Abstract
Vascular plants transport energy in the form of sugars from the leaves where they are produced to sites of active growth. The mass flow of sugars through the phloem vascular system is determined by the sap flow rate and the sugar concentration. If the concentration is low, little energy is transferred from source to sink. If it is too high, sap viscosity impedes flow. An interesting question is therefore at which concentration is the sugar flow optimal. Optimization of sugar flow and transport efficiency predicts optimal concentrations of 23.5 per cent (if the pressure differential driving the flow is independent of concentration) and 34.5 per cent (if the pressure is proportional to concentration). Data from more than 50 experiments (41 species) collected from the literature show an average concentration in the range from 18.2 per cent (all species) to 21.1 per cent (active loaders), suggesting that the phloem vasculature is optimized for efficient transport at constant pressure and that active phloem loading may have developed to increase transport efficiency.
Keywords: phloem transport, sugar translocation, optimal concentration theory
1. Introduction
Flows of matter, energy and information are ubiquitous. Whether biological (vascular systems of plants and animals), geological (rivers, oceans and glaciers) or engineered (pipes, roads, electrical grids and the Internet), they serve the purpose of moving matter, energy or information from one place to another. Often, we find that such flows are constrained by a desire to either maximize the flow of material or minimize the energy dissipated by the flow. While a higher mass flow can be achieved by increasing the concentration, this happens at the expense of increased impedance which eventually causes the volume flow to decrease. At an intermediate optimum value of concentration the mass flow is therefore at a maximum, a phenomena well known in blood flow where the volume fraction of erythrocytes (haematocrit) that is optimal for transporting a maximum amount of oxygen is approximately 45% v/v [1] and in nectar feeding animals where the optimum concentration (approx. 30–50% wt/wt) depends on the drinking strategy employed [2]. Less is known, however, about the situation in plants where a concentrated solution of sugars dissolved in water transfer energy between distal parts of the plant.
Sugars produced by photosynthesis are transported in the phloem vascular system of plants. Transport is initiated in the leaves where sugars are either passively or actively loaded into phloem. In active loading species, the process is driven by membrane transporters and sugar polymerization and occurs against a sugar concentration gradient. However, in passive loading species, sugars move into the phloem without the use of metabolic energy by travelling down a concentration gradient from the mesophyll to phloem [3]. In the phloem, an aqueous solution of sugars, amino acids, proteins, ions and signalling molecules flow through a series of narrow elongated cylindrical cells, known as sieve tube elements, lying end-to-end forming a microfluidic network spanning the entire length of the plant. The solution moves with a flow speed 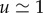 m h−1 [4], and while the sieve tube radius a varies among species, it is often of the order
m h−1 [4], and while the sieve tube radius a varies among species, it is often of the order 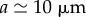 [5]. Typically, the total solute concentration is approximately 20% wt/wt, and sugars, of which sucrose is the most abundant type, constitute 80–90% of this [6]. The flow in the phloem is driven by differences in hydrostatic pressure between source (leaves) and sink (e.g. roots or fruits) tissues believed to be generated by gradients in osmotic potential between distal parts of the plant according to the Münch pressure flow hypothesis [7,8].
[5]. Typically, the total solute concentration is approximately 20% wt/wt, and sugars, of which sucrose is the most abundant type, constitute 80–90% of this [6]. The flow in the phloem is driven by differences in hydrostatic pressure between source (leaves) and sink (e.g. roots or fruits) tissues believed to be generated by gradients in osmotic potential between distal parts of the plant according to the Münch pressure flow hypothesis [7,8].
As in animals, it is likely to be physiologically favourable when a maximum amount of energy is transported through a given section of the plant vasculature. The existence of an optimum sugar concentration for this process was first proposed by Passioura [9] who argued that efficient transport of sugar requires concentrations in the range 14–35% based on increased viscous friction at high concentrations. Later, Lang [10] conducted a theoretical comparison of different sugars and sugar alcohols, and concluded that sucrose at c ∼ 25% is the most advantageous substance to transport, since it chemically stable, highly soluble and only generates a modest osmotic pressure when compared with other sugars, e.g. glucose and fructose. To our knowledge, however, no one has tested these predictions against measured values of phloem sap sugar concentrations. Also, it is not clear how the relation between driving pressure and sugar concentration influence the predictions given above, or how phloem loading mechanisms may be related to transport efficiency.
In this paper, we derive general criteria for determining the concentrations that maximize the mass flow and efficiency of sugar transport in plants. Our analysis thus extends the work of Passioura [9] and Lang [10] by providing a general framework for evaluating the efficiency of sugar transport in plants. We compare the predictions of the model with measured sugar concentrations from more than 50 experiments (41 species) collected from the literature, and consider the effects of sugar loading mode.
2. Mathematical model
Sugar is produced in the leaves during photosynthesis and is then transferred to the phloem vascular system by an either active or passive loading mechanism as discussed in §1. Once loaded, the mass flow of sugar J through a given section of phloem tissue can be expressed in terms of the volumetric flow rate Q of phloem sap, the sugar mass fraction (concentration) c and the density ρ of the solution
| 2.1 |
The magnitude of the Reynolds number 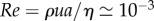 allows us to assume a laminar Poiseuille pipe flow where the volume flow rate Q is determined by the geometry of the phloem channel, the pressure differential driving the flow Δp and the viscosity of the sugar solution η
allows us to assume a laminar Poiseuille pipe flow where the volume flow rate Q is determined by the geometry of the phloem channel, the pressure differential driving the flow Δp and the viscosity of the sugar solution η
| 2.2 |
Here, X is a geometric parameter which for cylindrical phloem cells of radius a and length L is given by X = πa4/(8L). In a plant of length L = 10 m and sieve tube radius a = 10 μm, the geometric factor 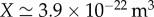 .
.
In general, both the pressure differential Δp, density ρ and viscosity η in equations (2.1) and (2.2) depend on the sugar concentration c. The viscosity and density are known functions of concentration, plotted in figure 1a,b. The pressure differential Δp required to drive the phloem flow is of the order of 0.1–1 MPa and is believed to be generated by osmotic pressure gradients [7,8]. Other mechanisms have been proposed, e.g. electroosmotic or protoplasmic streaming, but these have been less successful in explaining experimental data [12]. Notwithstanding the mechanism driving the flow, we divide the problem into two categories: those in which the pressure Δp = Δp0 does not depend on sugar concentration, and those in which pressure does depend on sugar concentration, Δp = Δp(c). An example of a concentration-dependent pressure is found in the classical interpretation of the Münch hypothesis [13–19], where the pressure differential Δp is the osmotic pressure difference associated with the molar sugar concentration gradient Δ(ρc)/M between source and sink, i.e. Δp(c) = RTΔ(ρc)/M, where M is the molar mass of the solute, R the gas constant and T the absolute temperature. Here, we use the van't Hoff value RTΔ(ρc)/M for the osmotic pressure, which is valid only for dilute (ideal) solutions. If sugars are unloaded effectively at the sink region, it is reasonable to take the sugar concentration difference to be approximately given by the characteristic concentration itself 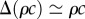 and so the pressure is proportional to concentration Δp(c) = RTρc/M. For simplicity, we do not consider variations in viscosity along the pathway associated with this estimate of the osmotic driving pressure.
and so the pressure is proportional to concentration Δp(c) = RTρc/M. For simplicity, we do not consider variations in viscosity along the pathway associated with this estimate of the osmotic driving pressure.
