Abstract
Although antibiotic resistance represents a public health emergency, the pipeline of new antibiotics is running dry. Repurposing of old drugs for new clinical applications is an attractive strategy for drug development. We used the bacterial pathogen Pseudomonas aeruginosa as a target for the screening of antivirulence activity among marketed drugs. We found that the antimycotic agent flucytosine inhibits the expression of the iron-starvation σ-factor PvdS, thereby repressing the production of major P. aeruginosa virulence factors, namely pyoverdine, PrpL protease, and exotoxin A. Flucytosine administration at clinically meaningful dosing regimens suppressed P. aeruginosa pathogenicity in a mouse model of lung infection. The in vitro and in vivo activity of flucytosine against P. aeruginosa, combined with its desirable pharmacological properties, paves the way for clinical trials on the anti-P. aeruginosa efficacy of flucytosine in humans.
Keywords: antivirulence drug, cystic fibrosis, drug repositioning, iron uptake, selective optimization of side activities (SOSA) approach
Only 70 y after the introduction of antibiotics in the clinical practice, the development and spread of resistance among pathogenic bacteria are limiting the therapeutic efficacy of these magic bullets. Inhibition of bacterial virulence, rather than growth, is an alternative approach to the development of new antimicrobials. Antivirulence drugs disarm rather than kill pathogens. In principle, they combat bacterial infections without exerting the strong selective pressure for resistance imposed by conventional antibiotics, with no predictable detrimental effect on the host microbiota (1). In the last decade, many antivirulence strategies have been proven effective in animal models of infection (reviewed in ref. 2), although no antivirulence compound has yet been tested in large-scale clinical trials.
A shortcut to the development of new drugs is searching for side activities in old drugs already approved for use in humans and for which safety issues have extensively been considered (3). This drug-repurposing strategy has a high probability of yielding safe and bioavailable hit compounds, which can move straightforward into clinical trials or be used as leads for drug optimization programs (3).
The Gram-negative bacterium Pseudomonas aeruginosa is one of the most dreaded nosocomial pathogens and the leading cause of chronic lung infection in patients with cystic fibrosis (CF) (4). Multidrug-resistant P. aeruginosa has become increasingly frequent in healthcare settings and poses a tremendous challenge to traditional antibiotic therapy (5). Because P. aeruginosa has a large armamentarium of virulence factors (6), inhibition of master regulatory networks controlling its pathogenicity, rather than individual virulence traits, is more likely to cause an overall attenuation of virulence (7).
The siderophore pyoverdine represents a promising target for antivirulence compounds. Pyoverdine is not only the primary iron carrier during P. aeruginosa infection and biofilm formation (8, 9) but also, a master signal molecule that controls virulence gene expression through a mechanism called surface signaling (10). Interaction of iron-loaded pyoverdine with its cognate outer membrane receptor FpvA triggers a signal through the inner membrane-spanning anti–σ-factor FpvR, leading to full activation of the alternative σ-factor PvdS, which is responsible for expression not only of pyoverdine genes but also, key virulence factors (i.e., exoproteases and exotoxin A) (10). Pyoverdine synthesis is stimulated by iron deficiency, a nutritional condition characterizing the biological fluids of infected mammals (11), whereas negative control of pyoverdine synthesis is exerted by the global regulator of bacterial iron homeostasis Fur, which represses pvdS transcription under high-iron conditions (12). Although the role of pyoverdine in pathogenicity has been known for years, this system has so far been ignored as a target for antivirulence drugs. Only recently, an enzymatic screening assay allowed the identification of two compounds inhibiting the in vitro activity of PvdQ, a periplasmic hydrolase that is required for pyoverdine maturation (13). However, the antipyoverdine activity of these inhibitors has not been tested in bacterial cultures or in vivo.
The aim of the present work was to apply a drug-repurposing approach to identify antipyoverdine compounds that could represent good candidates for in vivo use as antivirulence drugs against P. aeruginosa. By using a specific biosensor for pyoverdine inhibitors, we screened a chemical library of marketed drugs and identified a promising US Food and Drug Administration-approved compound that resulted effective in suppressing P. aeruginosa virulence in vitro and in an animal model of pulmonary infection.
Results and Discussion
Identification of a Pyoverdine Synthesis Inhibitor.
