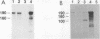Abstract
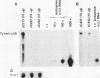
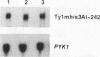
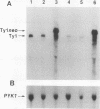
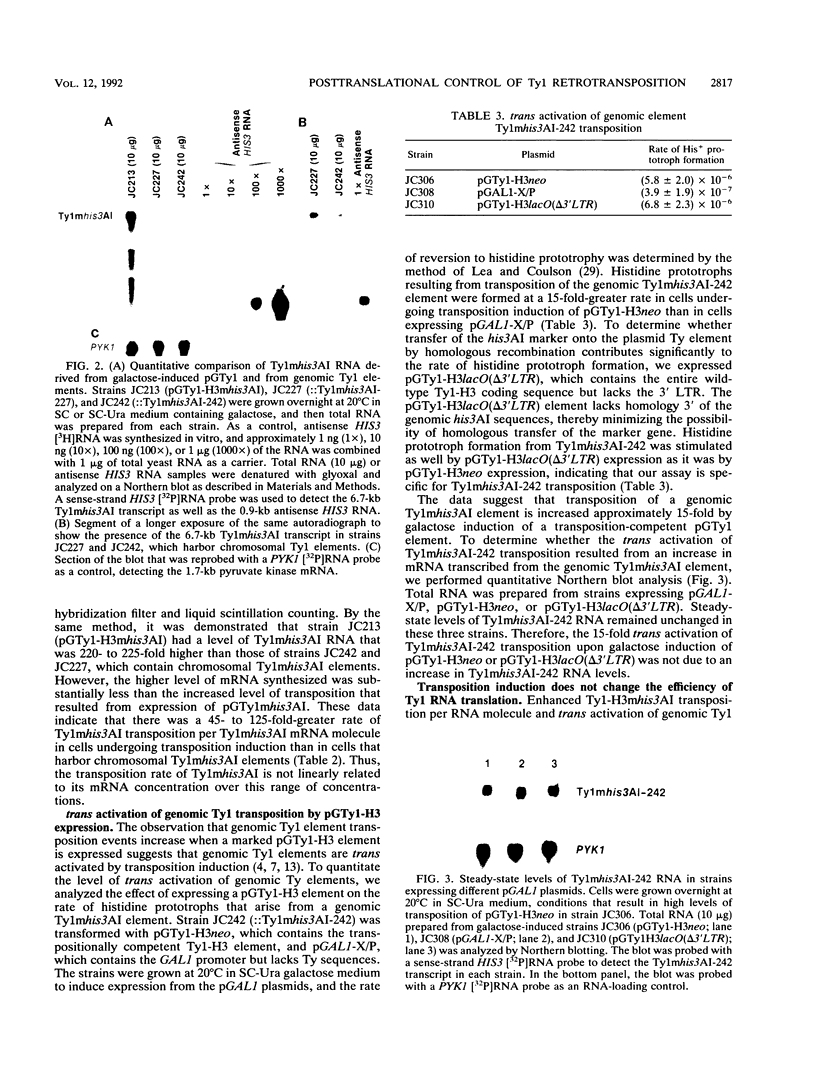
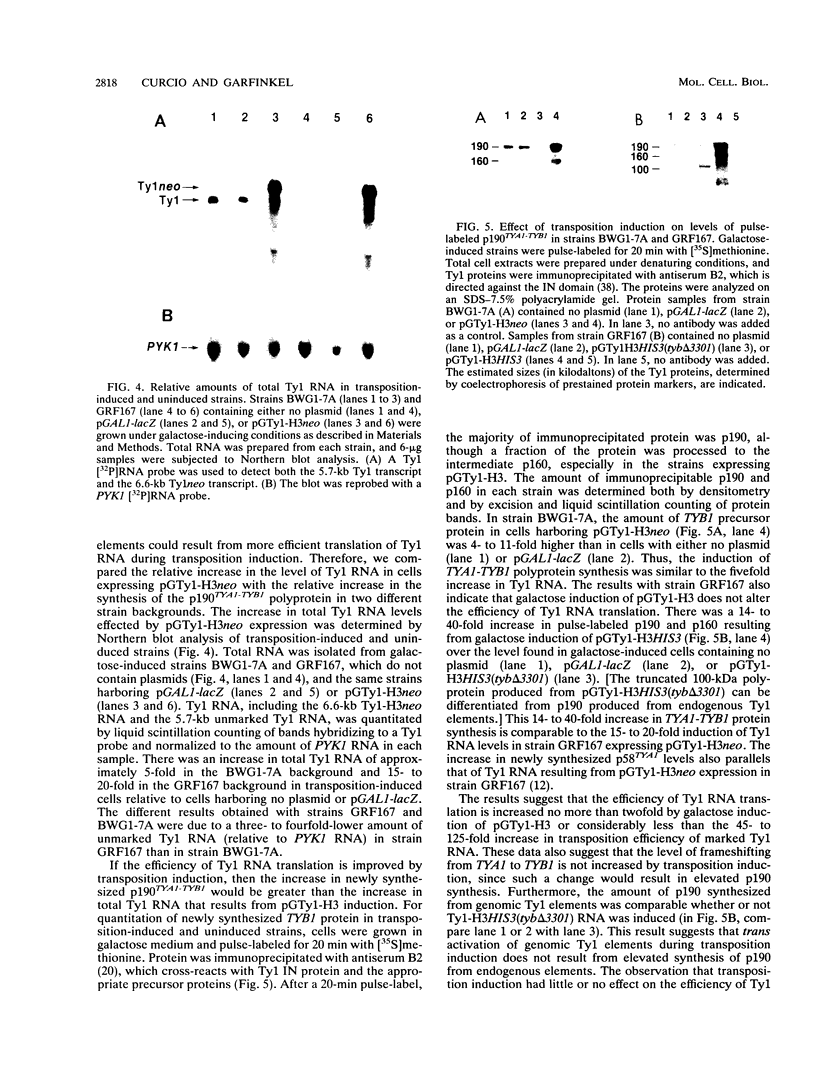
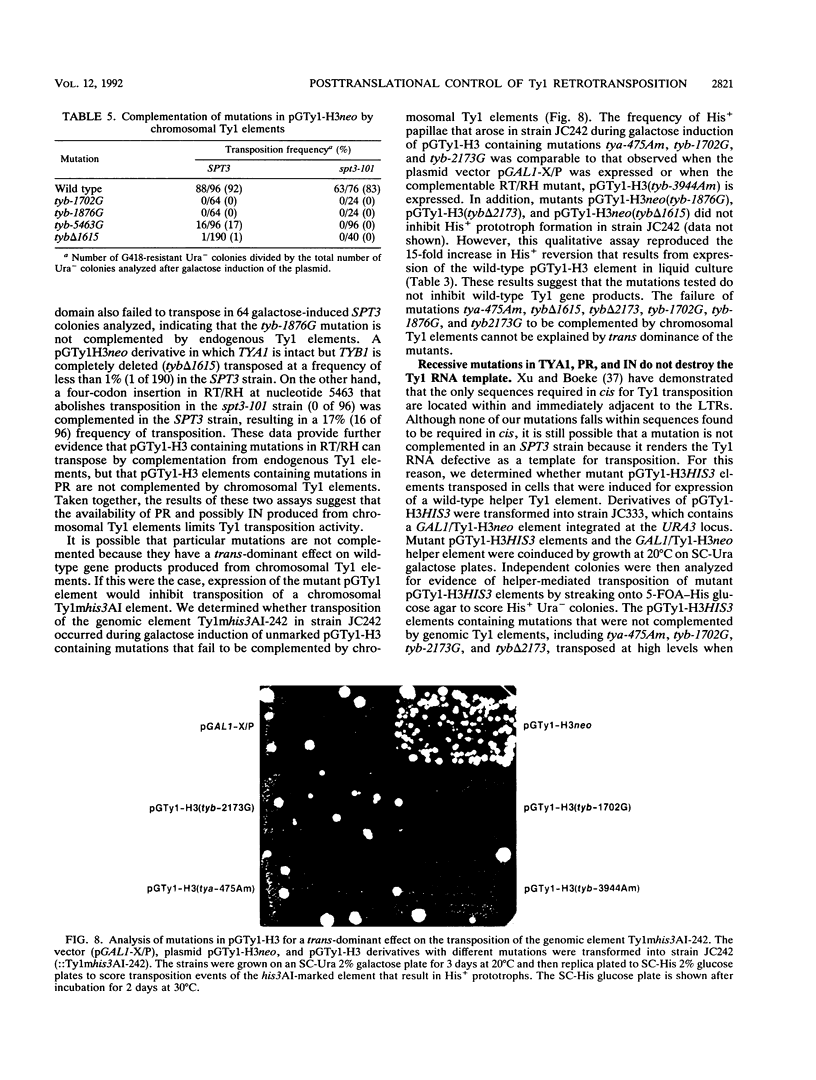
High-level expression of a transpositionally competent Ty1 element fused to the inducible GAL1 promoter on a 2 microns plasmid (pGTy1) overcomes transpositional dormancy in Saccharomyces cerevisiae. To investigate the mechanisms controlling the rate of Ty1 retrotransposition, we quantitated transposition and Ty1 gene products in cells induced and uninduced for expression of pGTy1. The increase in Ty1 transposition was 45- to 125-fold greater than the increase in Ty1 RNA effected by pGTy1 induction. Translational efficiency of Ty1 RNA was not altered in transposition-induced cells, since p190TYA1-TYB1 protein synthesis increased in proportion to steady-state Ty1 RNA levels. Therefore, expression of a pGTy1 element increases the efficiency of Ty1 transposition at a posttranslational level. Galactose induction of pGTy1 enhanced TYA1 protein processing and allowed detection of processed TYB1 proteins, which are normally present at very low levels in uninduced cells. When the ability of genomic Ty1 elements to complement defined mutations in HIS3-marked pGTy1 elements was examined, mutations in the protease domain or certain mutations in the integrase domain failed to be complemented, but mutations in the reverse transcriptase domain were partially complemented by genomic Ty1 elements. Therefore, the activity of Ty1 elements in yeast cells may be limited by the availability of Ty1 protease and possibly integrase. These results suggest that Ty1 transposition is regulated at the level of protein processing and that this regulation is overcome by expression of a pGTy1 element.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Mellor J., Gull K., Sim R. B., Tuite M. F., Kingsman S. M., Kingsman A. J. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell. 1987 Apr 10;49(1):111–119. doi: 10.1016/0092-8674(87)90761-6. [DOI] [PubMed] [Google Scholar]