Figure 1.
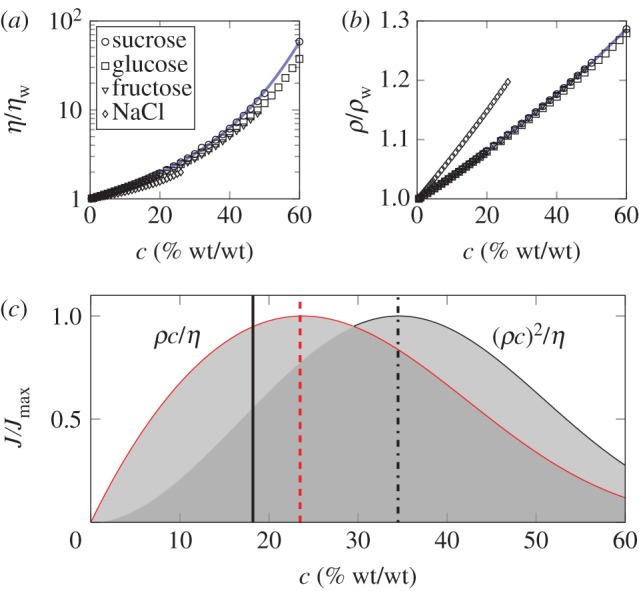
Optimal concentration for sugar transport in plants. (a) Viscosity η in units of the viscosity of pure water ηw plotted as a function of mass fraction c for sucrose (circles) and other solutes as indicated in the legend. Solid line is fit to sucrose data. (b) Density ρ in units of the density of pure water ρw plotted as a function of mass fraction c for sucrose (circles) and other solutes as indicated in the legend of (a). Solid line is fit to sucrose data. Data in (a) and (b) are from [11], see appendix A. (c) Normalized mass flow curves J ∝ ρc/η (equation (2.3)) and J ∝ (ρc)2/η (equation (2.4)) plotted as a function of sucrose concentration c with η and ρ taken from data fits in (a) and (b). The maximum rates of sugar transport are found at 23.5% for constant pressure Δp = Δp0 (dashed vertical line) and at 34.5% for variable pressure Δp = RTρc/M (dash-dotted vertical line). The average value of the sugar concentrations reported in table 2 (18.2%) is indicated by the solid vertical line. (Online version in colour.)
From equations (2.1) and (2.2), we can finally write the sugar flow rate J as
| 2.3 |
if the pressure Δp = Δp0 is constant, and as
| 2.4 |
if the pressure Δp(c) = RTρc/M is proportional to concentration.
2.1. Maximizing flux
To optimize sugar mass flow, we must maximize the expressions in equations (2.3) and (2.4), i.e. find solutions of ∂J/∂c = 0 and ∂2J/∂c2 < 0. As shown in figure 1c, the optima for these two cases occur at 23.5 per cent and 34.5 per cent, respectively. Optimal concentrations for the case where the pressure Δp is linear combination of the forms given above (i.e. Δp = Δp0+RTρc/M) must necessarily lie between these two values.
2.2. Maximizing efficiency
Having established the concentration that maximizes sugar mass flow, we will now consider the efficiency of the transport process. The energy E transferred per unit time by the movement of sugars between distal parts of the plant depends on the sugar flow J and energy content k per unit mass of solute E = kJ = kXΔpρc/η. Several factors contribute to the energetic cost of transporting sugar: maintaining the pressure gradient, building and preserving the vasculature, loading and unloading of solutes, and power dissipated by the viscous flow. While not all these are easily quantifiable, the viscous power p = XΔp2/η dissipated by the flow is known to strongly influence transport in other biological systems [20–22]. Assuming this will be the dominant source of loss, the net energy 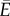 available at the sugar sink is therefore
available at the sugar sink is therefore
| 2.5 |
The pressure difference Δp required to drive the observed flow speeds (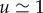 m h–1, [4]) is of the order of Δp = 0.1−1 MPa. With c = 0.2 and k = 104 J g–1 [23], we find
m h–1, [4]) is of the order of Δp = 0.1−1 MPa. With c = 0.2 and k = 104 J g–1 [23], we find 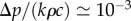 so the energy lost to viscous friction is almost negligible (i.e.
so the energy lost to viscous friction is almost negligible (i.e. 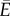 ), and optimizing the net energy transfer
), and optimizing the net energy transfer 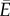 in equation (2.5) is equivalent to maximizing J in equations (2.3) and (2.4).
in equation (2.5) is equivalent to maximizing J in equations (2.3) and (2.4).
Similarly, we can define the efficiency 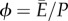 as the amount of useful energy the plant can extract from the flow per unit energy spent on the transport process. Using the estimates given above, we find
as the amount of useful energy the plant can extract from the flow per unit energy spent on the transport process. Using the estimates given above, we find 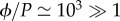 and conclude that optimizing the efficiency ϕ is equivalent to optimizing the flow J in equations (2.3) and (2.4).
and conclude that optimizing the efficiency ϕ is equivalent to optimizing the flow J in equations (2.3) and (2.4).
3. Material and methods
3.1. Phloem sap sugar concentration data
Phloem sap sugar concentration from 41 species (55 experiments) was collected from the literature. The data are listed in table 2 and plotted in figure 2. Phloem sap is challenging to collect because disruption of the cell membrane releases the hydrostatic pressure inside a sieve tube, which can potentially damage intracellular structures [24], block phloem transport [25] and contaminate fluid samples with apoplastic material. Currently, there are two commonly used methods for collecting phloem sap: bleeding and stylectomy. Bleeding is achieved by making diagonal incisions into the bark of woody species [26] or by transversely cutting through entire organs, such as petioles, pedicels or branches [27]. This technique is primarily used on trees and a handful of herbs that readily bleed from incisions (e.g. Ricinus communis [28], cucurbits [29] and some legumes [30]). Bleeding techniques that rely on EDTA and measurements on cucurbits were not included in this meta-analysis because of debate over the purity of their exudates [31–34]. Stylectomy [35] uses the severed stylets (mouthparts) of phloem-feeding insects (e.g. aphids [36,37], planthoppers [38,39] and scale insects [40]). These insects feed on individual sieve elements without disrupting phloem transport, thereby creating a direct tap into functional sieve tubes with their stylets [41]. Once the stylets are cut, droplets of relatively pure phloem sap can be collected with capillary tubes.
Table 2.
