Abstract
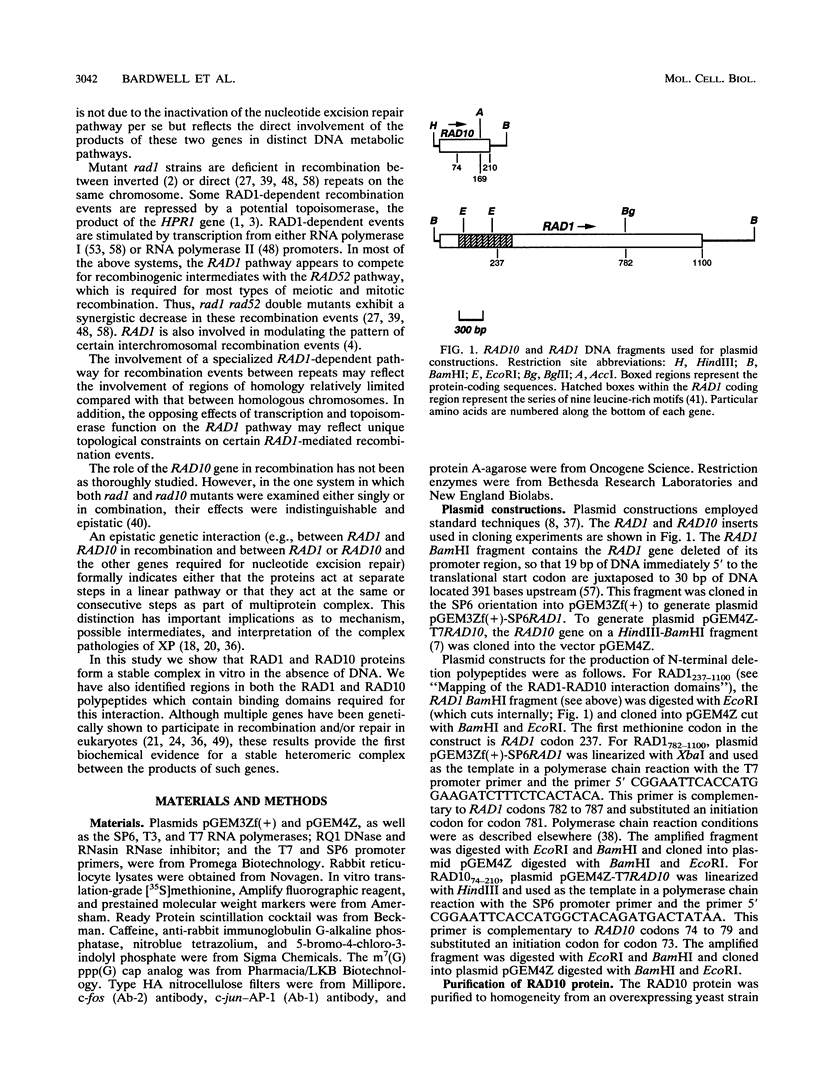
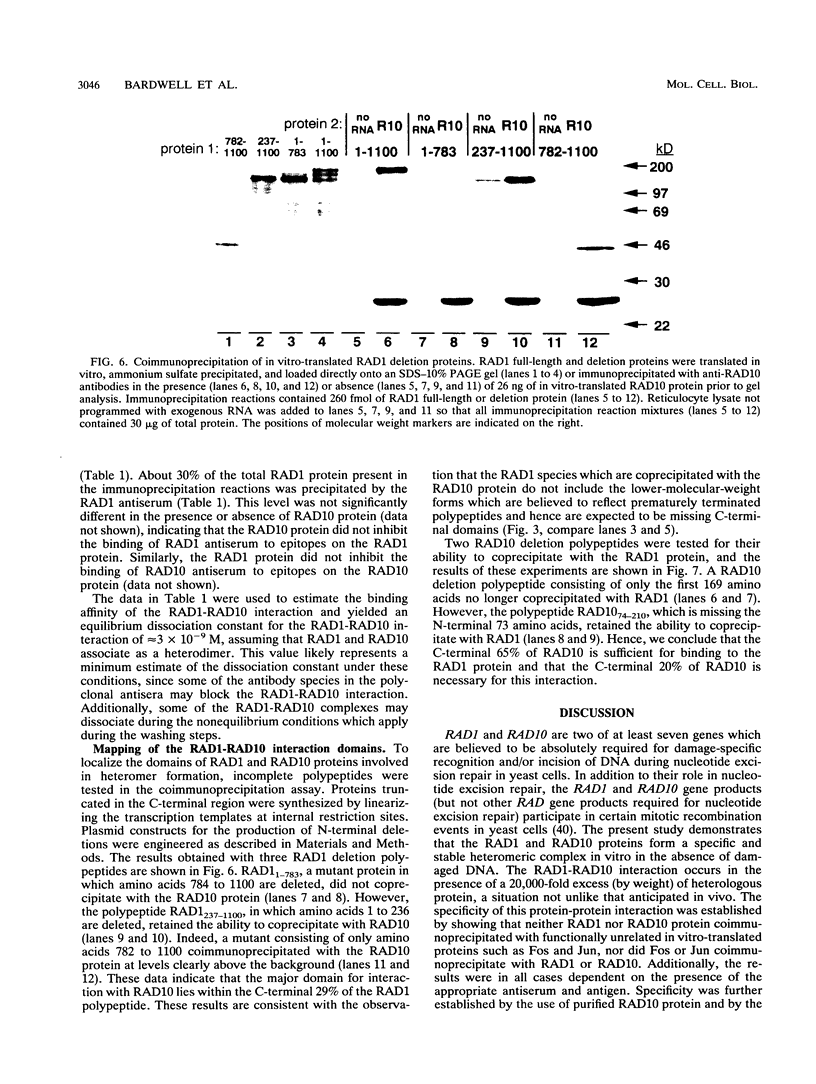
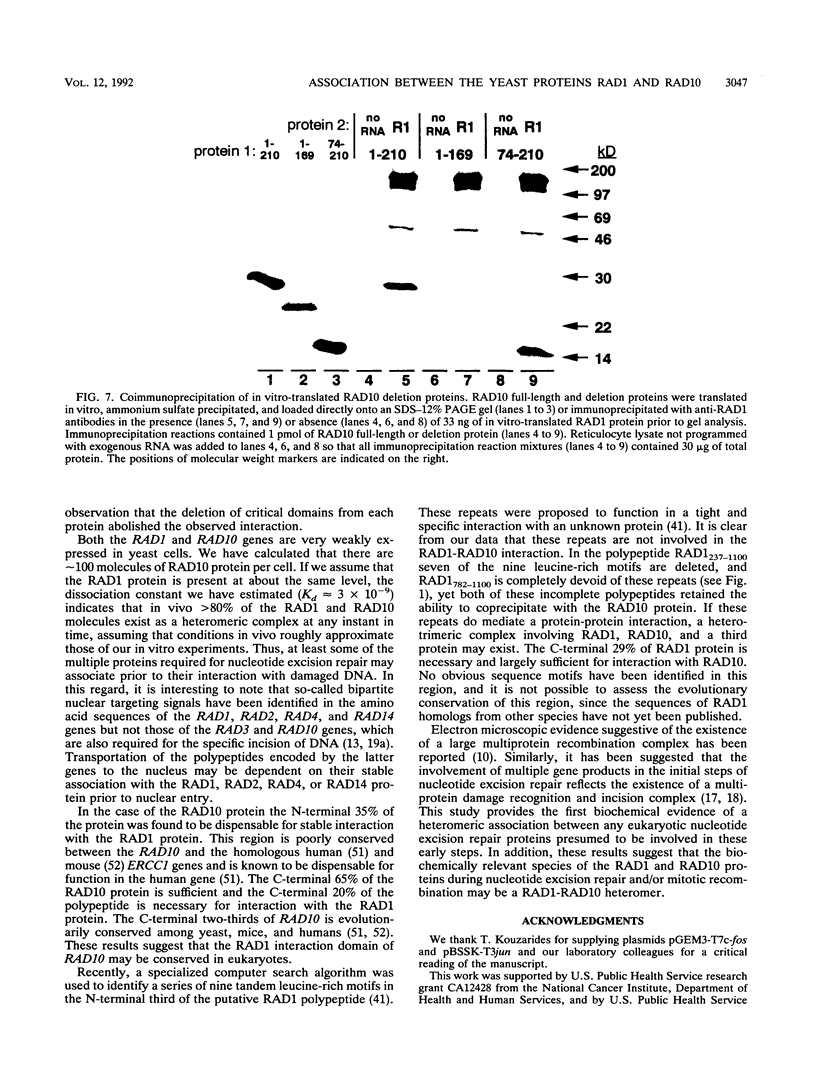
The RAD1 and RAD10 genes of Saccharomyces cerevisiae are two of at least seven genes which are known to be required for damage-specific recognition and/or damage-specific incision of DNA during nucleotide excision repair. RAD1 and RAD10 are also involved in a specialized mitotic recombination pathway. We have previously reported the purification of the RAD10 protein to homogeneity (L. Bardwell, H. Burtscher, W. A. Weiss, C. M. Nicolet, and E. C. Friedberg, Biochemistry 29:3119-3126, 1990). In the present studies we show that the RAD1 protein, produced by in vitro transcription and translation of the cloned gene, specifically coimmunoprecipitates with the RAD10 protein translated in vitro or purified from yeast. Conversely, in vitro-translated RAD10 protein specifically coimmunoprecipitates with the RAD1 protein. The sites of this stable and specific interaction have been mapped to the C-terminal regions of both polypeptides. This portion of RAD10 protein is evolutionarily conserved. These results are the first biochemical evidence of a specific association between any eukaryotic proteins genetically identified as belonging to a recombination or DNA repair pathway and suggest that the RAD1 and RAD10 proteins act at the same or consecutive biochemical steps in both nucleotide excision repair and mitotic recombination.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera A., Klein H. L. Genetic and molecular analysis of recombination events in Saccharomyces cerevisiae occurring in the presence of the hyper-recombination mutation hpr1. Genetics. 1989 Jul;122(3):503–517. doi: 10.1093/genetics/122.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A., Klein H. L. HPR1, a novel yeast gene that prevents intrachromosomal excision recombination, shows carboxy-terminal homology to the Saccharomyces cerevisiae TOP1 gene. Mol Cell Biol. 1990 Apr;10(4):1439–1451. doi: 10.1128/mcb.10.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A., Klein H. L. Yeast intrachromosomal recombination: long gene conversion tracts are preferentially associated with reciprocal exchange and require the RAD1 and RAD3 gene products. Genetics. 1989 Dec;123(4):683–694. doi: 10.1093/genetics/123.