Abstract
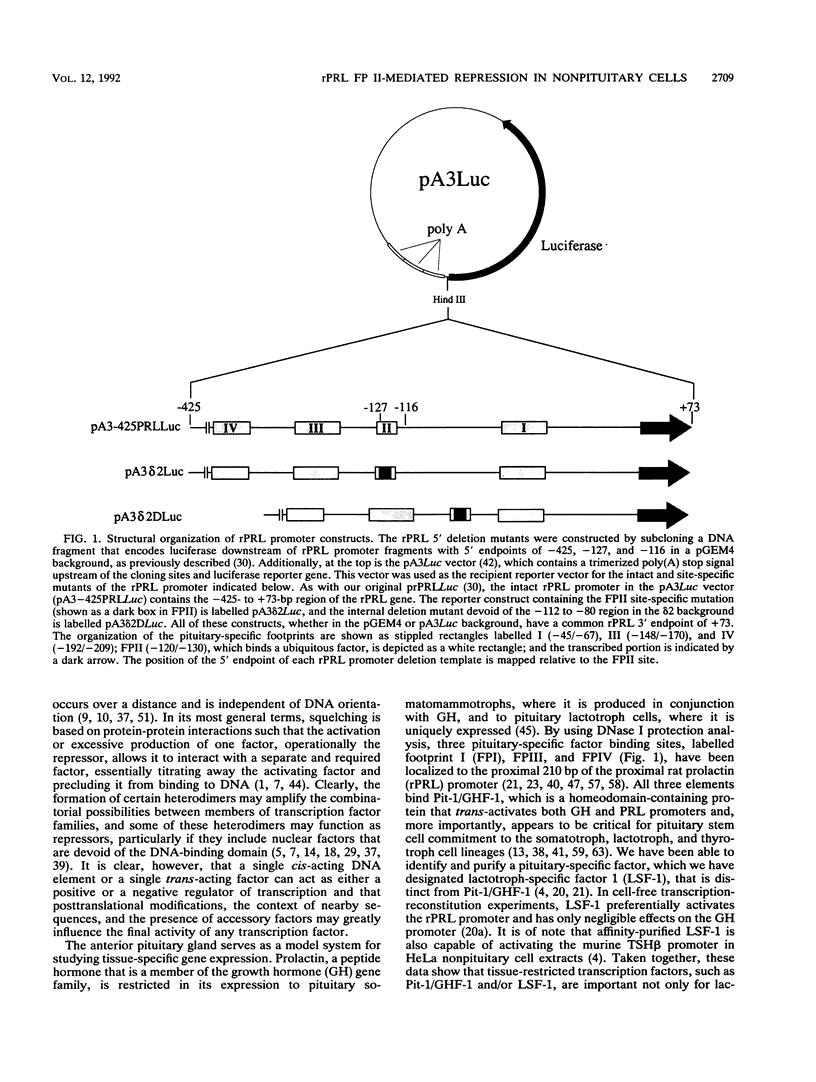
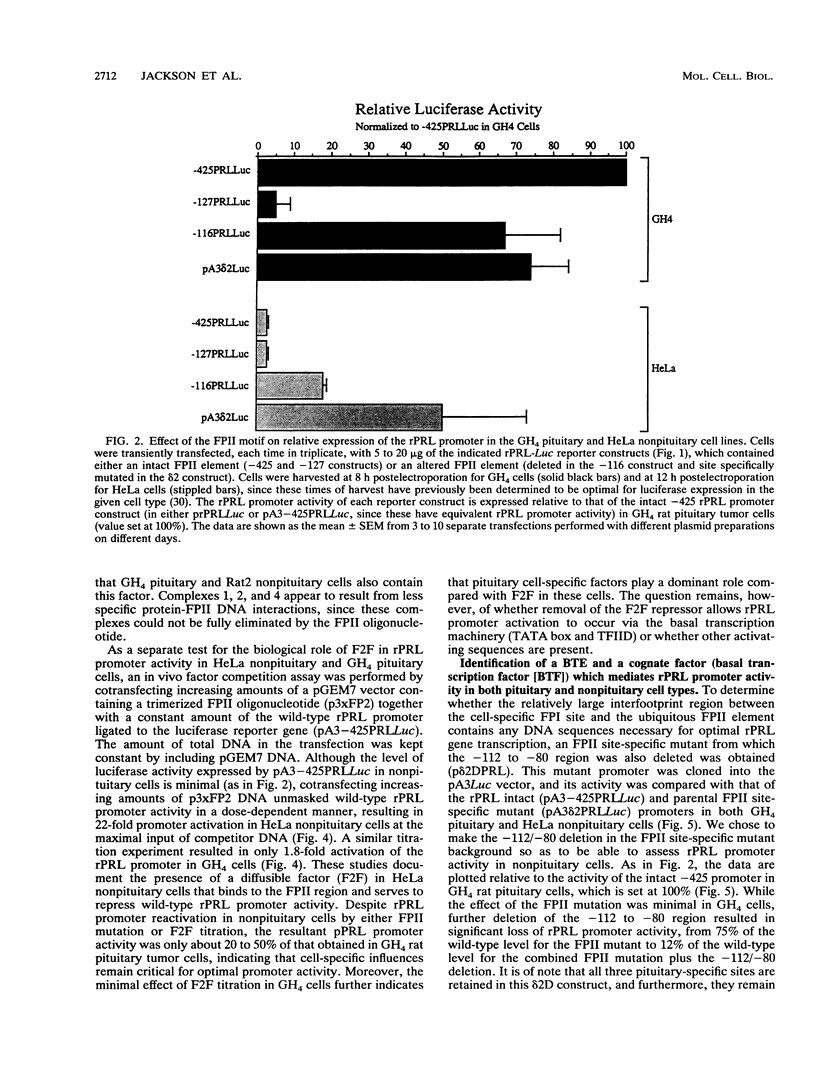
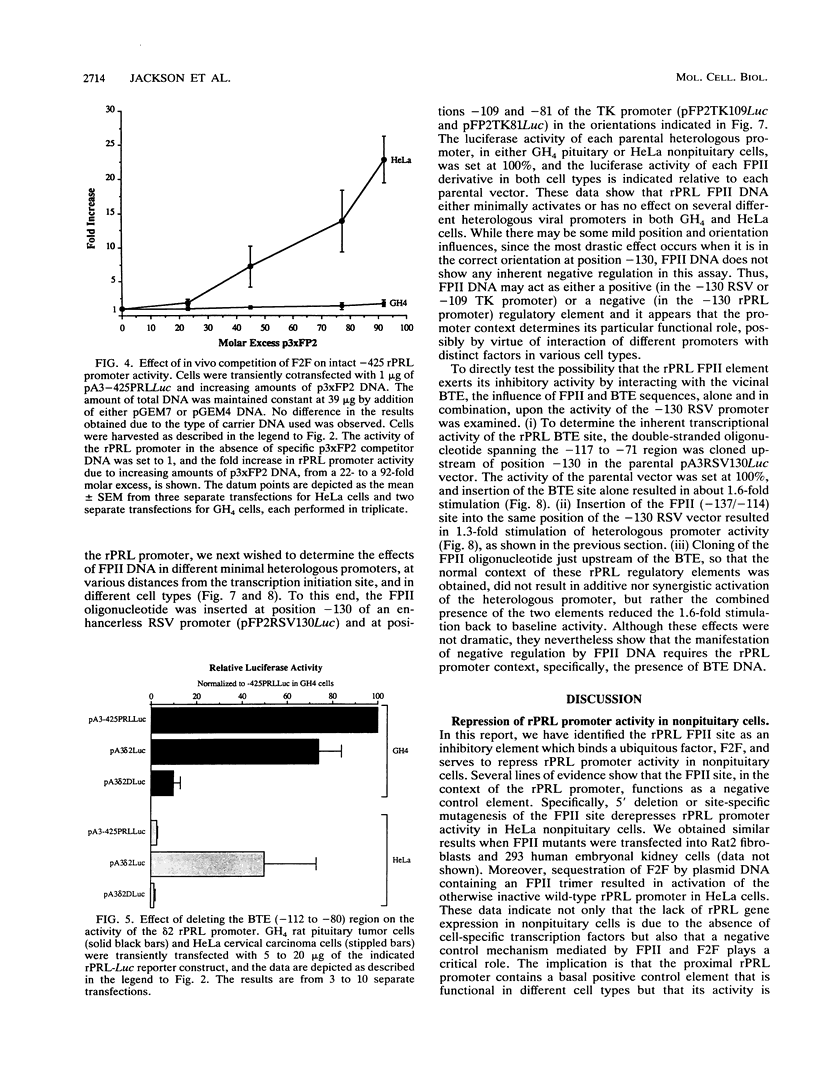
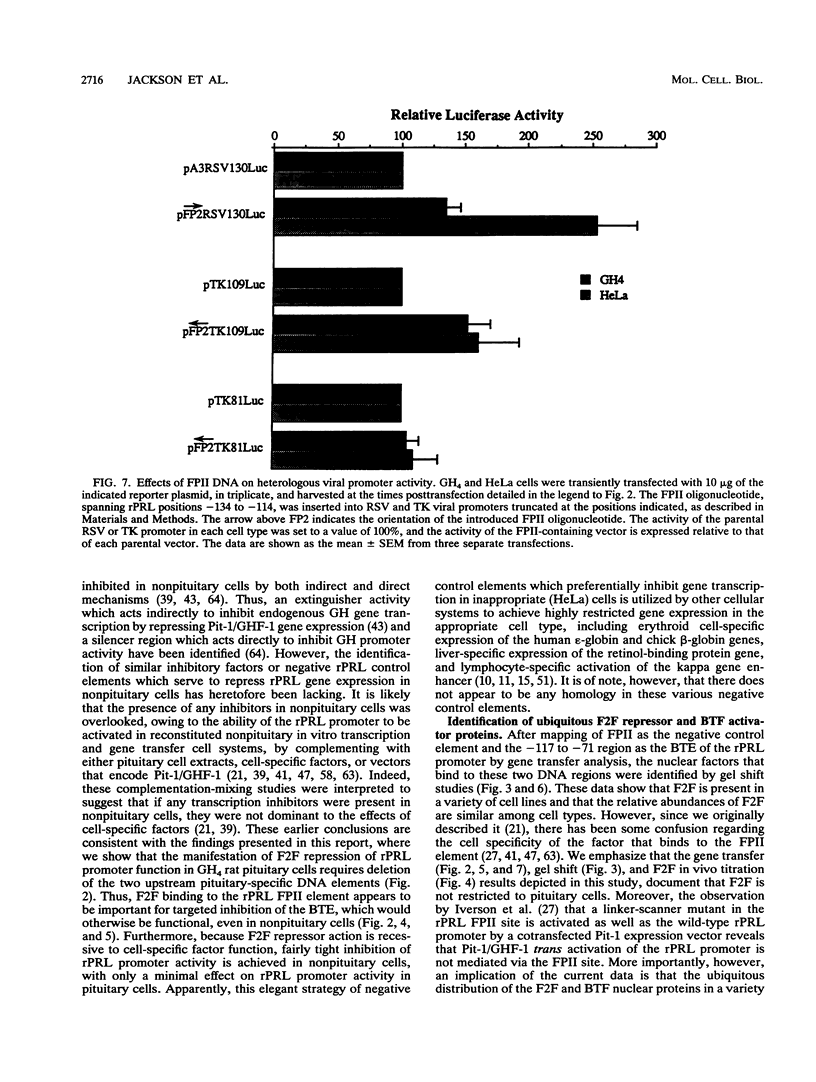
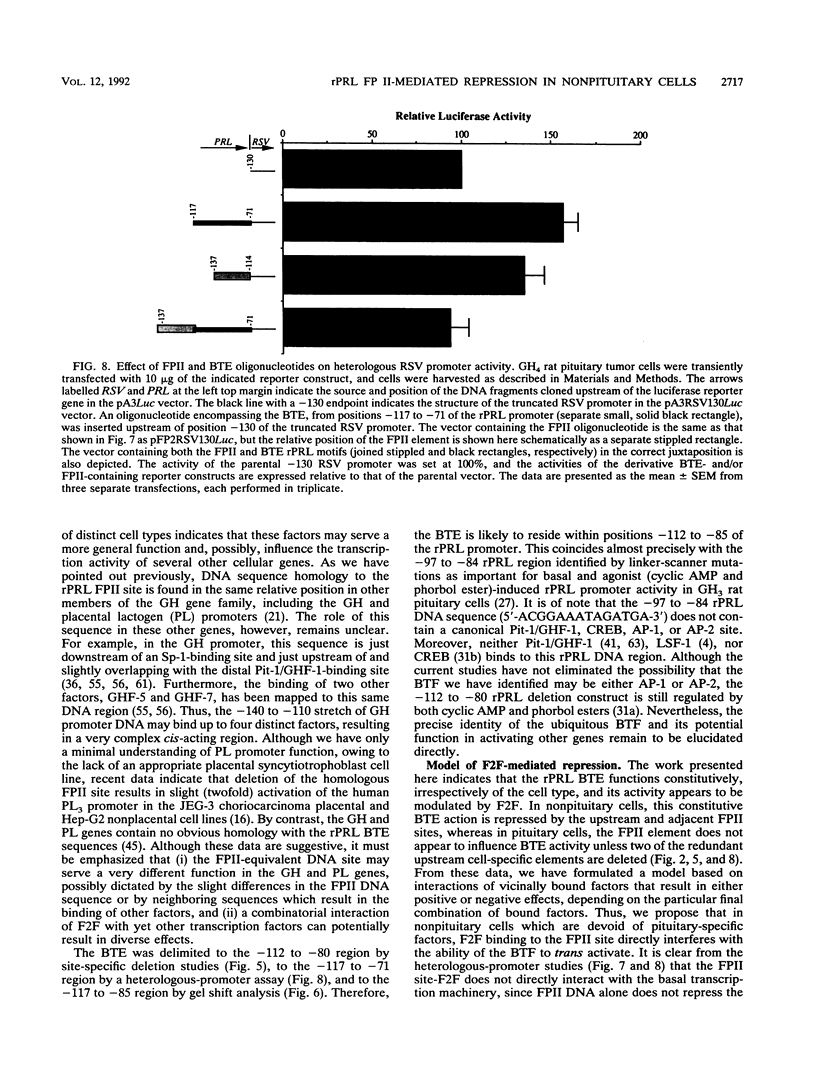
The proximal rat prolactin (rPRL) promoter contains three cell-specific elements, designated footprints I, III, and IV, which restrict rPRL gene expression to anterior pituitary lactotroph cells. Footprint II (-130 to -120) binds a factor, which we have termed F2F, present in pituitary and nonpituitary cell types. Here we demonstrate that a key role of the footprint II site is to inhibit rPRL promoter activity in nonpituitary cells, specifically, by interfering with the basal activating function of a vicinal element. Gene transfer analysis revealed 20-fold activation of the rPRL promoter in nonpituitary cell types when footprint II was either deleted or specifically mutated. Similar activation of the intact rPRL promoter was obtained by in vivo F2F titration studies. In GH4 rat pituitary cells, the footprint II inhibitory activity was masked by the redundant, positively acting cell-specific elements and was inhibitory only if the two upstream sites, footprints III and IV, were deleted. Deletion of the -112 to -80 region in the footprint II site-specific mutant background resulted in complete loss of rPRL promoter activity in both pituitary and nonpituitary cell types, mapping a basal activating element that is operative irrespective of cell type to this region. While the basal activating element imparted an activating function in a heterologous promoter assay, the footprint II sequence did not display any inherent repressor function and actually induced several minimal heterologous promoters. However, the inhibitory activity of the footprint II site was detected only if it was in context with the basal activating element. These data underscore the importance of ubiquitous activating and inhibitory factors in establishing cell-specific gene expression and further emphasize the complexity of the molecular mechanisms which restrict gene expression to specific cell types. We provide a novel paradigm to study rPRL promoter function and hormone responsiveness independently of lactotroph cell-specific requirements.