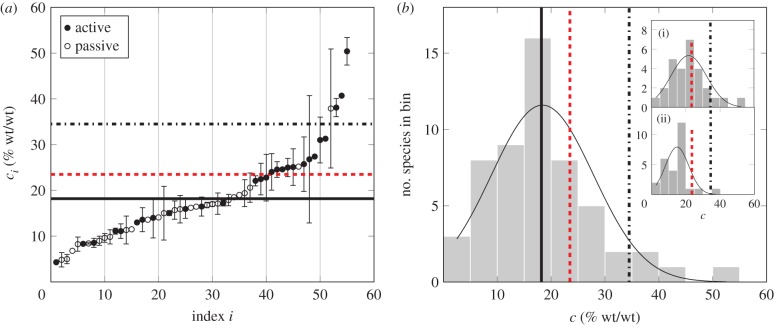
Phloem sap sugar concentration data. Sugar mass fraction ci and standard error/range δci obtained from reference Ref I using either bleeding (b) or stylectomy (s) protocols, as indicated in column PC. The protocol for determining the loading mechanism (PLM) was based on studies examining plasmodesmata (1), autoradiographs (2), leaf sugar concentration (3), osmolality of leaf sap (4), incipient plasmolysis (5) and sugar transporters (6) from reference Ref II. Whenever possible, species (S)- and genus (G)-level studies were used to infer loading type. For several species loading type was determined based on plant family (F). Index i refers to the position of the species along the coordinate axis in figure 2a.
| i | species | common name | ci | (δci) | PC | ref I | loading mechanism | PLM | ref II |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Brassica oleracea L. | cabbage | 4.3 | (b) | [53] | active | G(1) | [54] | |
| 2 | Salix L. sp. | willow | 4.8 | (1.6) | (s) | [55] | passive | G(1,2,3,5) | [56] |
| 3 | Salix viminalis L. | basket willow | 5.0 | (1.0) | (s) | [37] | passive | G(1,2,3,5) | [56] |
| 4 | Humulus lupulus L. | common hops | 6.8 | (b) | [7] | passive | F(1) | [54] | |
| 5 | Ricinus communis L. | castor bean | 8.3 | (1.6) | (b) | [57] | passive | S(1) | [54] |
| 6 | Triticum aestivum L. | wheat | 8.3 | (s) | [39] | active | S(6) | [58] | |
| 7 | Salix acutifolia Willd | littletree willow | 8.4 | (0.1) | (s) | [36] | passive | G(1,2,3,5) | [56] |
| 8 | Fraxinus americana L. | white ash | 8.5 | (1.0) | (b) | [29] | active | S(1,2,3) | [59] |
| 9 | Ricinus communis L. | castor bean | 9.0 | (1.0) | (b) | [28] | passive | S(1) | [54] |
| 10 | Platanus occidentalis L. | American sycamore | 9.6 | (0.9) | (b) | [29] | passive | G(1,2,3) | [59] |
| 11 | Ricinus communis L. | castor bean | 9.9 | (1.6) | (b) | [60] | passive | S(1) | [54] |
| 12 | Fraxinus pennsylvanica Marshall | green ash | 11.1 | (0.7) | (b) | [29] | active | G(1,2,3) | [59] |
| 13 | Arabidopsis thaliana (L.) Heynh. | mouse-ear cress | 11.1 | (1.6) | (s) | [61] | active | S(6) | [62] |
| 14 | Ricinus communis L. | castor bean | 11.4 | (3.1) | (b) | [45] | passive | S(1) | [54] |
| 15 | Pinus sylvestris L. | scots pine | 11.5 | (b) | [7] | passive | G(1) | [54] | |
| 16 | Medicago sativa L. | clover | 13.0 | (s) | [63] | active | G(1) | [54] | |
| 17 | Robinia pseudoacacia L. | black locust | 13.6 | (2.6) | (b) | [64] | active | G(1) | [54] |
| 18 | Quercus pedunculata Willd. | pedunculate oak | 13.6 | (b) | [7] | passive | G(1,2,3,4) | [3] | |
| 19 | Lupinus albus L. | white lupine | 14.0 | (4.4) | (b) | [65] | active | G(1) | [54] |
| 20 | Arenga pinnata (Wurmb) Merr. | sugar palm | 14.1 | (b) | [66] | passive | F(1) | [54] | |
| 21 | Salix viminalis L. | basket willow | 15.0 | (5.9) | (s) | [67] | passive | G(1,2,3,5) | [56] |
| 22 | Nicotiana glauca Graham | tree tobacco | 15.1 | (0.6) | (b) | [68] | active | G(1,2,3,4) | [3] |
| 23 | Carpinus betulus L. | European hornbeam | 15.7 | (2.0) | (b) | [7] | passive | S(1) | [54] |
| 24 | Salix exigua Nutt. | narrowleaf willow | 15.9 | (3.0) | (s) | [40] | passive | G(1,2,3,5) | [56] |
| 25 | Lactuca sativa L. | lettuce | 16.0 | (2.1) | (s) | [32] | active | F(1,2,3,4) | [3] |
| 26 | Castanea sativa Mill. | European chestnut | 16.2 | (b) | [7] | passive | G(1) | [54] | |
| 27 | Oryza sativa L. var. Towada | rice | 16.5 | (0.1) | (s) | [69] | passive | S(1,3,6) | [70] |
| 28 | Robinia pseudoacacia L. | black locust | 16.5 | (2.1) | (b) | [29] | active | G(1) | [54] |
| 29 | Tilia platyphyllos Scop. | large-leaved lime | 16.8 | (0.6) | (b) | [64] | passive | G(1,2,3,4) | [3] |
| 30 | Quercus borealis Michx. f. | northern red oak | 17.0 | (0.5) | (b) | [64] | passive | G(1,2,3,4) | [3] |
| 31 | Quercus rubra L. | northern red oak | 17.1 | (2.3) | (b) | [7] | passive | S(1,2,3,4) | [3] |
| 32 | Triticum aestivum L. | wheat | 17.2 | (0.6) | (s) | [71] | active | S(6) | [58] |
| 33 | Acer platanoides L. | Norway maple | 17.9 | (1.2) | (b) | [64] | passive | G(1,2,3,4) | [3] |
| 34 | Tilia L. sp. | basswood | 18.5 | (b) | [7] | passive | G(1,2,3,4) | [3] | |
| 35 | Tilia parvifolia Ehrh. | small-leaved lime | 19.0 | (b) | [7] | passive | G(1,2,3,4) | [3] | |
| 36 | Oryza sativa L. var. Towada | rice | 19.4 | (2.9) | (s) | [38] | passive | S(1,3,6) | [70] |
| 37 | Acer pseudoplatanus L. | sycamore maple | 20.6 | (3.2) | (b) | [7] | passive | G(1,2,3,4) | [3] |
| 38 | Fraxinus americana L. | white ash | 22.1 | (1.1) | (b) | [72] | active | S(1,2,3) | [59] |
| 39 | Brassica nigra (L.) W. D. J. Koch | black mustard | 22.5 | (3.5) | (s) | [73] | active | G(1) | [54] |
| 40 | Robinia pseudoacacia L | black locust | 22.8 | (5.1) | (b) | [7] | active | G(1) | [54] |
| 41 | Lupinus mutabilis Sweet | Tarwi | 24.0 | (4.0) | (b) | [30] | active | G(1) | [54] |
| 42 | Avena sativa L. | common oat | 24.6 | (1.7) | (s) | [74] | active | F(1) | [54] |
| 43 | Spinacia oleracea L. | spinach | 24.6 | (s) | [75] | active | S(3) | [76] | |
| 44 | Fraxinus americana L. | white ash | 25.0 | (2.0) | (b) | [64] | active | S(1,2,3) | [59] |
| 45 | Eucalyptus globulus Labill. | Tansmanian bluegum | 25.1 | (4.0) | (b) | [77] | active | G(1,3) | [54,78] |
| 46 | Acer platanoides L. | Norway maple | 25.2 | (b) | [7] | passive | G(1,2,3,4) | [3] | |
| 47 | Zea mays L. | maize | 25.8 | (6.0) | (s) | [79] | active | S(1,3,5) | [80,81] |
| 48 | Tanacetum vulgare L. | tansy | 26.8 | (13.9) | (s) | [45] | active | F(1,2,3,4) | [3] |
| 49 | Zea mays L. | maize | 27.4 | (s) | [82] | active | S(1,3,5) | [80,81] | |
| 50 | Hordeum vulgare L. | barley | 31.0 | (5.0) | (s) | [83] | active | S(1,3) | [76,84] |
| 51 | Lolium perenne L. | ryegrass | 31.3 | (s) | [31] | active | S(1,3,5,6) | [31,85] | |
| 52 | Prunus persica L. | peach | 37.9 | (13) | (s) | [86] | passive | S(1,2,3) | [59] |
| 53 | Solanum tuberosum L. cv. Desiree | potato | 38.1 | (2.0) | (s) | [87] | active | S(1,2,3) | [59] |
| 54 | Zea mays L. | maize | 40.7 | (s) | [81] | active | S(1,3,5) | [80,81] | |
| 55 | Solanum tuberosum L. cv. Desiree | potato | 50.4 | (3.0) | (s) | [46] | active | S(1,2,3) | [59] |