A screening system for pyoverdine inhibitors, based on a P. aeruginosa PAO1 reporter strain carrying a transcriptional fusion between the PvdS-dependent pvdE promoter (PpvdE) and the luxCDABE operon inserted at a neutral chromosomal site, was constructed (Fig. S1). This system was used to screen a commercial library of 1,120 chemical compounds with known biological activities selected for their high chemical and pharmacological diversity and safety in humans (Prestwick Chemicals). Blind screening led to the identification of one compound that reproducibly reduced bioluminescence and pyoverdine production by the reporter strain under iron-depleted conditions. This compound was decoded as flucytosine [5-Fluorocytosine (5-FC)], a synthetic fluorinated pyrimidine used as an antimycotic drug with the brand name of Ancobon.
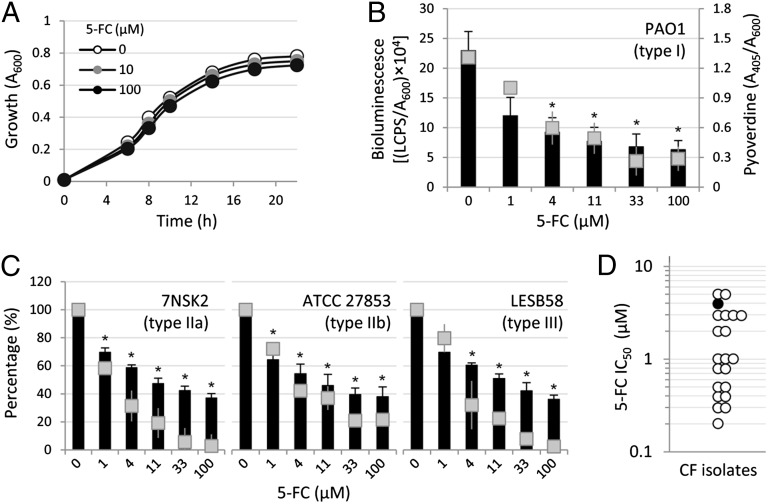
To confirm the antipyoverdine activity of 5-FC, the compound was purchased from a different supplier (Sigma-Aldrich) and used for additional investigation. Although 5-FC did not affect P. aeruginosa growth (Fig. 1A) (minimum inhibitory concentration > 10 mM), it had a very high inhibitory activity on PpvdE-dependent bioluminescence emission and pyoverdine production (Fig. 1B), with IC50 values of 2 and 3 µM, respectively. 5-FC also showed a similar inhibitory effect on the transcription of other pyoverdine biosynthetic genes (Fig. S2A), suggesting that 5-FC negatively affects the expression of the entire pyoverdine biosynthesis machinery. As a control, no variation in the expression of the housekeeping gene proC was observed in the presence of 5-FC (Fig. S2B).
Fig. 1.
5-FC inhibits pyoverdine production in P. aeruginosa. (A) P. aeruginosa PAO1 growth curve in TSBD in the presence of different 5-FC concentrations (0–100 µM). (B) Dose–response effect of 5-FC (0–100 µM) on bioluminescence (black bars, left y axis) and pyoverdine production (gray squares, right y axis) by PAO1 PpvdE::lux at 14 h of growth in TSBD. (C) Dose–response effect of 5-FC (0–100 µM) on bioluminescence (black bars) and pyoverdine production (gray squares) at 14 h of growth in TSBD by P. aeruginosa strains producing different pyoverdine types and pyoverdine receptors (IIa, IIb, or III) and carrying the PpvdE::lux fusion. Values are normalized to A600 of bacterial cultures and expressed as percentage of the corresponding untreated control values. Values represent the mean (± SD) of at least three independent assays. The specific pyoverdine receptor type expressed by each strain is indicated in B and C. (D) IC50 values (micromolar) of 5-FC for pyoverdine production in 20 P. aeruginosa CF isolates (white circles) compared with the PAO1 reference strain (black circle). *Statistically significant differences (P < 0.01, ANOVA) in both bioluminescence and pyoverdine production with respect to the corresponding untreated controls.
5-FC Inhibits Pyoverdine Synthesis in Diverse P. aeruginosa Strains.
Because P. aeruginosa strains can produce one of three different pyoverdine types (I, II, or III), which are recognized by cognate FpvA receptor variants (14), we assessed the effect of 5-FC on prototypic P. aeruginosa strains producing different types of pyoverdine and carrying the PpvdE::lux reporter construct. The inhibitory activity of 5-FC on both pyoverdine production and pvdE transcription was similar among P. aeruginosa strains producing type I, II, or III pyoverdine (Fig. 1 B and C), indicating that the antipyoverdine activity of 5-FC is independent of the chemical nature of the pyoverdine molecule and the structure of the ferripyoverdine receptor.
