Abstract
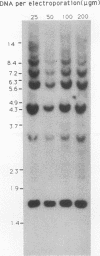
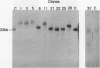
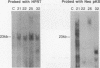
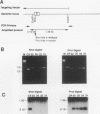
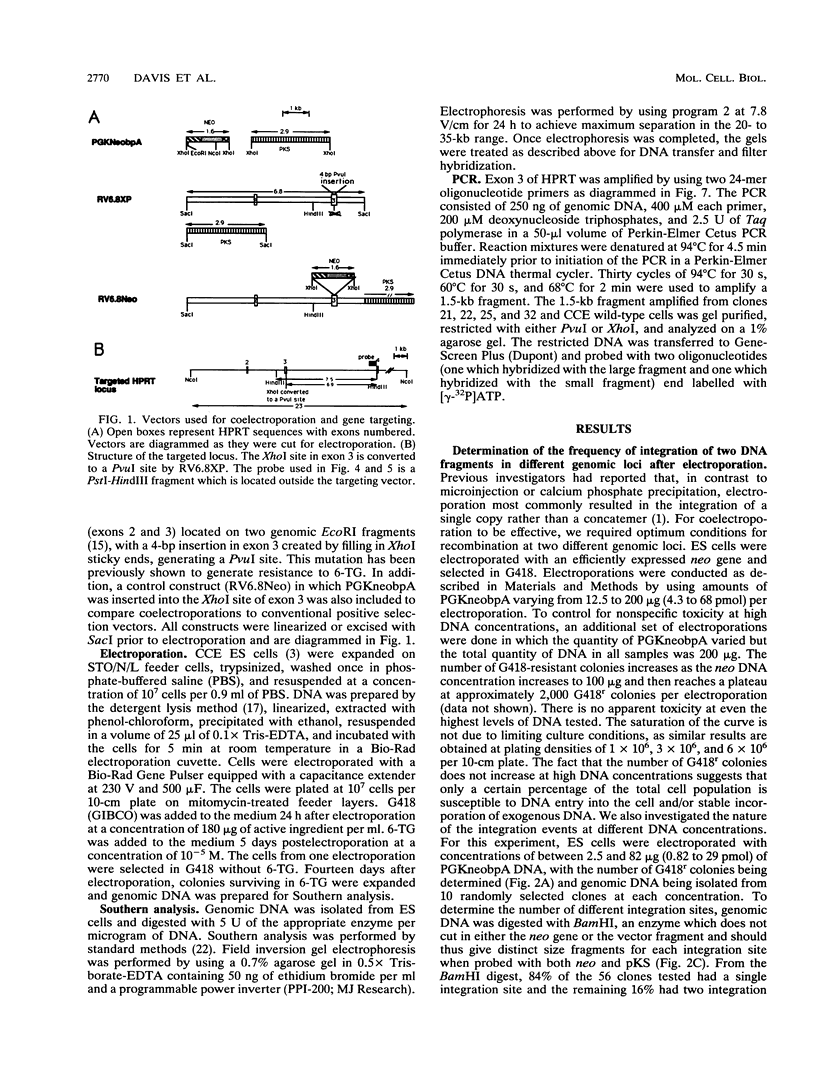
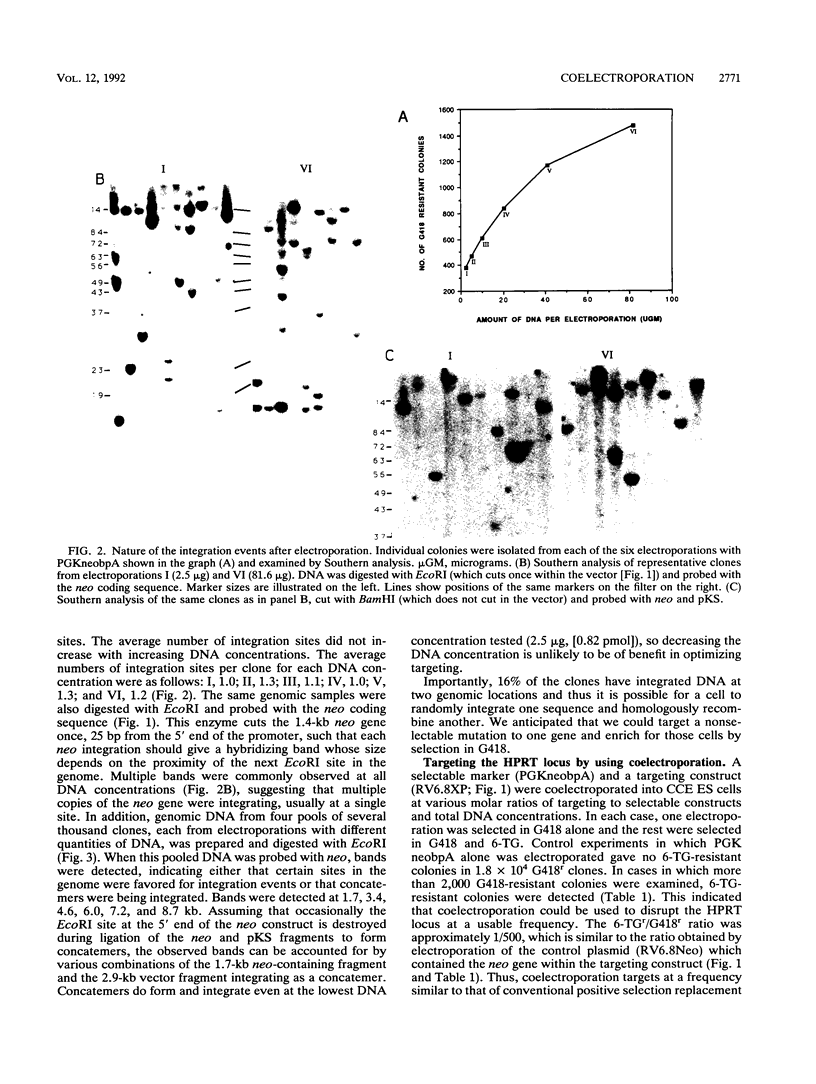
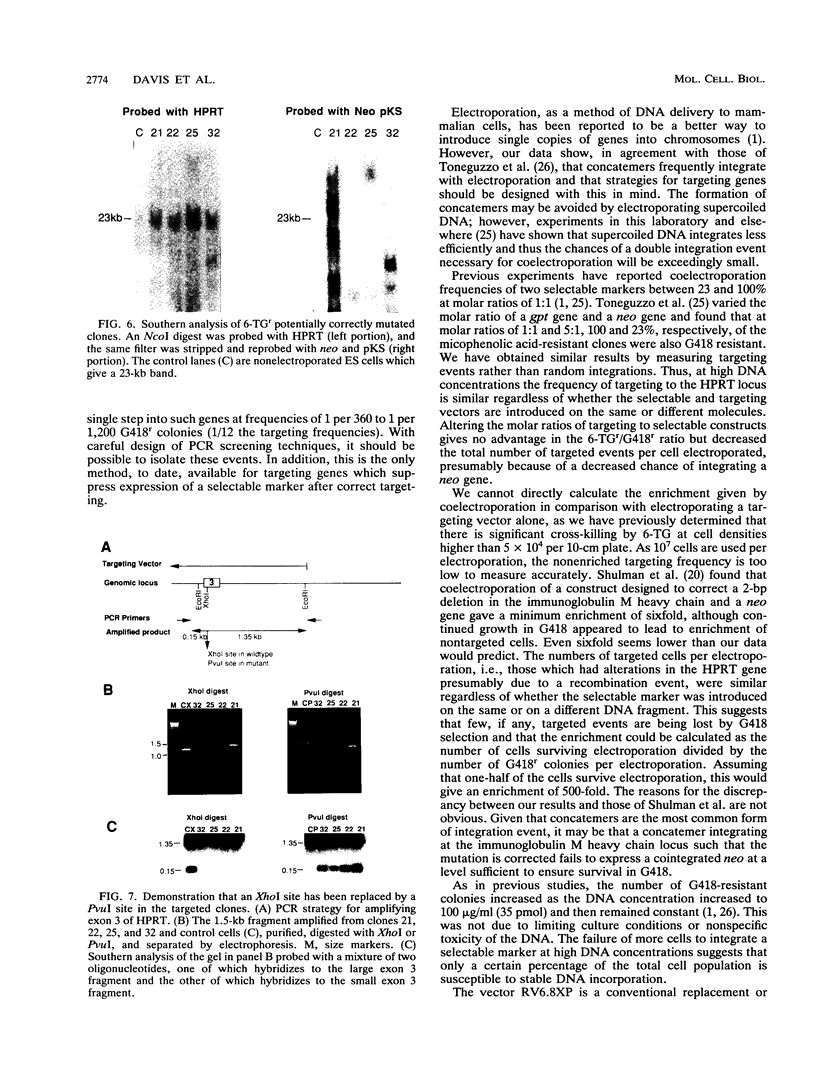
We have investigated coelectroporation as a method for introducing minor genetic changes into specific genes in embryonic stem cells. A selectable marker (neo) and a targeting replacement vector designed to insert a 4-bp insertion into exon 3 of the mouse hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene were coelectroporated into embryonic stem cells and selected in G418 and 6-thioguanine (6-TG). HPRT-negative clones were obtained at a frequency of approximately 1 per 520 G418r clones. Southern analysis and the polymerase chain reaction were used to demonstrate that 3 of 36 of the 6-TG-resistant clones had the desired 4-bp insertion without any other disruption of the HPRT locus. Initial studies indicated that the other 33 6-TG-resistant clones probably resulted from the targeted integration of a concatemer containing both the targeting construct and the selectable neo gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Fell H. P., Yarnold S., Hellström I., Hellström K. E., Folger K. R. Homologous recombination in hybridoma cells: heavy chain chimeric antibody produced by gene targeting. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8507–8511. doi: 10.1073/pnas.86.21.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Chang C., Bradley A. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol Cell Biol. 1991 Sep;11(9):4509–4517. doi: 10.1128/mcb.11.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Berg P. Homologous integration in mammalian cells without target gene selection. Genes Dev. 1988 Nov;2(11):1353–1363. doi: 10.1101/gad.2.11.1353. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Sheng M., Greenberg M. E., Kolodner R. D., Papaioannou V. E., Spiegelman B. M. Targeting of nonexpressed genes in embryonic stem cells via homologous recombination. Science. 1989 Sep 15;245(4923):1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989 Mar 9;338(6211):153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Lemna W. K., Feldman G. L., Kerem B., Fernbach S. D., Zevkovich E. P., O'Brien W. E., Riordan J. R., Collins F. S., Tsui L. C., Beaudet A. L. Mutation analysis for heterozygote detection and the prenatal diagnosis of cystic fibrosis. N Engl J Med. 1990 Feb 1;322(5):291–296. doi: 10.1056/NEJM199002013220503. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989 Nov 10;246(4931):799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Robertson E. J., Goff S. P. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3210–3214. doi: 10.1073/pnas.87.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Nissen L., Collins C. Homologous recombination in hybridoma cells: dependence on time and fragment length. Mol Cell Biol. 1990 Sep;10(9):4466–4472. doi: 10.1128/mcb.10.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Keating A., Glynn S., McDonald K. Electric field-mediated gene transfer: characterization of DNA transfer and patterns of integration in lymphoid cells. Nucleic Acids Res. 1988 Jun 24;16(12):5515–5532. doi: 10.1093/nar/16.12.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Testing an "in-out" targeting procedure for making subtle genomic modifications in mouse embryonic stem cells. Mol Cell Biol. 1991 Mar;11(3):1402–1408. doi: 10.1128/mcb.11.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]