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis A. M., Rothstein R. A defect in mismatch repair in Saccharomyces cerevisiae stimulates ectopic recombination between homeologous genes by an excision repair dependent process. Genetics. 1990 Nov;126(3):535–547. doi: 10.1093/genetics/126.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Sung P., Prakash L., Prakash S. DNA.RNA helicase activity of RAD3 protein of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9712–9716. doi: 10.1073/pnas.88.21.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankmann M., Prakash L., Prakash S. Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature. 1992 Feb 6;355(6360):555–558. doi: 10.1038/355555a0. [DOI] [PubMed] [Google Scholar]
- Bardwell L., Burtscher H., Weiss W. A., Nicolet C. M., Friedberg E. C. Characterization of the RAD10 gene of Saccharomyces cerevisiae and purification of Rad10 protein. Biochemistry. 1990 Mar 27;29(12):3119–3126. doi: 10.1021/bi00464a031. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. Synaptonemal complex and recombination nodules in wild-type Drosophila melanogaster females. Genetics. 1979 Jun;92(2):511–541. doi: 10.1093/genetics/92.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Beguinot L., Ishii S., Ma D. P., Roe B. A., Merlino G. T., Pastan I. Synthesis of epidermal growth factor (EGF) receptor in vitro using SP6 RNA polymerase-transcribed template mRNA. Biochim Biophys Acta. 1986 Aug 22;867(4):244–251. doi: 10.1016/0167-4781(86)90040-0. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Yeast genes involved in DNA-repair processes: new looks on old faces. Mol Microbiol. 1991 Oct;5(10):2303–2310. doi: 10.1111/j.1365-2958.1991.tb02074.x. [DOI] [PubMed] [Google Scholar]
- Garfin D. E. One-dimensional gel electrophoresis. Methods Enzymol. 1990;182:425–441. doi: 10.1016/0076-6879(90)82035-z. [DOI] [PubMed] [Google Scholar]
- Harosh I., Naumovski L., Friedberg E. C. Purification and characterization of Rad3 ATPase/DNA helicase from Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 5;264(34):20532–20539. [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Ishii S., Ihara M., Maekawa T., Nakamura Y., Uchida H., Imamoto F. The nucleotide sequence of the cloned nusA gene and its flanking region of Escherichia coli. Nucleic Acids Res. 1984 Apr 11;12(7):3333–3342. doi: 10.1093/nar/12.7.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagus R. Translation in cell-free systems. Methods Enzymol. 1987;152:267–276. doi: 10.1016/0076-6879(87)52030-4. [DOI] [PubMed] [Google Scholar]