- Belcourt M. F., Farabaugh P. J. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990 Jul 27;62(2):339–352. doi: 10.1016/0092-8674(90)90371-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Eichinger D., Castrillon D., Fink G. R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988 Apr;8(4):1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Styles C. A., Fink G. R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986 Nov;6(11):3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Xu H., Fink G. R. A general method for the chromosomal amplification of genes in yeast. Science. 1988 Jan 15;239(4837):280–282. doi: 10.1126/science.2827308. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Clare J., Farabaugh P. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2829–2833. doi: 10.1073/pnas.82.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M. J., Garfinkel D. J. Single-step selection for Ty1 element retrotransposition. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M. J., Hedge A. M., Boeke J. D., Garfinkel D. J. Ty RNA levels determine the spectrum of retrotransposition events that activate gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1990 Jan;220(2):213–221. doi: 10.1007/BF00260484. [DOI] [PubMed] [Google Scholar]
- Curcio M. J., Sanders N. J., Garfinkel D. J. Transpositional competence and transcription of endogenous Ty elements in Saccharomyces cerevisiae: implications for regulation of transposition. Mol Cell Biol. 1988 Sep;8(9):3571–3581. doi: 10.1128/mcb.8.9.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Elder R. T., St John T. P., Stinchcomb D. T., Davis R. W., Scherer S., Davis R. W. Studies on the transposable element Ty1 of yeast. I. RNA homologous to Ty1. II. Recombination and expression of Ty1 and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):581–591. doi: 10.1101/sqb.1981.045.01.075. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Curcio M. J., Youngren S. D., Sanders N. J. The biology and exploitation of the retrotransposon Ty in Saccharomyces cerevisiae. Genome. 1989;31(2):909–919. doi: 10.1139/g89-162. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Hedge A. M., Youngren S. D., Copeland T. D. Proteolytic processing of pol-TYB proteins from the yeast retrotransposon Ty1. J Virol. 1991 Sep;65(9):4573–4581. doi: 10.1128/jvi.65.9.4573-4581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Mastrangelo M. F., Sanders N. J., Shafer B. K., Strathern J. N. Transposon tagging using Ty elements in yeast. Genetics. 