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988 Mar 11;52(5):685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Alexander L. M., Gordon D. F., Wood W. M., Kao M. Y., Ridgway E. C., Gutierrez-Hartmann A. Identification of thyrotroph-specific factors and cis-acting sequences of the murine thyrotropin beta subunit gene. Mol Endocrinol. 1989 Jul;3(7):1037–1045. doi: 10.1210/mend-3-7-1037. [DOI] [PubMed] [Google Scholar]
- Alexander L. M., Williamson D. J., Wood W. M., Gordon D. F., Ridgway E. C., Gutierrez-Hartmann A. Activation of the murine thyrotropin beta-subunit promoter by GH4 rat pituitary cell-free extracts. Mol Endocrinol. 1990 Dec;4(12):1887–1896. doi: 10.1210/mend-4-12-1887. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990 Nov 16;63(4):815–825. doi: 10.1016/0092-8674(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Baumhueter S., Mendel D. B., Conley P. B., Kuo C. J., Turk C., Graves M. K., Edwards C. A., Courtois G., Crabtree G. R. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 1990 Mar;4(3):372–379. doi: 10.1101/gad.4.3.372. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. A purified Drosophila homeodomain protein represses transcription in vitro. Cell. 1989 Aug 11;58(3):433–440. doi: 10.1016/0092-8674(89)90424-8. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni V., Pirozzi A., Blance C., Cortese R. Negative control of liver-specific gene expression: cloned human retinol-binding protein gene is repressed in HeLa cells. EMBO J. 1987 Mar;6(3):631–636. doi: 10.1002/j.1460-2075.1987.tb04801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. Expression of GHF-1 protein in mouse pituitaries correlates both temporally and spatially with the onset of growth hormone gene activity. Cell. 1990 Mar 9;60(5):809–820. doi: 10.1016/0092-8674(90)90095-v. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Fong T. C. Erythroid-specific activation and derepression of the chick beta-globin promoter in vitro. Cell. 1989 Jun 30;57(7):1189–1200. doi: 10.1016/0092-8674(89)90056-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S. L., Walker W. H., Saunders G. F. DNA sequences involved in the transcriptional activation of a human placental lactogen gene. Mol Endocrinol. 1990 Dec;4(12):1815–1826. doi: 10.1210/mend-4-12-1815. [DOI] [PubMed] [Google Scholar]
- Frain M., Swart G., Monaci P., Nicosia A., Stämpfli S., Frank R., Cortese R. The liver-specific transcription factor LF-B1 contains a highly diverged homeobox DNA binding domain. Cell. 1989 Oct 6;59(1):145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A., Siddiqui S., Loukin S. Selective transcription and DNase I protection of the rat prolactin gene by GH3 pituitary cell-free extracts. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5211–5215. doi: 10.1073/pnas.84.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey C., Jackson S. M., Siddiqui S. K., Gutierrez-Hartmann A. Structure-function analysis of the rat prolactin promoter: phasing requirements of proximal cell-specific elements. Mol Endocrinol. 1991 Jun;5(6):836–843. doi: 10.1210/mend-5-6-836. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Scott M. P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990 Nov 30;63(5):883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Iverson R. A., Day K. H., d'Emden M., Day R. N., Maurer R. A. Clustered point mutation analysis of the rat prolactin promoter. Mol Endocrinol. 1990 Oct;4(10):1564–1571. doi: 10.1210/mend-4-10-1564. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Activation and repression of transcription by homoeodomain-containing proteins that bind a common site. Nature. 1988 Dec 22;336(6201):744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech C. A., Gutierrez-Hartmann A. Analysis of rat prolactin promoter sequences that mediate pituitary-specific and 3',5'-cyclic adenosine monophosphate-regulated gene expression in vivo. Mol Endocrinol. 1989 May;3(5):832–839. doi: 10.1210/mend-3-5-832. [DOI] [PubMed] [Google Scholar]