Figure 2.
Sugar concentration in phloem sap. (a) Sugar concentration ci for species number i as listed in table 2. Legend indicates phloem loading type. Error bars show standard deviation or range of concentrations reported. (b) Histogram showing distribution of sugar concentrations from (a). Inset shows histogram (i) for active and (ii) passive loaders. Thin solid lines are normal distributions fitted to the histograms as a guide to the eye. In (a,b), the thick solid line indicates the mean value 18.2% of phloem sap concentrations given in (a) (table 1). Dashed line indicates the optimum concentration 23.5% for flows driven by constant pressure while the dash-dotted lined indicates the optimum concentration 34.5% for flows driven by concentration-dependent pressure. (Online version in colour.)
Both bleeding and stylectomy have advantages and disadvantages as techniques for sampling phloem sap. Bleeding techniques are relatively simple to use but they are destructive and susceptible to contamination by apoplastic material and intracellular contents of neighbouring cells [31,42]. Samples produced by bleeding are also subject to dilution over time by water that is osmotically attracted to the incision [43], and may thus only provide a lower limit on the actual phloem concentration. Conversely, stylectomy yields more pure phloem sap because the sampling is less invasive, and although aphids can alter the amino acid composition of phloem exudates [44] there is no evidence that these changes directly affect sugar concentration. The disadvantage of this technique is that it is technically challenging and is susceptible to the effects of evaporation because of the small size of the exudate droplets [45]. Furthermore, it can only be used in plant species associated with sap-feeding insects [35], which could lead to biases in sample collection if these insects prefer certain types of plants. For example, research on transgenic potatoes [46] and in artificial systems [47] suggests that aphids may selectively feed on sieve tubes based on their high sugar concentration relative to neighbouring cells. If this is a common mechanism employed by sap-feeding insects, they may have a stronger affinity towards active loading plant species.
A least-squares regression model was used to examine whether there were systematic differences in the phloem sap sugar concentrations in samples collected by stylectomy and bleeding, and between passive and active loading species. The model considered the effect of each bivariate factor individually and their interaction, and multiple comparisons of all combinations of these variables were conducted using Tukey's HSD (α = 0.05). To avoid oversampling of common species, phloem sugar concentrations for each species were averaged across studies. For averages of the different groups, see table 1.
Table 1.
Average phloem sugar concentration (in %wt/wt) determined from the data in table 2 for all species, active loaders and passive loaders. In the species averaged column, the reported concentrations for each species were averaged across studies before taking the total average. In the including repeated species column, the reported concentrations for each species were not averaged before taking the total average.
| species averaged | including repeated species | |
|---|---|---|
| all species | 18.2 | 18.4 |
| active loaders | 21.1 | 21.8 |
| passive loaders | 15.4 | 14.9 |
4. Results and discussion
We have derived a general framework for determining sugar concentrations that optimizes phloem transport under different conditions, and a relatively complete physical picture of the process has emerged. First and foremost, the optimal sugar concentration for a given plant depends only on how the pressure differential Δp is maintained. At constant pressure, we find that the mass flow of sugar is optimal when c = 23.5%, in agreement with the predictions by Passioura [9] and Lang [10]. We have further shown that optimizing transport efficiency in this case leads to a similar prediction. On the other hand, if the pressure is proportional to the concentration, we have shown that the transport efficiency and mass flow of sugar is optimal when c = 34.5%.
From figure 1a,b, we observe that the mass fraction c is the major determinant of both viscosity and density of aqueous sugar solutions. It follows that all sugars which are dissolvable in water to a concentration of at least 23.5 per cent yield approximately the same mass flow at constant pressure, because equation (2.3) does not depend on the molar mass M. When the flow is driven primarily by the osmotic pressure of the sugar itself (equation (2.4)), however, light sugars such as fructose or glucose should have a distinct advantage over sucrose, since the sugar flow scales J ∝ M−1. To argue why sucrose is nevertheless the most abundant compound, one must therefore consider other effects, such as the chemical properties of sugars and sugar alcohols discussed by Lang [10], see §1.
The optimal concentrations discussed above provide a rationale for the observation that the mean sugar concentration in the phloem sap of the plants considered in this study is 18.2 per cent, which is closer to the predicted optimum concentration for sugar transport under constant pressure (figure 2 and table 1). When considering active sugar loaders separately the trend is even more clear: average concentration 21.1 per cent (figure 2b(i)). Interestingly, the fact that the observed values cluster around the optimum value for constant pressure suggests that the pressure difference Δp driving the flow may be constant, and consequently does not scale with the sugar concentration c. Under a scenario where translocation is driven by osmotic pressure generated by both sugars and ions, this implies that changes in the sugar concentration c are balanced by additional terms in the pressure, i.e. Δp = f(c)+RTρc/M = const., such that the total pressure Δp remains constant. This can be achieved by active loading and unloading of potassium ions [48–51] and may explain why pressure does not scale with plant size [52].
