Abstract
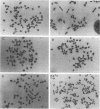
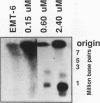
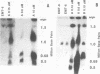
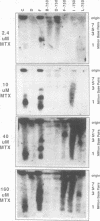
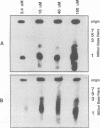
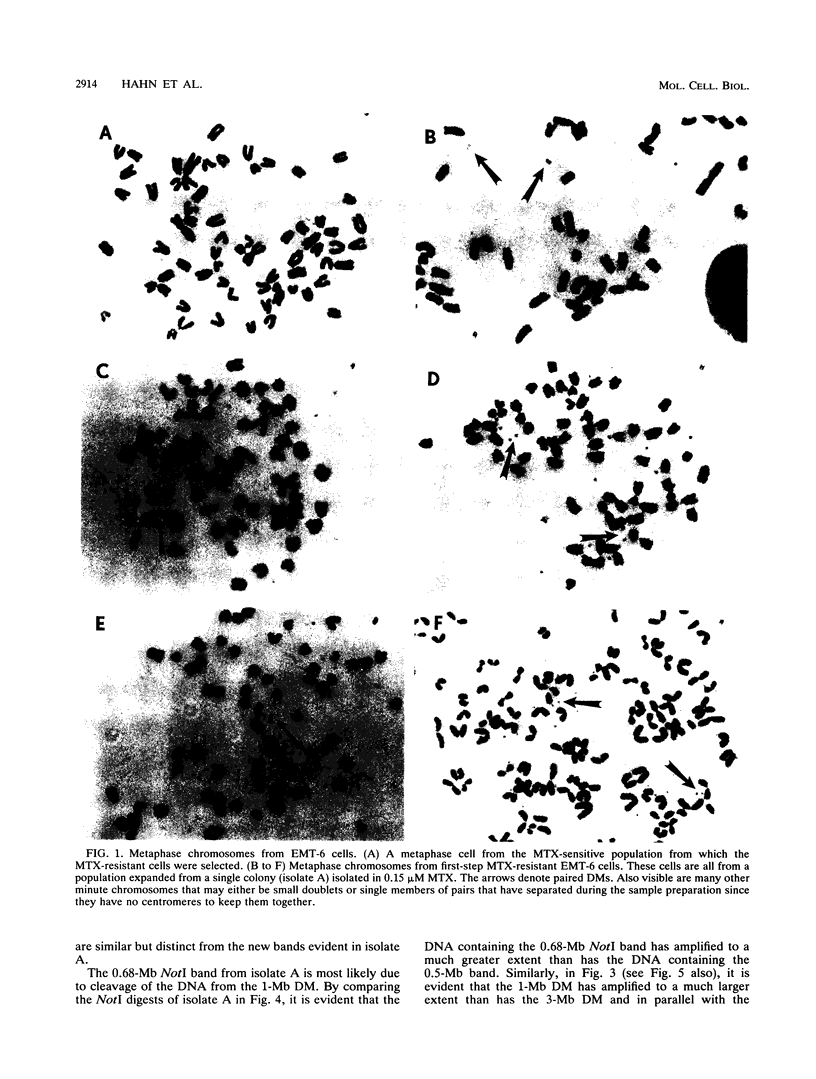
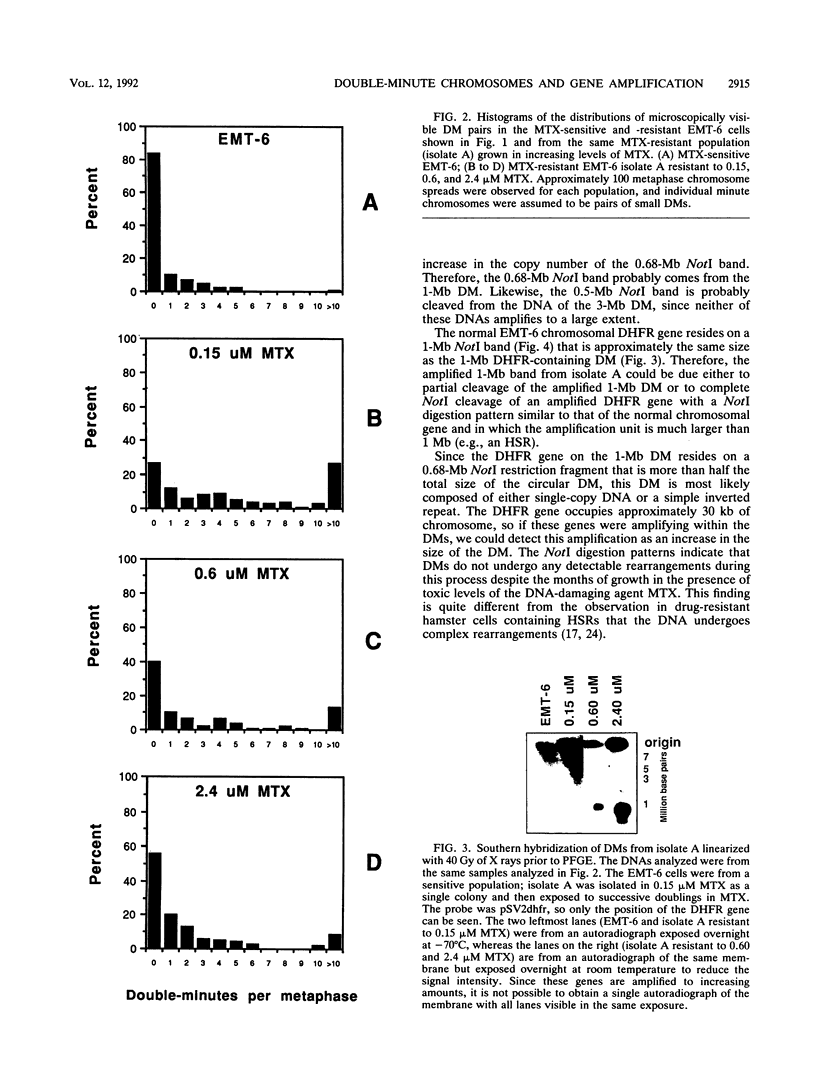
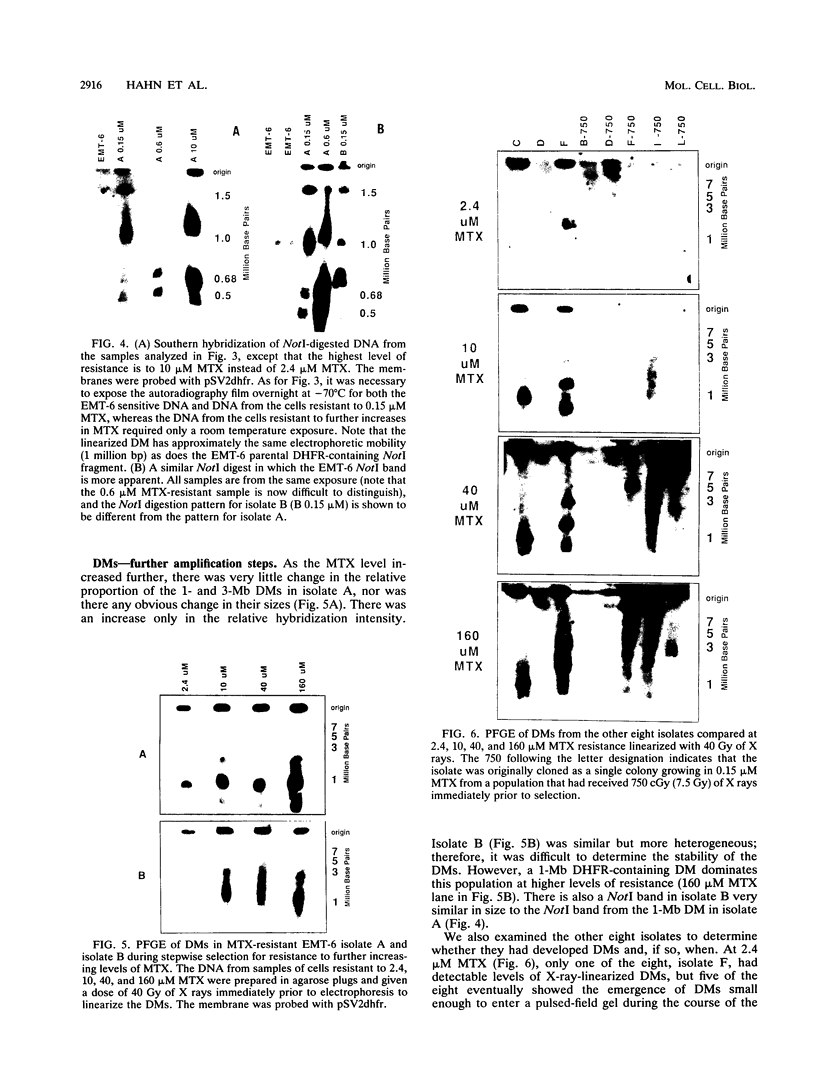
To determine whether microscopically visible double-minute chromosomes (DMs) are derived from submicroscopic precursors, we monitored the amplification of the dihydrofolate reductase (DHFR) gene in 10 independent isolates of methotrexate (MTX)-resistant mouse cells. At every other doubling in MTX concentration, the cells were examined both microscopically, to detect the presence of microscopically visible DMs, and by pulsed-field gel electrophoresis and hybridization to a DHFR-specific probe, to detect submicroscopic DMs. One of the cloned MTX-resistant isolates was examined in detail and was shown to originally contain amplified DHFR genes on circular DMs measuring 1 and 3 Mb in size; additionally, metaphase chromosome preparations from this cloned isolate were examined and were shown to contain microscopically visible DMs too large to enter a pulsed-field gel. During stepwise selection for increasing levels of MTX, the smaller DMs (not microscopically visible) were shown to be preferentially amplified, whereas the larger (microscopically visible) ones decreased in relative numbers. Rare-cutting NotI digestion patterns of total genomic DNA that includes the DMs containing the DHFR gene suggest that the DMs increase in copy number without any further significant rearrangements. We saw no evidence from any of the 10 isolates to suggest that microscopically visible DMs are formed from smaller submicroscopic precursors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn S. Y., Nevaldine B., Hahn P. J. Direct measurement by pulsed-field gel electrophoresis of induction and rejoining of X-ray-induced double-strand breaks in cultured mouse cells. Int J Radiat Biol. 1991 Mar;59(3):661–675. doi: 10.1080/09553009114550591. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Scotto K. W., Melera P. W., Spengler B. A. Chromosomal organization of amplified genes in multidrug-resistant Chinese hamster cells. Cancer Res. 1988 Jun 1;48(11):3179–3187. [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- Federspiel N. A., Beverley S. M., Schilling J. W., Schimke R. T. Novel DNA rearrangements are associated with dihydrofolate reductase gene amplification. J Biol Chem. 1984 Jul 25;259(14):9127–9140. [PubMed] [Google Scholar]
- Giulotto E., Saito I., Stark G. R. Structure of DNA formed in the first step of CAD gene amplification. EMBO J. 1986 Sep;5(9):2115–2121. doi: 10.1002/j.1460-2075.1986.tb04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P., Morgan W. F., Painter R. B. The role of acentric chromosome fragments in gene amplification. Somat Cell Mol Genet. 1987 Nov;13(6):597–608. doi: 10.1007/BF01534480. [DOI] [PubMed] [Google Scholar]
- Hahn P., Nevaldine B., Morgan W. F. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. 1990 Sep;16(5):413–423. doi: 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Ma C. The mammalian dihydrofolate reductase locus. Biochim Biophys Acta. 1990 Oct 23;1087(2):107–125. doi: 10.1016/0167-4781(90)90195-8. [DOI] [PubMed] [Google Scholar]
- Hyrien O., Debatisse M., Buttin G., de Saint Vincent B. R. The multicopy appearance of a large inverted duplication and the sequence at the inversion joint suggest a new model for gene amplification. EMBO J. 1988 Feb;7(2):407–417. doi: 10.1002/j.1460-2075.1988.tb02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney J. E., Hamlin J. L. Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol. 1987 Feb;7(2):569–577. doi: 10.1128/mcb.7.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Looney J. E., Leu T. H., Hamlin J. L. Organization and genesis of dihydrofolate reductase amplicons in the genome of a methotrexate-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1988 Jun;8(6):2316–2327. doi: 10.1128/mcb.8.6.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passananti C., Davies B., Ford M., Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification. EMBO J. 1987 Jun;6(6):1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletti G., Lai E., Attardi G. Early appearance and long-term persistence of the submicroscopic extrachromosomal elements (amplisomes) containing the amplified DHFR genes in human cell lines. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2955–2959. doi: 10.1073/pnas.87.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Choi K. H., von Hoff D. D., Roninson I. B., Wahl G. M. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Mol Cell Biol. 1989 Jan;9(1):109–115. doi: 10.1128/mcb.9.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Chromosomal destabilization during gene amplification. Mol Cell Biol. 1990 Jun;10(6):3056–3066. doi: 10.1128/mcb.10.6.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Formation of an inverted duplication can be an initial step in gene amplification. Mol Cell Biol. 1988 Oct;8(10):4302–4313. doi: 10.1128/mcb.8.10.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Groves R., Giulotto E., Rolfe M., Stark G. R. Evolution and stability of chromosomal DNA coamplified with the CAD gene. Mol Cell Biol. 1989 Jun;9(6):2445–2452. doi: 10.1128/mcb.9.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Hamlin J. L. Early dihydrofolate reductase gene amplification events in CHO cells usually occur on the same chromosome arm as the original locus. Genes Dev. 1989 Dec;3(12A):1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- VanDevanter D. R., Piaskowski V. D., Casper J. T., Douglass E. C., Von Hoff D. D. Ability of circular extrachromosomal DNA molecules to carry amplified MYCN proto-oncogenes in human neuroblastomas in vivo. J Natl Cancer Inst. 1990 Dec 5;82(23):1815–1821. doi: 10.1093/jnci/82.23.1815. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Needham-VanDevanter D. R., Yucel J., Windle B. E., Wahl G. M. Amplified human MYC oncogenes localized to replicating submicroscopic circular DNA molecules. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4804–4808. doi: 10.1073/pnas.85.13.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- Windle B., Draper B. W., Yin Y. X., O'Gorman S., Wahl G. M. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991 Feb;5(2):160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]