5-FC–dependent pyoverdine inhibition was also tested on a small collection of P. aeruginosa CF isolates (n = 20), including clonal variants isolated from the same CF patients during a period of more than 15 y (15) (Table S1). The IC50 values of 5-FC for CF isolates were comparable with or even lower than the IC50 determined for the laboratory strain PAO1 (Fig. 1D), suggesting that susceptibility to 5-FC is conserved in CF isolates.
5-FC Inhibits pvdS Gene Expression.
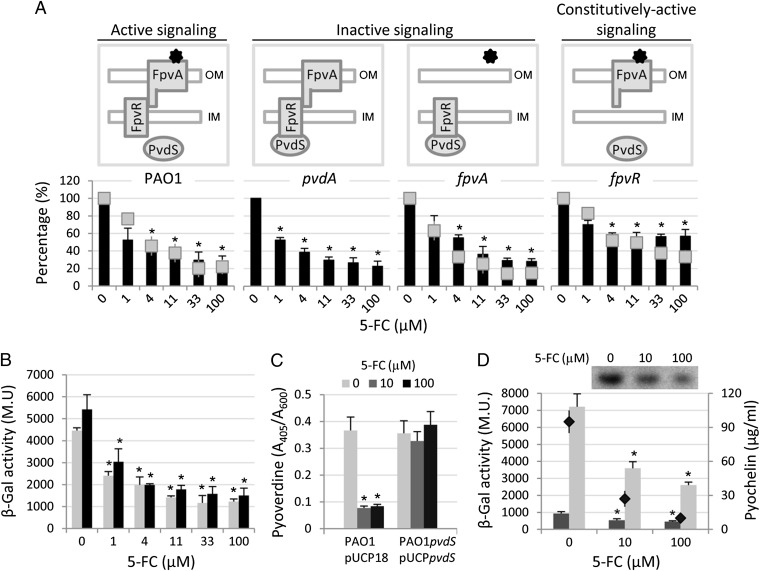
To investigate the effect of 5-FC on pyoverdine signaling, we determined the antipyoverdine activity of 5-FC on a set of P. aeruginosa mutants impaired in different steps of the pyoverdine signaling cascade (Fig. 2A). The FpvA- and PvdA-deficient mutants are impaired in pyoverdine uptake and synthesis, respectively, and they are, therefore, unable to activate the PvdS σ-factor through pyoverdine-mediated signaling (signaling-off mutants). In contrast, the FpvR-deficient mutant cannot suppress PvdS activity in the absence of pyoverdine signaling, thus resulting in signaling-insensitive up-regulation of PvdS-dependent genes (constitutive signaling-on mutant) (Fig. 2A). 5-FC inhibited pyoverdine production and pvdE gene expression in all mutants tested (Fig. 2A), indicating that pyoverdine signaling is not the target of 5-FC. However, 5-FC seemed to be slightly less effective against the fpvR mutant (Fig. 2A). Because the constitutively active state of pyoverdine signaling in the fpvR mutant results in maximal activation of the PvdS intracellular pool (16), the lower activity of 5-FC in the fpvR background suggests that intracellular levels and/or activity of PvdS are critical for the inhibitory activity of 5-FC.
Fig. 2.
5-FC down-regulates pvdS transcription. (A) Dose–response effect of 5-FC (0–100 µM) on bioluminescence emission (black bars) and pyoverdine production (gray squares) at 14 h of growth in TSBD by P. aeruginosa WT and mutant strains defective in different steps of the pyoverdine signaling cascade and carrying the PpvdE::lux reporter fusion. Values are normalized to the cell density of the bacterial cultures and expressed as percentage of the corresponding untreated control values. The different behavior of mutants with respect to pyoverdine signaling is illustrated in Upper (black stars represent pyoverdine). (B) Dose–response effect of 5-FC (0–100 µM) on β-gal expression by PAO1 PpvdS::lacZ during exponential (gray bars) and stationary phase of growth in TSBD (black bars). (C) Effect of 5-FC (0–100 µM) on pyoverdine production at 8 h of growth in TSBD by P. aeruginosa PAO1 carrying the empty vector (pUCP18) and its isogenic pvdS mutant constitutively expressing PvdS (pUCPpvdS). (D) Effect of 5-FC (0–100 µM) on β-gal expression by P. aeruginosa PAO1 PpchR::lacZ (dark gray histograms, left y axis) and PAO1 PpchE::lacZ (light gray histograms, left y axis) and pyochelin production by PAO1 WT (black diamonds, right y axis) after 14 h of growth in TSBD. Values represent the mean (± SD) of three independent assays. Inset shows ferripyochelin yields following separation of PAO1 culture extracts on a representative TLC plate. *Statistically significant differences (P < 0.01, ANOVA) with respect to the corresponding untreated controls.