- Klein H. L. Different types of recombination events are controlled by the RAD1 and RAD52 genes of Saccharomyces cerevisiae. Genetics. 1988 Oct;120(2):367–377. doi: 10.1093/genetics/120.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Translation of exogenous mRNAs in reticulocyte lysates. Methods Enzymol. 1983;101:606–615. doi: 10.1016/0076-6879(83)01041-1. [DOI] [PubMed] [Google Scholar]
- Naegeli H., Bardwell L., Friedberg E. C. The DNA helicase and adenosine triphosphatase activities of yeast Rad3 protein are inhibited by DNA damage. A potential mechanism for damage-specific recognition. J Biol Chem. 1992 Jan 5;267(1):392–398. [PubMed] [Google Scholar]
- Naegeli H., Bardwell L., Harosh I., Freidberg E. C. Substrate specificity of the Rad3 ATPase/DNA helicase of Saccharomyces cerevisiae and binding of Rad3 protein to nucleic acids. J Biol Chem. 1992 Apr 15;267(11):7839–7844. [PubMed] [Google Scholar]
- Nicolet C. M., Chenevert J. M., Friedberg E. C. The RAD2 gene of Saccharomyces cerevisiae: nucleotide sequence and transcript mapping. Gene. 1985;36(3):225–234. doi: 10.1016/0378-1119(85)90177-5. [DOI] [PubMed] [Google Scholar]
- Nicolet C. M., Friedberg E. C. Overexpression of the RAD2 gene of S. cerevisiae: identification and preliminary characterization of Rad2 protein. Yeast. 1987 Sep;3(3):149–160. doi: 10.1002/yea.320030303. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Prakash S. RAD1, an excision repair gene of Saccharomyces cerevisiae, is also involved in recombination. Mol Cell Biol. 1988 Sep;8(9):3619–3626. doi: 10.1128/mcb.8.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Prakash S. RAD10, an excision repair gene of Saccharomyces cerevisiae, is involved in the RAD1 pathway of mitotic recombination. Mol Cell Biol. 1990 Jun;10(6):2485–2491. doi: 10.1128/mcb.10.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Schweiger M. The yeast DNA repair proteins RAD1 and RAD7 share similar putative functional domains. FEBS Lett. 1991 Jun 3;283(2):203–206. doi: 10.1016/0014-5793(91)80588-t. [DOI] [PubMed] [Google Scholar]
- Stefanini M., Collins A. R., Riboni R., Klaude M., Botta E., Mitchell D. L., Nuzzo F. Novel Chinese hamster ultraviolet-sensitive mutants for excision repair form complementation groups 9 and 10. Cancer Res. 1991 Aug 1;51(15):3965–3971. [PubMed] [Google Scholar]