1988 Sep;120(1):95–108. doi: 10.1093/genetics/120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Nelböck-Hochstetter P., Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucleic Acids Res. 1985 Apr 25;13(8):2745–2758. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Schneider H., Zybarth G., Carter C. A., Wimmer E. Processing of in vitro-synthesized gag precursor proteins of human immunodeficiency virus (HIV) type 1 by HIV proteinase generated in Escherichia coli. J Virol. 1988 Nov;62(11):4393–4397. doi: 10.1128/jvi.62.11.4393-4397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Brühl K. H., Freidel K., Kowallik K. V., Ciriacy M. Processing of TY1 proteins and formation of Ty1 virus-like particles in Saccharomyces cerevisiae. Mol Gen Genet. 1987 May;207(2-3):421–429. doi: 10.1007/BF00331610. [DOI] [PubMed] [Google Scholar]
- Müller F., Laufer W., Pott U., Ciriacy M. Characterization of products of TY1-mediated reverse transcription in Saccharomyces cerevisiae. Mol Gen Genet. 1991 Apr;226(1-2):145–153. doi: 10.1007/BF00273598. [DOI] [PubMed] [Google Scholar]
- Picologlou S., Brown N., Liebman S. W. Mutations in RAD6, a yeast gene encoding a ubiquitin-conjugating enzyme, stimulate retrotransposition. Mol Cell Biol. 1990 Mar;10(3):1017–1022. doi: 10.1128/mcb.10.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbauch P., Sulica A., Givol D. General method for the detection of cells producing antibodies against haptens and proteins. Nature. 1970 Jul 4;227(5253):68–69. doi: 10.1038/227068a0. [DOI] [PubMed] [Google Scholar]
- Winston F., Durbin K. J., Fink G. R. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984 Dec;39(3 Pt 2):675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- Xu H., Boeke J. D. Localization of sequences required in cis for yeast Ty1 element transposition near the long terminal repeats: analysis of mini-Ty1 elements. Mol Cell Biol. 1990 Jun;10(6):2695–2702. doi: 10.1128/mcb.10.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren S. D., Boeke J. D., Sanders N. J., Garfinkel D. J. Functional organization of the retrotransposon Ty from Saccharomyces cerevisiae: Ty protease is required for transposition. Mol Cell Biol. 1988 Apr;8(4):1421–1431. doi: 10.1128/mcb.8.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]