The effect of 5-FC on pvdS gene expression was investigated in P. aeruginosa carrying a transcriptional fusion between the pvdS promoter and the β-gal gene. 5-FC reduced pvdS promoter activity in a dose-dependent manner (Fig. 2B), suggesting that 5-FC acts as an inhibitor of pvdS transcription and consequently, reduces PvdS intracellular levels, expression of pyoverdine genes, and ultimately, pyoverdine production. To verify this hypothesis, the effect of 5-FC on pyoverdine production was assessed in a pvdS-deficient P. aeruginosa mutant carrying the pvdS coding sequence on a multicopy plasmid under the control of a constitutive promoter. Although 5-FC inhibited pyoverdine production by the WT strain carrying the empty vector, it had no effect on the strain constitutively expressing PvdS (Fig. 2C). Interestingly, 5-FC also repressed the transcription of the Fur-Fe2+–regulated gene pchR (Fig. 2D), encoding an AraC/XylS-like transcriptional regulator essential for production of the second P. aeruginosa siderophore pyochelin (17). Accordingly, the expression of the PchR-regulated gene pchE and production of pyochelin were also reduced in the presence of 5-FC (Fig. 2D). 5-FC also inhibited the transcription of additional iron-repressible genes, namely feoA and foxA (Fig. S2 C and D), which are directly and indirectly controlled by Fur-Fe2+, respectively (12). However, 5-FC–dependent suppression of pyoverdine production was also observed in a P. aeruginosa PAO1 fur mutant (Fig. S2E), suggesting that 5-FC could repress iron uptake genes through a Fur-independent mechanism.
5-FC Down-Regulates PvdS-Dependent Expression of Virulence Genes.
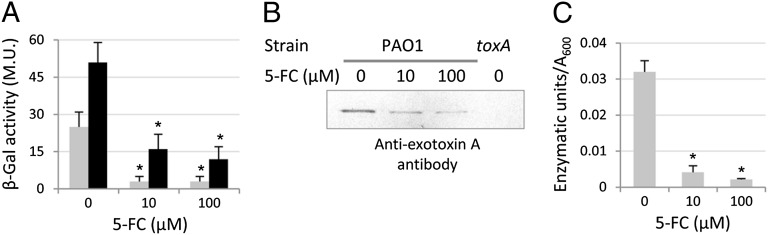
The finding that 5-FC inhibits pvdS transcription implies that this compound could also affect the expression of PvdS-regulated virulence factors other than pyoverdine. To verify this hypothesis, we investigated the effect of 5-FC on the expression of two major virulence factors of P. aeruginosa, the endoprotease PrpL and exotoxin A, which are directly and indirectly regulated by PvdS, respectively (12). PrpL and exotoxin A were monitored at the expression level using PprpL::lacZ and PtoxA::lacZ fusions and at the protein level using antiexotoxin A Western blot analysis and a PrpL enzyme activity assay. As for pyoverdine, the expression of toxA and prpL genes was down-regulated in 5-FC–treated cultures with respect to untreated controls (Fig. 3A), consistent with the strongly reduced ToxA and PrpL levels in culture supernatants (Fig. 3 B and C).
Fig. 3.
5-FC inhibits PvdS-dependent virulence gene expression. Effect of 5-FC (0–100 µM) on (A) β-gal expression by PAO1 PtoxA::lacZ (gray bars) and PAO1 PprpL::lacZ (black bars) and (B) exotoxin A levels and (C) PrpL enzymatic activity in 10 μL PAO1 culture supernatants after 8 h of growth in TSBD. Values represent the mean (± SD) of three independent assays, whereas the Western blot is representative of two independent experiments giving similar results. *Statistically significant differences (P < 0.01, ANOVA) with respect to the corresponding untreated controls.
5-FC Suppresses P. aeruginosa Pathogenicity in Vivo.