Together, loading type and sampling method appear to impact the sugar concentrations measured in the phloem (ANOVA, F = 3.37, p = 0.028). This effect is due to the significant difference (Tukey HSD, t = 2.94, p = 0.027) between active loaders measured with stylectomy (24.3±2.3) and passive loaders sampled by bleeding (15.2±2.1). Although there is not a direct interaction between loading type and sampling method (ANOVA, F = 1.95, p = 0.17), these factors are potentially confounding because of bias in the sampling method used for each loading type (table 2). As a result, differences in the sampling methods (described in §3a) may partially explain the higher sugar concentrations observed in the active loaders compared with the passive loaders (figure 2b(i),(ii)). However, if the higher sugar concentrations measured in the active loaders are not an artefact of differences in these methods, the data would suggest that active loaders achieve more optimal concentrations for transport than passive loading species, thus providing a new perspective on recent discussions regarding the potential advantages of using an active loading method [52,88]. If this loading method allows for more efficient transport, it may facilitate a faster growth habit and explain why active loaders tend to be faster growing herbaceous species and passive loaders tend to be slower growing trees [89]. Plants with the highest reported sugar concentration are broadly characterized by rapid growth, and several of those presented here are crop plants (e.g. potato (c = 50.4%) and maize (c = 40.7%)). These extreme concentrations may thus be an artefact of selective breeding.
Several caveats are in order, however. The optimal concentration predicted here might differ from that observed in nature due to the limited availability of light, water and nutrients. Although some plant species maintain fairly constant sugar concentrations in their phloem (e.g. Ricinus), other species appear to exhibit diurnal and seasonal changes in sap chemistry. In the autumn, growth cessation leads to reduced sink activity, which coincides with a 1.5–5-fold increase in the sugar content of the phloem exudates of some deciduous [40,67,90] and evergreen species [91]. In these species, the phloem may serve as a reservoir of carbon that plants can access without the use of temperature-sensitive enzymes [92]. Higher sugar concentrations may also prevent desiccation during extracellular freezing and facilitate supercooling during the winter [40]. On a smaller temporal scale, sugar concentrations in the phloem can increase by 25–160% between the night and the day [45,71,87] and can change in response to disturbance such as defoliation [31]. Therefore, some of the variation observed in phloem sugar concentration could be the result of differences in growth and sampling conditions used in each study. Optimal concentrations suggested by models thus still need to be carefully scrutinized in attempts to understand the evolutionary implications of sugar loading strategies.
Acknowledgements
The authors wish thank Maciej Zwieniecki, John Bush, Wonjung Kim, Nick Carroll, Kenneth Ho and David Weitz. This work was supported by the NSF (grant no. 1021779) and the Materials Research Science and Engineering Center (MRSEC, grant no. DMR-0820484) at Harvard University.
Appendix A. Viscosity and density of phloem sap
Phloem sap consists of an aqueous solution of sugars, amino acids, proteins, ions and signalling molecules. Typically, the total solute concentration is approximately 20% wt/wt, and sugars, of which sucrose is the most abundant type, constitute 80–90% of this [6]. To approximate the viscosity η and density ρ of phloem sap, we used data from sucrose, glucose, fructose and NaCl solutions of concentration c obtained from [11]. The data are plotted in figure 1a,b where least-square fits to sucrose data yield the approximate expressions for viscosity η = ηw exp[0.032c−(0.012c)2+(0.023c)3] and density ρ = ρw(1+0.0038c+(0.0037c)2+(0.0033c)3), shown as solid lines in figure 1a,b. We note that viscosity data from all solutes are well approximated by the fit suggesting that only the mass fraction c, and not the type of solute, plays a role as the major determinant of viscosity.
References
- 1.Stark H, Schuster S. 2012. Comparison of various approaches to calculating the optimal hematocrit in vertebrates. J. Appl. Physiol. 113, 355–367 10.1152/japplphysiol.00369.2012 (doi:10.1152/japplphysiol.00369.2012) [DOI] [PubMed] [Google Scholar]
- 2.Kim W, Gilet T, Bush JWM. 2011. Optimal concentrations in nectar feeding. Proc. Natl Acad. Sci. USA 104, 20 167–20 172 10.1073/pnas.1108642108 (doi:10.1073/pnas.1108642108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rennie EA, Turgeon R. 2009. A comprehensive picture of phloem loading strategies. Proc. Natl Acad. Sci. USA 106, 14 162–14 167 10.1073/pnas.0902279106 (doi:10.1073/pnas.0902279106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windt CW, Vergeldt FJ, de Jager PA, van As H. 2006. MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ. 29, 1715–1729 10.1111/j.1365-3040.2006.01544.x (doi:10.1111/j.1365-3040.2006.01544.x) [DOI] [PubMed] [Google Scholar]
- 5.Jensen KH, Liesche J, Bohr T, Schulz A. 2012. Universality of phloem transport in seed plants. Plant Cell Environ. 35, 1065–1076 10.1111/j.1365-3040.2011.02472.x (doi:10.1111/j.1365-3040.2011.02472.x) [DOI] [PubMed] [Google Scholar]
- 6.Pate J. 1976. Nutrients and metabolites of fluids recovered from xylem and phloem: significance in relation to long-distance transport in plants. In Transport and transfer processes in plants (eds Wardlaw IF, Passioura JB.), pp. 253–281 New York, NY: Academic Press [Google Scholar]
- 7.Münch E. 1930. Die Stoffbewegungen in der Pflanze. Jena: Gustav Fisher [Google Scholar]
- 8.Knoblauch M, Peters WS. 2010. Münch, morphology, microfluidics: our structural problem with the phloem. Plant Cell Environ. 33, 1439–1452 10.1111/j.1365-3040.2010.02177.x (doi:10.1111/j.1365-3040.2010.02177.x) [DOI] [PubMed] [Google Scholar]
- 9.Passioura JB. 1976. Translocation and the diffusion equation. In Transport and transfer processes in plants (eds Wardlaw IF, Passioura JB.), pp. 357–361 New York, NY: Academic Press [Google Scholar]