- Keech C. A., Gutierrez-Hartmann A. Insulin activation of rat prolactin promoter activity. Mol Cell Endocrinol. 1991 Jun;78(1-2):55–60. doi: 10.1016/0303-7207(91)90185-u. [DOI] [PubMed] [Google Scholar]
- Keleher C. A., Goutte C., Johnson A. D. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell. 1988 Jun 17;53(6):927–936. doi: 10.1016/s0092-8674(88)90449-7. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Saffman E. E., Kornfeld K., Hogness D. S. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Bancroft C. Identification by cell fusion of gene sequences that interact with positive trans-acting factors. Science. 1987 Jul 17;237(4812):283–286. doi: 10.1126/science.3474782. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Jackson A. E., Pan W. T., Bancroft C. Proximal rat prolactin promoter sequences direct optimal, pituitary cell-specific transcription. Mol Endocrinol. 1989 Mar;3(3):559–566. doi: 10.1210/mend-3-3-559. [DOI] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Harrison G. S., Wood W. M., Maxwell F. A DNA cassette containing a trimerized SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. Biotechniques. 1989 Mar;7(3):276–280. [PubMed] [Google Scholar]
- McCormick A., Wu D., Castrillo J. L., Dana S., Strobl J., Thompson E. B., Karin M. Extinction of growth hormone expression in somatic cell hybrids involves repression of the specific trans-activator GHF-1. Cell. 1988 Oct 21;55(2):379–389. doi: 10.1016/0092-8674(88)90061-x. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Eberhardt N. L. Structure and evolution of the growth hormone gene family. Endocr Rev. 1983 Spring;4(2):97–130. doi: 10.1210/edrv-4-2-97. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Binding site-dependent direct activation and repression of in vitro transcription by Drosophila homeodomain proteins. Cell. 1990 May 4;61(3):475–484. doi: 10.1016/0092-8674(90)90529-n. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Engrailed, a homeodomain protein, can repress in vitro transcription by competition with the TATA box-binding protein transcription factor IID. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2289–2293. doi: 10.1073/pnas.87.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G., Finney M. Regulation of transcription and cell identity by POU domain proteins. Cell. 1991 Feb 8;64(3):475–478. doi: 10.1016/0092-8674(91)90227-p. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Smith D. L., Johnson A. D. Flexibility of the yeast alpha 2 repressor enables it to occupy the ends of its operator, leaving the center free. Genes Dev. 1988 Jul;2(7):807–816. doi: 10.1101/gad.2.7.807. [DOI] [PubMed] [Google Scholar]
- Schaufele F., West B. L., Reudelhuber T. L. Overlapping Pit-1 and Sp1 binding sites are both essential to full rat growth hormone gene promoter activity despite mutually exclusive Pit-1 and Sp1 binding. J Biol Chem. 1990 Oct 5;265(28):17189–17196. [PubMed] [Google Scholar]
- Schaufele F., West B. L., Reudelhuber T. Somatotroph- and lactotroph-specific interactions with the homeobox protein binding sites in the rat growth hormone gene promoter. Nucleic Acids Res. 1990 Sep 11;18(17):5235–5243. doi: 10.1093/nar/18.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W. A., Treacy M. N., Martin F. Tissue specific trans-acting factor interaction with proximal rat prolactin gene promoter sequences. EMBO J. 1988 Jun;7(6):1721–1733. doi: 10.1002/j.1460-2075.1988.tb03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp Z. D., Helsel S., Cao Z. D., Barron E. A., Sanchez Y. DNA recognition element required for PUF-I mediated cell-type-specific transcription of the rat prolactin gene. Nucleic Acids Res. 1989 Apr 11;17(7):2705–2722. doi: 10.1093/nar/17.