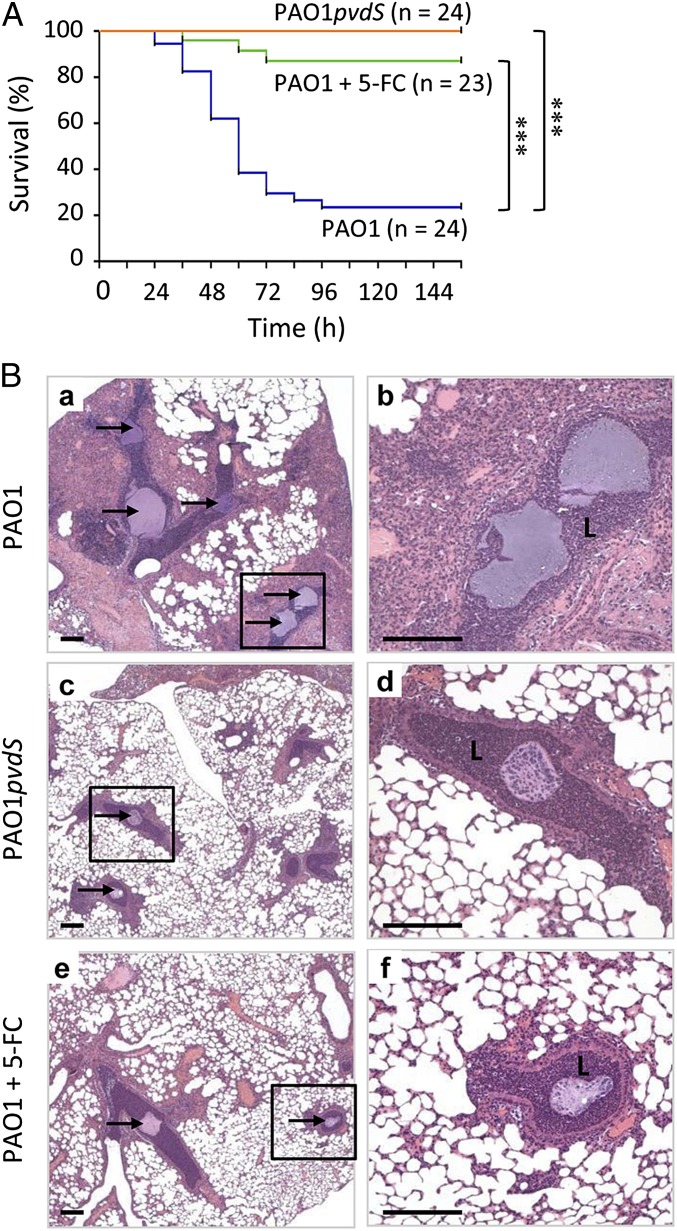
The promising antivirulence activity of 5-FC in vitro led us to investigate the efficacy of 5-FC as an anti-P. aeruginosa drug in a mouse model of pulmonary infection. Mice were infected intratracheally with ca. 106 P. aeruginosa PAO1 cells embedded in agar beads and then treated two times daily with i.p. administration of either a therapeutic dose of 5-FC (30 mg/kg per day) or the placebo (saline). As a control, mice were also infected with an isogenic pvdS mutant and treated with saline. Although 75% of placebo-treated mice were killed within 4 d of PAO1 infection, 5-FC treatment almost completely protected mice from the P. aeruginosa lethal challenge (Fig. 4A). Notably, all mice infected with the pvdS mutant survived the challenge (Fig. 4A), highlighting the importance of PvdS as a major pathogenicity determinant in P. aeruginosa pulmonary infection. After 6 d of infection, the bacterial load in lungs of surviving mice was comparable between mice infected with PAO1 and PAO1pvdS as well as between 5-FC–treated and -untreated mice (Fig. S3), confirming that 5-FC inhibits virulence rather than cell viability. Moreover, lung histopathology revealed that lesions and inflammation in bronchi and pulmonary parenchyma were similarly reduced in both 5-FC–treated and PAO1pvdS-infected mice compared with untreated mice infected with WT PAO1 (Fig. 4B).
Fig. 4.