- Struhl K. Reverse biochemistry: methods and applications for synthesizing yeast proteins in vitro. Methods Enzymol. 1991;194:520–535. doi: 10.1016/0076-6879(91)94039-f. [DOI] [PubMed] [Google Scholar]
- Sung P., Prakash L., Matson S. W., Prakash S. RAD3 protein of Saccharomyces cerevisiae is a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8951–8955. doi: 10.1073/pnas.84.24.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Prakash L., Prakash S. Renaturation of DNA catalysed by yeast DNA repair and recombination protein RAD10. Nature. 1992 Feb 20;355(6362):743–745. doi: 10.1038/355743a0. [DOI] [PubMed] [Google Scholar]
- Sung P., Prakash L., Weber S., Prakash S. The RAD3 gene of Saccharomyces cerevisiae encodes a DNA-dependent ATPase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6045–6049. doi: 10.1073/pnas.84.17.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. The genetic control of direct-repeat recombination in Saccharomyces: the effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptionally regulated gene. Genetics. 1989 Dec;123(4):725–738. doi: 10.1093/genetics/123.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Shiomi T., Salazar E. P., Stewart S. A. An eighth complementation group of rodent cells hypersensitive to ultraviolet radiation. Somat Cell Mol Genet. 1988 Nov;14(6):605–612. doi: 10.1007/BF01535314. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Somatic cell genetics approach to dissecting mammalian DNA repair. Environ Mol Mutagen. 1989;14(4):264–281. doi: 10.1002/em.2850140409. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wood R. D. DNA repair. Seven genes for three diseases. Nature. 1991 Mar 21;350(6315):190–190. doi: 10.1038/350190a0. [DOI] [PubMed] [Google Scholar]
- Zehfus B. R., McWilliams A. D., Lin Y. H., Hoekstra M. F., Keil R. L. Genetic control of RNA polymerase I-stimulated recombination in yeast. Genetics. 1990 Sep;126(1):41–52. doi: 10.1093/genetics/126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Duin M., van den Tol J., Warmerdam P., Odijk H., Meijer D., Westerveld A., Bootsma D., Hoeijmakers J. H. Evolution and mutagenesis of the mammalian excision repair gene ERCC-1. Nucleic Acids Res. 1988 Jun 24;16(12):5305–5322. doi: 10.1093/nar/16.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]