- 10.Lang A. 1978. A model of mass flow in the phloem. Aust. J. Plant Physiol. 5, 535–546 10.1071/PP9780535 (doi:10.1071/PP9780535) [DOI] [Google Scholar]
- 11.Lide DR. 2012. CRC handbook of chemistry and physics. Boca Raton, FL: CRC Press [Google Scholar]
- 12.MacRobbie EAC. 1971. Phloem translocation. Facts and mechanisms: a comparative survey. Biol. Rev. 46, 429–481 10.1111/j.1469-185X.1971.tb01053.x (doi:10.1111/j.1469-185X.1971.tb01053.x) [DOI] [Google Scholar]
- 13.Horwitz L. 1958. Some simplified mathematical treatments of translocation in plants. Plant Physiol. 33, 81–93 10.1104/pp.33.2.81 (doi:10.1104/pp.33.2.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minchin P, Thorpe M, Farrar J. 1993. A simple mechanistic model of phloem transport which explains sink priority. J. Exp. Bot. 44, 947–955 10.1093/jxb/44.5.947 (doi:10.1093/jxb/44.5.947) [DOI] [Google Scholar]
- 15.Thompson M, Holbrook NM. 2003. Application of a single-solute non-steady-state phloem model to the study of long-distance assimilate transport. J. Theor. Biol. 220, 419–455 10.1006/jtbi.2003.3115 (doi:10.1006/jtbi.2003.3115) [DOI] [PubMed] [Google Scholar]
- 16.Pickard WF, Abraham-Shrauner B. 2009. A simplest steady-state Münch-like model of phloem translocation, with source and pathway and sink. Func. Plant Biol. 36, 629–644 10.1071/FP08278 (doi:10.1071/FP08278) [DOI] [PubMed] [Google Scholar]
- 17.Lacointe A, Minchin PEH. 2008. Modelling phloem and xylem transport within a complex architecture. Func. Plant Biol. 35, 772–780 10.1071/FP08085 (doi:10.1071/FP08085) [DOI] [PubMed] [Google Scholar]
- 18.Pickard WF. 2012. Münch without tears: a steady-state Münch-like model of phloem so simplified that it requires only algebra to predict the speed of translocation. Func. Plant Biol. 39, 531–537 10.1071/FP12004 (doi:10.1071/FP12004) [DOI] [PubMed] [Google Scholar]
- 19.Jensen KH, Berg-Sørensen K, Friis SMM, Bohr T. 2012. Analytic solutions and universal properties of sugar loading models in Münch phloem flow. J. Theor. Biol. 304, 286–296 10.1016/j.jtbi.2012.03.012 (doi:10.1016/j.jtbi.2012.03.012) [DOI] [PubMed] [Google Scholar]
- 20.Murray CD. 1926. The physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proc. Natl Acad. Sci. USA 12, 207–214 10.1073/pnas.12.3.207 (doi:10.1073/pnas.12.3.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman TF. 1981. On connecting large vessels to small. The meaning of Murray's law. J. Gen. Physiol. 78, 431–453 10.1085/jgp.78.4.431 (doi:10.1085/jgp.78.4.431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBarbera M. 1990. Principles of design of fluid transport systems in zoology. Science 249, 992–1000 10.1126/science.2396104 (doi:10.1126/science.2396104) [DOI] [PubMed] [Google Scholar]
- 23.Donato K, Hegsted DM. 1985. Efficiency of utilization of various sources of energy for growth. Proc. Natl Acad. Sci. USA 82, 4866–4870 10.1073/pnas.82.15.4866 (doi:10.1073/pnas.82.15.4866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehlers K, Knoblauch M, van Bel AJE. 2000. Ultrastructural features of well-preserved and injured sieve elements: minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214, 80–92 10.1007/BF02524265 (doi:10.1007/BF02524265) [DOI] [Google Scholar]
- 25.Knoblauch M, Stubenrauch M, van Bel AJE, Peters WS. 2012. Forisome performance in artificial sieve tubes. Plant Cell Environ. 35, 1419–1427 10.1111/j.1365-3040.2012.02499.x (doi:10.1111/j.1365-3040.2012.02499.x) [DOI] [PubMed] [Google Scholar]
- 26.Hartig T. 1860. Beitrage zur physiologischen forstbotanik. Forst und Jagdzeitung 36, 257–263 [Google Scholar]
- 27.Crafts AS. 1939. The relation between structure and function of the phloem. Am. J. Bot. 26, 172–177 10.2307/2436534 (doi:10.2307/2436534) [DOI] [Google Scholar]
- 28.Hall SM, Baker DA, Milburn JA. 1971. Phloem transport of 14C-labelled assimilates in Ricinus. Planta 100, 200–207 10.1007/BF00387036 (doi:10.1007/BF00387036) [DOI] [PubMed] [Google Scholar]
- 29.Moose C. 1938. Chemical and spectroscopic analysis of phloem exudate and parenchyma sap from several species of plants. Plant Physiol. 13, 365–380 10.1104/pp.13.2.365 (doi:10.1104/pp.13.2.365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins C. 1999. Spontaneous phloem exudation accompanying abscission in Lupinus mutabilis (Sweet). J. Exp. Bot. 50, 805–812 10.1093/jexbot/50.335.805 (doi:10.1093/jexbot/50.335.805) [DOI] [Google Scholar]
- 31.Amiard V, Morvan-Bertrand A, Cliquet J-B, Billard J-P, Huault C, Sandström JP, Prud'homme M-P. 2004. Carbohydrate and amino acid composition in phloem sap of Lolium perenne L. before and after defoliation. Can. J. Bot. 82, 1594–1601 10.1139/b04-117 (doi:10.1139/b04-117) [DOI] [Google Scholar]
- 32.Helden M, Tjallingh WF, Beek TA. 1994. Phloem sap collection from lettuce (Lactuca sativa L.): chemical comparison among collection methods. J. Chem. Ecol. 20, 3191–3206 10.1007/BF02033720 (doi:10.1007/BF02033720) [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Tolstikov V, Turnbull C, Hicks LM, Fiehn O. 2010. Divergent metabolome and proteome suggest functional independence of dual phloem transport systems in cucurbits. Proc. Natl Acad. Sci. USA 107, 13 532–13 537 10.1073/pnas.0910558107 (doi:10.1073/pnas.0910558107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C, Yu X, Ayre BG, Turgeon R. 2012. The origin and composition of cucurbit ‘phloem’ exudate. Plant Physiol. 158, 1873–1882 10.1104/pp.112.194431 (doi:10.1104/pp.112.194431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher DB, Frame JM. 1984. A guide to the use of the exuding-stylet technique in phloem physiology. Planta 161, 385–393 10.1007/BF00394567 (doi:10.1007/BF00394567) [DOI] [PubMed] [Google Scholar]
- 36.Mittler TE. 1958. Studies on the feeding and nutrition of Tuberolachnus Salignus (Gmelin) (Homoptera, Aphididae) II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. J. Exp. Biol. 35, 74–84 [Google Scholar]
- 37.Peel AJ, Weatherly PE. 1959. Composition of sieve-tube sap. Nature 184, 1955–1956 10.1038/1841955a0 (doi:10.1038/1841955a0) [DOI] [Google Scholar]
- 38.Fukumorita T, Chino M. 1982. Sugar, amino acid and inorganic contents in rice phloem sap. Plant Cell Physiol. 23, 273–283 [Google Scholar]