5-FC suppresses P. aeruginosa virulence in vivo. (A) Effect of 5-FC on P. aeruginosa PAO1 lethality in a mouse model of pulmonary infection. Mice were infected intratracheally with P. aeruginosa PAO1 embedded in agar beads and treated with i.p. administrations of 30 mg/kg per day 5-FC (green lines) or saline (blue lines). As control, mice infected with PAO1pvdS and treated with saline were used (orange lines). Data were pooled from two independent experiments (n indicates the total number of mice). ***P < 0.0001 (Mantel–Cox test). (B) Murine lung histology. Four additional mice per group were infected with P. aeruginosa PAO1 or PAO1pvdS embedded in agar beads, treated with 5-FC or saline as described in A, and euthanized at day 2 postinfection (10). Lung sections were stained with H&E. PAO1-infected mice showed a massive bronchiolitis and huge interstitial/alveolar inflammation. In PAO1pvdS- and PAO1-infected mice treated with 5-FC (+5-FC), the inflammation was focal, and most of alveolar spaces were spared. Beads, indicated by arrows, are visible in the bronchial lumen (L), and P. aeruginosa macrocolonies can be observed into the beads. b, d, and f are enlargements of the boxed areas in a, c, and e. (Scale bars: 200 μm.)
Antivirulence Activity of 5-FC Requires Metabolic Conversion to 5-Fluorouracil.
The antimycotic compound 5-FC is a prodrug that is taken up by fungi through one or more cytosine permeases, deaminated to 5-fluorouracil by a cytosine deaminase, and subsequently, converted to 5-fluoro-UMP and 5-fluoro-dUMP, ultimately causing perturbation of DNA and protein synthesis (18). Although 5-FC by itself is not toxic, 5-fluorouracil is highly cytotoxic. Therefore, the direct use of 5-fluorouracil in medicine is restricted to the treatment of solid tumors (19).
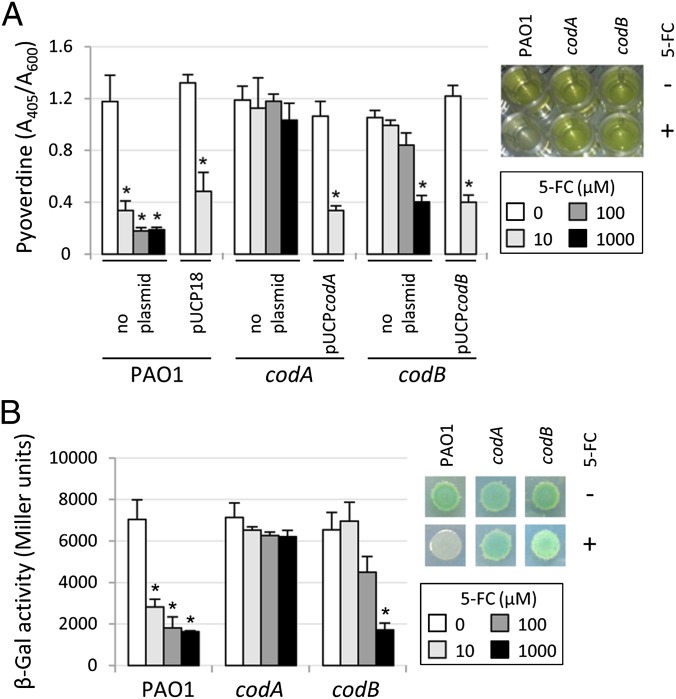
All P. aeruginosa genomes sequenced so far contain homologs of the codA and codB genes of Escherichia coli (www.pseudomonas.com), encoding a cytosine deaminase and a cytosine permease, respectively (20). To assess whether conversion to 5-fluorouracil is essential for the antipyoverdine activity of 5-FC, we tested 5-FC against individual P. aeruginosa codA and codB deletion mutants. Inhibition of pyoverdine production and pvdS gene expression by 5-FC was strongly reduced in the PAO1codB mutant and completely abrogated in the PAO1codA mutant, indicating that 5-FC uptake and conversion to 5-fluorouracil are essential for 5-FC activity in P. aeruginosa (Fig. 5). Interestingly, a very high 5-FC concentration (1 mM) retained some activity against the PAO1codB mutant (Fig. 5), suggesting that 5-FC can also enter P. aeruginosa cells through low-affinity secondary systems or by passive diffusion.
Fig. 5.
