Native Canadian population with a very high risk of type 2 diabetes (42).
A potential limitation of our study is the higher prevalence of a family history of type 2 diabetes in blacks. However, this is merely a reflection of the national data, which, even including normal-weight individuals, show that AAs, young or old, have higher rates of type 2 diabetes (40). Thus, in an at-risk OW/OB population of AA youth, it is not surprising for this to be the case also. In our statistical analyses, we have appropriately used adjustments for a family history of diabetes. Another perceived limitation is using the hyperglycemic clamp to derive insulin sensitivity and insulin secretion instead of a separate hyperinsulinemic-euglycemic clamp for insulin sensitivity, one during NS and another during IL infusion. In support of using the hyperglycemic clamp is our observation that in obese adolescents, insulin sensitivity from the hyperglycemic clamp correlates with insulin sensitivity from the euglycemic clamp (r = 0.80, P < .001) and DI derived from a single hyperglycemic clamp correlates with DI from 2 clamps, a euglycemic clamp and a hyperglycemic clamp (r = 0.74, P < .001) (30). Moreover, a 4-clamp design, 2 hyperglycemic and 2 hyperinsulinemic-euglycemic, NS vs IL conditions, would have increased youth participant burden tremendously and would have made recruitment for the study impossible. Another potential limitation is that our blood specimen tubes did not contain a triglyceride hydrolysis inhibitor, which may lead to falsely elevated FFA levels. We do not believe this would impact our findings because of our sample processing procedures detailed above and because any FFA elevation in the sample tubes would be expected to be comparable between AAs and AWs.
In conclusion, our study demonstrates that AA overweight/obese adolescents are not more susceptible to FFA-induced β-cell lipotoxicity than their AW counterparts, as a potential explanation for their heightened risk of type 2 diabetes. Further, despite an approximately 30% decline in insulin sensitivity with overnight IL infusion, there was no compensatory increase in first- or second-phase insulin secretion in either group of overweight/obese youth. Future investigations with longer durations of IL infusion and/or prolonged lipid-rich diets may unravel race-related contrasts in β-cell lipotoxicity.
Acknowledgments
We express our gratitude to the study participants and their parents; Sabrina Kadri and Nancy Guerra, certified registered nurse practitioner (project coordinators), the laboratory expertise of Resa Stauffer and the Pediatric Clinical and Translational Research Center nursing staff for their excellent contributions, all at Children's Hospital of Pittsburgh of the University of Pittsburgh Medical Center. The contribution of Dr Linda Boselli in modeling glucose/C-peptide data at the University of Verona is gratefully acknowledged. K.S.H. analyzed the data and wrote the manuscript. R.C.B. provided the C-peptide modeling analysis and critically reviewed/edited the manuscript. S.L. contributed the laboratory/analytical tools. S.F.M. assisted with the data analyses and reviewed/edited the manuscript. S.A.A. provided the study concept and design, obtained funding, acquired data, participated in the data analyses, provided the administrative, technical and material support, supervised the study, and critically reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 28th Annual Meeting of the Obesity Society, San Diego, California, October 8–12, 2010.
This work was supported by U.S. Public Health Service Grants RO1-HD-27503 and K24-HD-01357 (to S.A.A.); the Richard L. Day Endowed Chair by the University of Pittsburgh Medical Center (to S.A.A.); Clinical and Translational Science Award UL1-RR-024153, T32-DK-07729, National Institutes of Health T32 scholar (to K.H.); Department of Defense Grant FA7014-09-2-0008 (to S.A.A., S.L., and S.F.M.); Italian Ministry of University and Research Grant PRIN 2008CJ7CTW_002 (to R.C.B.); and research funds from the University of Verona (R.C.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- African American
- AW
- American white
- BMI
- body mass index
- CV
- coefficient of variation
- DI
- disposition index
- FFA
- free fatty acid
- FFM
- fat-free mass
- FOX
- fat oxidation
- GOX
- glucose oxidation
- GSIS
- glucose-stimulated insulin secretion
- IL
- intralipid
- NS
- normal saline
- OW/OB
- overweight/obese
- TG
- triglyceride
- VAT
- visceral adipose tissue.