- 39.Hayashi H, Chino M. 1986. Collection of pure phloem sap from wheat and its chemical composition. Plant Cell Physiol. 27, 1387–1393 [Google Scholar]
- 40.Fisher DB. 1983. Year-round collection of willow sieve-tube exudate. Planta 159, 529–533 10.1007/BF00409142 (doi:10.1007/BF00409142) [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann MH. 1961. Movement of organic substances in trees: photosynthates are translocated in a layer of bark only a fraction of a millimeter thick. Science 133, 73–79 10.1126/science.133.3446.73 (doi:10.1126/science.133.3446.73) [DOI] [PubMed] [Google Scholar]
- 42.Turgeon R, Wolf S. 2009. Phloem transport: cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 60, 207–221 10.1146/annurev.arplant.043008.092045 (doi:10.1146/annurev.arplant.043008.092045) [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann MH. 1957. Translocation of organic substances in trees. II. On the translocation mechanism in the phloem of white ash (Fraxinus americana L.). Plant Physiol. 32, 399–404 10.1104/pp.32.5.399 (doi:10.1104/pp.32.5.399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandström J, Telang A, Moran N. 2000. Nutritional enhancement of host plants by aphids: a comparison of three aphid species on grasses. J. Insect Physiol. 46, 33–40 10.1016/S0022-1910(99)00098-0 (doi:10.1016/S0022-1910(99)00098-0) [DOI] [PubMed] [Google Scholar]
- 45.Kallarackal J, Bauer SN, Nowak H, Hajirezaei M-R, Komor E. 2012. Diurnal changes in assimilate concentrations and fluxes in the phloem of castor bean (Ricinus communis L.) and tansy (Tanacetum vulgare L.). Planta 236, 209–223 10.1007/s00425-012-1600-7 (doi:10.1007/s00425-012-1600-7) [DOI] [PubMed] [Google Scholar]
- 46.Pescod KV, Quick WP, Douglas AE. 2007. Aphid responses to plants with genetically manipulated phloem nutrient levels. Physiol. Entomol. 32, 253–258 10.1111/j.1365-3032.2007.00577.x (doi:10.1111/j.1365-3032.2007.00577.x) [DOI] [Google Scholar]
- 47.Hewer A, Will T, van Bel AJE. 2010. Plant cues for aphid navigation in vascular tissues. J. Exp. Biol. 213, 4030–4042 10.1242/jeb.046326 (doi:10.1242/jeb.046326) [DOI] [PubMed] [Google Scholar]
- 48.Hoad G, Peel A. 1964. Studies on the movement of solutes between the sieve tubes and surrounding tissues in willow I. Interference between solutes and rate of translocation measurements. J. Exp. Bot. 16, 433–451 10.1093/jxb/16.3.433 (doi:10.1093/jxb/16.3.433) [DOI] [Google Scholar]
- 49.Smith JAC, Milburn JA. 1980. Osmoregulation and the control of phloem-sap composition in Ricinus communis L. Planta 148, 28–34 10.1007/BF00385438 (doi:10.1007/BF00385438) [DOI] [PubMed] [Google Scholar]
- 50.Lang A. 1983. Turgor-regulated translocation. Plant Cell Environ. 6, 683–689 [Google Scholar]
- 51.Thompson M, Zwieniecki M. 2005. The role of potassium in long distance transport in plants. In Vascular transport in plants (eds Holbrook NM, Zwieniecki MA.), pp. 221–240 Burlington, MA: Elsevier Academic Press [Google Scholar]
- 52.Turgeon R. 2010. The puzzle of phloem pressure. Plant Physiol. 154, 578–581 10.1104/pp.110.161679 (doi:10.1104/pp.110.161679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pate J. 1973. Uptake, assimilation and transport of nitrogen compounds by plants. Soil Biol. Biochem. 5, 109–119 10.1016/0038-0717(73)90097-7 (doi:10.1016/0038-0717(73)90097-7) [DOI] [Google Scholar]
- 54.Gamalei Y. 1989. Structure and function of leaf minor veins in trees and herbs. Trees 3, 96–110 10.1007/BF01021073 (doi:10.1007/BF01021073) [DOI] [Google Scholar]
- 55.Canny M. 1961. Measurements of the velocity of translocation. Ann. Bot. 25, 153–167 [Google Scholar]
- 56.Turgeon R, Medville R. 1998. The absence of phloem loading in willow leaves. Proc. Natl Acad. Sci. USA 95, 12 055–12 060 10.1073/pnas.95.20.12055 (doi:10.1073/pnas.95.20.12055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vreugdenhil D, Koot-Gronsveld EAM. 1989. Measurements of pH, sucrose and potassium ions in the phloem sap of castor bean (Ricinus communis) plants. Physiol. Plantarum 77, 385–388 10.1111/j.1399-3054.1989.tb05657.x (doi:10.1111/j.1399-3054.1989.tb05657.x) [DOI] [Google Scholar]
- 58.Braun DM, Slewinski TL. 2009. Genetic control of carbon partitioning in grasses: roles of sucrose transporters and tie-dyed loci in phloem loading. Plant Physiol. 149, 71–81 10.1104/pp.108.129049 (doi:10.1104/pp.108.129049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Q, Cheng L, Guo Y, Turgeon R. 2011. Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 157, 1518–1527 10.1104/pp.111.184820 (doi:10.1104/pp.111.184820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JAC, Milburn JA. 1980. Phloem transport, solute flux and the kinetics of sap exudation in Ricinus communis L. Planta 148, 35–41 10.1007/BF00385439 (doi:10.1007/BF00385439) [DOI] [PubMed] [Google Scholar]
- 61.Deeken R, Geiger D, Fromm J, Koroleva O, Ache P, Langenfeld-Heyser R, Sauer N, May ST, Hedrich R. 2002. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 216, 334–344 10.1007/s00425-002-0895-1 (doi:10.1007/s00425-002-0895-1) [DOI] [PubMed] [Google Scholar]
- 62.DeWitt ND, Harper JF, Sussman MR. 1991. Evidence for a plasma membrane proton pump in phloem cells of higher plants. Plant J. 1, 121–128 10.1111/j.1365-313X.1991.00121.x (doi:10.1111/j.1365-313X.1991.00121.x) [DOI] [PubMed] [Google Scholar]
- 63.Girousse C, Bonnemain J-L, Delrot S, Bournoville R. 1991. Sugar and amino acid composition of phloem sap of Medicago sativa: a comparative study of two collecting methods. Plant Physiol. Biochem. 29, 41–48 [Google Scholar]
- 64.Ziegler H. 1956. Untersuchungen Über die Leitung und Sekretion der Assimilate. Planta 47, 447–500 10.1007/BF01935416 (doi:10.1007/BF01935416) [DOI] [Google Scholar]
- 65.Pate J, Atkins C, Hamel K. 1979. Transport of organic solutes in phloem and xylem of a nodulated legume. Plant Physiol. 63, 1082–1088 10.1104/pp.63.6.1082 (doi:10.1104/pp.63.6.1082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canny M. 1973. Phloem translocation. London, UK: Cambridge University Press [Google Scholar]
- 67.Rogers S, Peel AJ. 1975. Some evidence for the existence of turgor pressure gradients in the sieve tubes of willow. Planta 126, 259–267 10.1007/BF00388967 (doi:10.1007/BF00388967) [DOI] [PubMed] [Google Scholar]