Enzymatic conversion to 5-fluorouracil is essential for the antipyoverdine activity of 5-FC. Effect of 5-FC (0–1,000 μM) on (A) pyoverdine production by PAO1, PAO1codA (codA), and PAO1codB (codB) containing or not containing the plasmid pUCP18, pUCPcodA, or pUCPcodB as indicated and (B) β-gal activity by the same strains containing the PpvdS::lacZ fusion construct grown for 14 h in TSBD. Insets show (A) pyoverdine production in M9 medium (green fluorescence) and (B) β-gal activity (blue color) in M9 agar plates containing the chromogenic substrate X-gal after 14 h of growth in the absence (−) or presence (+) of 100 μM 5-FC. *Statistically significant differences (P < 0.01, ANOVA) with respect to the corresponding untreated controls.
Conclusions
This work represents proof that the pyoverdine system is a suitable target for the development of antivirulence compounds against P. aeruginosa. We showed that the antimycotic drug 5-FC inhibits the production of critical virulence factors, like pyoverdine, exotoxin A, and protease PrpL, by down-regulating pvdS gene expression. 5-FC also suppressed P. aeruginosa pathogenicity in a mouse model of lung infection, consistent with the essential role played by PvdS during pulmonary infection (Fig. 4). The molecular mechanisms by which 5-FC inhibits pvdS transcription are unknown at the moment, although we showed that (i) 5-FC has an inhibitory effect on the expression of iron uptake genes and (ii) 5-FC uptake and metabolic conversion to 5-fluorouracil are essential steps for 5-FC activity. Cytosine deaminase is typically produced by microorganisms and has no counterpart in higher eukaryotes, including mammals. These features confer to 5-FC selective activity on those species capable of assimilating and activating the prodrug.
Our results gain additional relevance if the pharmacological properties of 5-FC are taken into account. 5-FC is currently used combined with other antifungal agents for the treatment of systemic mycoses and fungal pneumonias (21, 22). Orally administered 5-FC is almost completely adsorbed, reaches peak concentrations in serum within 1–2 h, and easily reaches most body sites (21). 5-FC is also well-tolerated and has very low toxicity as long as serum concentrations are maintained below 50 µg/mL (388 µM) (21, 23). This serum level is almost 40-fold higher than the 5-FC concentration (10 µM) able to exert the maximal inhibitory effect in vitro on P. aeruginosa virulence gene expression (Figs. 1, 2, and 3). 5-FC has also been successfully used to treat fungal infections in CF patients, including a case of pulmonary candidiasis, without causing side effects (24, 25). These issues raise the possibility that currently recommended 5-FC dosing regimens would also be effective as antivirulence therapy against P. aeruginosa. We hope that our findings will foster clinical investigations aimed at verifying the efficacy of 5-FC in the treatment of P. aeruginosa infections, offering the unique chance of assessing the clinical impact of an antivirulence drug.
Materials and Methods
Bacteria, Media, and Chemicals.
Bacterial strains and plasmids used in this work are listed in Table S2. P. aeruginosa CF isolates are described in Table S1. Bacteria were grown in LB (26) for general genetic procedures, whereas they were grown in the low-iron media trypticase soy broth dialysate (TSBD) (27) or M9 minimal medium supplemented with succinate (26) for specific assays. 5-FC was purchased from Sigma-Aldrich. Exogenous pyoverdine was added as pyoverdine-conditioned medium (8).
General Genetic Procedures.
E. coli was routinely used for recombinant DNA manipulations. The PpvdE::lux construct was generated by cloning in plasmid mini–CTX-lux (28) the SalI-HindIII DNA fragment encompassing the pvdE promoter region excised from pMP190::PpvdE (29). The PpvdE::lux construct was integrated into the genome of P. aeruginosa strains as described (30). The PAO1pvdS mutant was generated by replacement of the entire pvdS coding sequence with a GmR cassette using a previously described strategy (31). The in-frame deletion mutants PAO1codA and PAO1codB were generated using the suicide vector pDM4 as described (32). The complementing plasmids pUCPcodA and pUCPcodB were generated by cloning the codA and codB coding sequence, including their putative ribosome binding site, downstream to the lac promoter in the pUCP18 plasmid (Table S2). The PpchR::lacZ and PfeoA::lacZ transcriptional fusions were generated by cloning a PCR-amplified DNA fragment encompassing the entire promoter region of pchR and feoA genes, respectively, into the promoter probe plasmid pMP220 (Table S2). Primers and restriction enzymes used for cloning of PCR products are listed in Table S3.
Screening for Pyoverdine Inhibitors.