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld M. G., Swanson L. W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990 May;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Tansey W. P., Catanzaro D. F. Sp1 and thyroid hormone receptor differentially activate expression of human growth hormone and chorionic somatomammotropin genes. J Biol Chem. 1991 May 25;266(15):9805–9813. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theill L. E., Castrillo J. L., Wu D., Karin M. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989 Dec 21;342(6252):945–948. doi: 10.1038/342945a0. [DOI] [PubMed] [Google Scholar]
- Tripputi P., Guérin S. L., Moore D. D. Two mechanisms for the extinction of gene expression in hybrid cells. Science. 1988 Sep 2;241(4870):1205–1207. doi: 10.1126/science.2842865. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Sparkes R. S., Bertolotti R. Expression of differentiated functions in hepatoma cell hybrids: IX extinction and reexpression of liver-specific enzymes in rat hepatoma-Chinese hamster fibroblast hybrids. Somatic Cell Genet. 1975 Jan;1(1):27–40. doi: 10.1007/BF01538730. [DOI] [PubMed] [Google Scholar]
- West B. L., Catanzaro D. F., Mellon S. H., Cattini P. A., Baxter J. D., Reudelhuber T. L. Interaction of a tissue-specific factor with an essential rat growth hormone gene promoter element. Mol Cell Biol. 1987 Mar;7(3):1193–1197. doi: 10.1128/mcb.7.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. M., Ocran K. W., Kao M. Y., Gordon D. F., Alexander L. M., Gutierrez-Hartmann A., Ridgway E. C. Protein factors in thyrotropic tumor nuclear extracts bind to a region of the mouse thyrotropin beta-subunit promoter essential for expression in thyrotropes. Mol Endocrinol. 1990 Dec;4(12):1897–1904. doi: 10.1210/mend-4-12-1897. [DOI] [PubMed] [Google Scholar]
- Yu H., Porton B., Shen L. Y., Eckhardt L. A. Role of the octamer motif in hybrid cell extinction of immunoglobulin gene expression: extinction is dominant in a two enhancer system. Cell. 1989 Aug 11;58(3):441–448. doi: 10.1016/0092-8674(89)90425-x. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]