- 68.Hocking P. 1980. The composition of phloem exudate and xylem sap from tree tobacco (Nicotiana glauca Grah.). Ann. Bot. 45, 633–643 [Google Scholar]
- 69.Kawabe S, Fukumorita T, Chino M. 1980. Collection of rice phloem sap from stylets of homopterous insects severed by YAG laser. Plant Cell Physiol. 21, 1319–1327 [Google Scholar]
- 70.Eom J-S, Choi S-B, Ward JM, Jeon J-S. 2012. The mechanism of phloem loading in rice (Oryza sativa). Mol. Cells 33, 431–438 10.1007/s10059-012-0071-9 (doi:10.1007/s10059-012-0071-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher DB, Gifford RM. 1986. Accumulation and conversion of sugars by developing wheat grains VI. Gradients along the transport pathway from the peduncle to the endosperm cavity during grain filling. Plant Physiol. 82, 1024–1030 10.1104/pp.82.4.1024 (doi:10.1104/pp.82.4.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann MH. 1962. Translocation of organic substances in trees. V. Experimental double interruption of phloem in white ash (Fraxinus americana L.). Plant Physiol. 37, 527–530 10.1104/pp.37.4.527 (doi:10.1104/pp.37.4.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merritt SZ. 1996. Within-plant variation in concentrations of amino acids, sugar, and sinigrin in phloem sap of black mustard, Brassica nigra (L.) Koch (Cruciferae). J. Chem. Ecol. 22, 1133–1145 10.1007/BF02027950 (doi:10.1007/BF02027950) [DOI] [PubMed] [Google Scholar]
- 74.Kuo-Sell H-L. 1989. Aminosäuren und Zucker im Phloemsaft verschiedener Pflanzenteile von Hafer (Avena sativa) in Beziehung zur Saugortpräferenz von Getreideblattläusen (Hom, Aphididae). J. Appl. Entomol. 108, 54–63 10.1111/j.1439-0418.1989.tb00432.x (doi:10.1111/j.1439-0418.1989.tb00432.x) [DOI] [Google Scholar]
- 75.Riens B, Lohaus G, Heineke D, Heldt H. 1991. Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 97, 227–233 10.1104/pp.97.1.227 (doi:10.1104/pp.97.1.227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lohaus G, Winter H, Riens B, Heldt HW. 1995. Further studies of the phloem loading process in leaves of barley and spinach: the comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes. Botanica Acta 108, 270–275 [Google Scholar]
- 77.Pate J, Shedley E, Arthur D, Adams M. 1998. Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus. Oecologia 117, 312–322 10.1007/s004420050664 (doi:10.1007/s004420050664) [DOI] [PubMed] [Google Scholar]
- 78.Merchant A, Peuke AD, Keitel C, Macfarlane C, Warren CR, Adams MA. 2010. Phloem sap and leaf δ13C, carbohydrates, and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. J. Exp. Bot. 61, 1785–1793 10.1093/jxb/erq045 (doi:10.1093/jxb/erq045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lohaus G, Hussmann M, Pennewiss K, Schneider H, Zhu JJ, Sattelmacher B. 2000. Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. J. Exp. Bot. 51, 1721–1732 10.1093/jexbot/51.351.1721 (doi:10.1093/jexbot/51.351.1721) [DOI] [PubMed] [Google Scholar]
- 80.Evert RF, Eschrich W, Heyser W. 1978. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta 138, 279–294 10.1007/BF00386823 (doi:10.1007/BF00386823) [DOI] [PubMed] [Google Scholar]
- 81.Weiner H, Blechschmidt-Schneider S, Mohme H, Eschrich W, Heldt H. 1991. Phloem transport of amino acids. Comparison of amino acid contents of maize leaves and of the sieve tube exudate. Plant Physiol. Biochem. 29, 19–23 (doi:10.1104.97.1.227) [Google Scholar]
- 82.Ohshima T, Hayashi H, Chino M. 1990. Collection and chemical composition of pure phloem sap from Zea mays L. Plant Cell Physiol. 31, 735–737 [Google Scholar]
- 83.Winter H, Lohaus G, Heldt HW. 1992. Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiol. 99, 996–1004 10.1104/pp.99.3.996 (doi:10.1104/pp.99.3.996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Botha CEJ, Cross RHM. 1997. Plasmodesmatal frequency in relation to short-distance transport and phloem loading in leaves of barley (Hordeum vulgare). Phloem is not loaded directly from the symplast. Physiologia Plantarum 99, 355–362 10.1111/j.1399-3054.1997.tb00547.x (doi:10.1111/j.1399-3054.1997.tb00547.x) [DOI] [Google Scholar]
- 85.Berthier A, Desclos M, Amiard V, Morvan-Bertrand A, Demmig-Adams B, Adams WW, Turgeon R, Prud'homme M-P, Noiraud-Romy N. 2009. Activation of sucrose transport in defoliated Lolium perenne L.: an example of apoplastic phloem loading plasticity. Plant Cell Physiol. 50, 1329–1344 10.1093/pcp/pcp081 (doi:10.1093/pcp/pcp081) [DOI] [PubMed] [Google Scholar]
- 86.Moing A, Carbonne F, Rashad MH, Gaudillère JP. 1992. Carbon fluxes in mature peach leaves. Plant Physiol. 100, 1878–1884 10.1104/pp.100.4.1878 (doi:10.1104/pp.100.4.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kehr J, Hustiak F, Walz C, Willmitzer L, Fisahn J. 1998. Transgenic plants changed in carbon allocation pattern display a shift in diurnal growth pattern. Plant J. 16, 497–503 10.1046/j.1365-313x.1998.00318.x (doi:10.1046/j.1365-313x.1998.00318.x) [DOI] [PubMed] [Google Scholar]
- 88.Turgeon R. 2010. The role of phloem loading reconsidered. Plant Physiol. 152, 1817–1823 10.1104/pp.110.153023 (doi:10.1104/pp.110.153023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davidson A, Keller F, Turgeon R. 2011. Phloem loading, plant growth form, and climate. Protoplasma 248, 153–163 10.1007/s00709-010-0240-7 (doi:10.1007/s00709-010-0240-7) [DOI] [PubMed] [Google Scholar]
- 90.Hill GP. 1962. Exudation from aphid stylets during the period from dormancy to bud break in Tilia americana (L.). J. Exp. Bot. 13, 144–151 10.1093/jxb/13.1.144 (doi:10.1093/jxb/13.1.144) [DOI] [Google Scholar]
- 91.Hoffmann-Thoma G, van Bel AJE, Ehlers K. 2001. Ultrastructure of minor-vein phloem and assimilate export in summer and winter leaves of the symplasmically loading evergreens Ajuga reptans L, Aucuba japonica Thunb, and Hedera helix L. Planta 212, 231–242 10.1007/s004250000382 (doi:10.1007/s004250000382) [DOI] [PubMed] [Google Scholar]
- 92.Bachmann M, Matile P, Keller F. 1994. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation, and sink to source transition: discovery of chain elongation enzyme. Plant Physiol. 105, 1335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]