References
- 1. DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care. 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Libman IM, Arslanian SA. Prevention and treatment of type 2 diabetes in youth. Horm Res. 2007;67(1):22–34 [DOI] [PubMed] [Google Scholar]
- 4. Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32(1):100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss R, Caprio S. β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54(6):1735–1743 [DOI] [PubMed] [Google Scholar]
- 6. Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes. 2005;6:95–99 [DOI] [PubMed] [Google Scholar]
- 7. Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose induced insulin secretion and β-cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–1589 [DOI] [PubMed] [Google Scholar]
- 8. Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes. 1995;44:863–870 [DOI] [PubMed] [Google Scholar]
- 9. Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits β-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–1590 [DOI] [PubMed] [Google Scholar]
- 10. Zhou YP, Grill VE. Long term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGarry JD, Dobbins RL. Fatty acids, lipotoxicity, and insulin secretion. Diabetologia. 1999;42:128–138 [DOI] [PubMed] [Google Scholar]
- 12. Jensen MD. Adipose tissue as an endocrine organ: implications of its distribution on free fatty acid metabolism. Eur Heart J. 2006;suppl 8:B13–B19 [Google Scholar]
- 13. Groop LC, Bonadonna RC, Simonson DC, Petrides AS, Shank M, DeFronzo RA. Effect of insulin on oxidative and nonoxidative pathway of free fatty acid metabolism in human obesity. Am J Physiol. 1992;263:E79–E84 [DOI] [PubMed] [Google Scholar]
- 14. Björntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover in obesity. Acta Med Scand. 1969;185:351–356 [DOI] [PubMed] [Google Scholar]
- 15. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 16. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American White peers despite similar insulin sensitivity. Diabetes Care. 2008;31:1445–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540 [DOI] [PubMed] [Google Scholar]
- 18. Arslanian SA, Bacha F, Saad R, Gungor N. Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth. Diabetes Care. 2005;28:115–119 [DOI] [PubMed] [Google Scholar]
- 19. Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 20. Bacha F, Gungor N, Arslanian SA. Measures of β-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr. 2008;152:618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burns S, Kelsey S, Arslanian S. Effects of an intravenous lipid challenge and free fatty acid elevation on in vivo insulin sensitivity in African American versus Caucasian adolescents. Diabetes Care. 2009;32:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SJ, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab. 2010;95:2426–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 24. Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927 [DOI] [PubMed] [Google Scholar]
- 25. Cali' A, Bonadonna R, Trombetta M, Weiss R, Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab. 2008;93:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mari A, Camastra S, Toschi E, et al. A model for glucose control of insulin secretion during 24 h of free living. Diabetes. 2001;50(suppl 1):S164–S168 [DOI] [PubMed] [Google Scholar]
- 27. Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51(suppl 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- 28. Steil GM, Rebrin K, Janowski R, Darwin C, Saad MF. Modeling β-cell insulin secretion: implications for closed-loop glucose homeostasis. Diabetes Technol Ther. 2003;5:953–964 [DOI] [PubMed] [Google Scholar]
- 29. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–723 [Google Scholar]
- 30. Sjaarda L, Lee SJ, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring β-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? [published online December 28, 2012] Diabetes Care. doi:10.2337/dc12-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haffner SM, D'Agostino R, Goff D, et al. LDL size in African Americans, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Arterioscler Thromb Vasc Biol. 1999;19:2234–2240 [DOI] [PubMed] [Google Scholar]
- 32. Amery CM, Round RA, Smith JM, Nattrass M. Elevation of plasma fatty acids by ten-hour intralipid infusion has no effect on basal or glucose-stimulated insulin secretion in normal man. Metabolism. 2000;49:450–454 [DOI] [PubMed] [Google Scholar]
- 33. Balent B, Goswami G, Goodloe G, et al. Acute elevation of NEFA causes hyperinsulinemia without effect on insulin secretion rate in healthy human subjects. Ann NY Acad Sci. 2002;967:535–543 [DOI] [PubMed] [Google Scholar]
- 34. Magnan C, Cruciani C, Clement L, et al. Glucose-induced insulin hypersecretion in lipid-infused healthy subjects is associated with a decrease in plasma norepinephrine concentration and urinary excretion. J Clin Endocrinol Metab. 2001;86:4901–4907 [DOI] [PubMed] [Google Scholar]
- 35. Giacca A, Xiao C, Oprescu A, Carpentier A, Lewis G. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–E262 [DOI] [PubMed] [Google Scholar]
- 36. Paolisso G, Gambardella A, Amato L, et al. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295–1299 [DOI] [PubMed] [Google Scholar]
- 37. Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242 [DOI] [PubMed] [Google Scholar]
- 38. Carpentier A, Mittelman S, Bergman R, Giacca A, Lewis G. Prolonged elevation of plasma free fatty acids impairs pancreatic β-cell function in obese nondiabetic humans not in individuals with type 2 diabetes. Diabetes. 2000;49:399–408 [DOI] [PubMed] [Google Scholar]
- 39. Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 40. Pleis JR, Lucas JW, Ward BW. Summary health statistics for US adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009;242:1–157 [PubMed] [Google Scholar]
- 41. Edeoga C, Chapp-Jumbo E, Ammons A, Nyenwe E, Dagogo-Jack S. Pathobiology of prediabetes in a biracial cohort (POP-ABC): interim report on early progressors. Diabetes. 2011;60(suppl 1):A375 [Google Scholar]
- 42. Carpentier A, Zinman B, Leung N, et al. Free fatty acid-mediated impairment of glucose-stimulated insulin secretion in nondiabetic Oji-Cree individuals from the Sandy Lake community of Ontario, Canada: a population at very high risk for developing type 2 diabetes. Diabetes. 2003;52(6):1485–1495 [DOI] [PubMed] [Google Scholar]