Overnight cultures of PAO1 PpvdE::lux were diluted to A600 = 0.003 in the iron-poor TSBD medium, and growth at 37 °C in microtiter plates in the presence or absence of 50 or 5 μg/mL each Prestwick compound (200 μL final volume) was monitored for up to 20 h. A600 and bioluminescence light counts per second (LCPS) were measured in a Victor3V plate reader (Perkin-Elmer) as described (30). Pyoverdine fluorescence was assessed as emission at 460 nm after excitation at 405 nm (33). Luminescence and fluorescence values were normalized by the cell density and subtracted of untreated PAO1pvdA PpvdE::lux values. Criteria used for the selection of hit compounds were (i) ≥50% inhibition of normalized bioluminescence emission and/or pyoverdine-specific fluorescence and (ii) ≤20% alteration of growth relative to the untreated control. Criterion ii was aimed at avoiding any unspecific effect of altered growth on bioluminescence and/or pyoverdine production. For promising compounds, pyoverdine was also quantified in diluted cell-free culture supernatants (see below).
Miscellaneous Assays.
Pyoverdine levels in culture supernatants were measured as A405 in 100 mM Tris⋅HCl (pH 8) and normalized by the cell density (A600) of the bacterial cultures (33). Exotoxin A was detected in 10 μL culture supernatants by SDS/PAGE followed by Western blot with a polyclonal antiexotoxin A antibody (Sigma-Aldrich). PrpL and β-gal enzymatic activities were determined as previously described (34, 35). Pyochelin was isolated by ethyl acetate extraction of acidified culture supernatants, resuspended in methanol, and resolved by TLC on silica gel (36). Pyochelin was detected by spraying with 0.1 M FeCl3 and quantified by A520 readings of ferripyochelin eluted with methanol from TLC plates (37). Anti-PvdA Western blot analysis was performed using the 3H6D12 monoclonal antibody as described (38).
Mouse Model of P. aeruginosa Lung Infection.
C57BL/6 male mice (Charles River) were infected intratracheally with 106 P. aeruginosa viable cells embedded in agar beads as described (15), except for the use of TSBD agar instead of TSB agar for beads preparation. Mice were treated two times daily (starting 2 h postinfection) by i.p. administration of 50 µL 50 mM 5-FC in saline or 50 µL saline as control. Two 50-µL doses/d of 50 mM 5-FC correspond to a daily dosage of about 30 mg/kg (mouse weight was 20–22 g), which is within or below the dosage range recommended for humans; the dosage ranges for humans are 25–100 mg/kg per day for infants (<1 mo) and 50–150 mg/kg per day for children and adults (http://www.drugs.com/dosage/flucytosine.html). Mortality was monitored for a 6-d time period. Surviving mice were killed at day 6 postinfection, and lungs were excised, homogenized, and plated to determine the number of viable cells per lung. Four additional mice per group were infected with P. aeruginosa PAO1 or PAO1pvdS and treated with 5-FC or saline as described above, and they were euthanized at day 2 postinfection for lung histology. Lungs were removed en bloc, fixed in 4% (wt/vol) paraformaldehyde/PBS, and processed for paraffin embedding. Longitudinal sections of 5 μm taken at regular intervals were obtained using a microtome from the middle of the five lung lobes and stained with H&E. Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute Institutional Animal Care and Use Committee.
Statistical Analysis.
Statistical analysis was performed with the software GraphPad Instat using one-way ANOVA. Survival curves for the mouse infection assay were analyzed using the log-rank Mantel–Cox test.
Supplementary Material
Acknowledgments
We thank Martina Pasqua for assistance in mutagenesis experiments during the preparation of her bachelor thesis and Camilla Riva and Ida De Fino for assistance in experimental infections. We also thank Paolo Landini for the Prestwick Chemical Library, Pierre Cornelis for the type II pyoverdine strains, Burkhard Tümmler for CF strains, Cornelia Reimmann for the PpchE and PproC::lacZ, and Mike Vasil for the PpvdS, PtoxA, and PprpL::lacZ promoter probe plasmids. This work was supported by Italian Cystic Fibrosis Foundation Grants FFC#13/2011 (to F.I.) and FFC#8/2008 (to P.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222706110/-/DCSupplemental.
References
- 1.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6(1):17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9(2):117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 3.Wermuth CG. Selective optimization of side activities: The SOSA approach. Drug Discov Today. 2006;11(3–4):160–164. doi: 10.1016/S1359-6446(05)03686-X. [DOI] [PubMed] [Google Scholar]
- 4.Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67(3):351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Page MG, Heim J. Prospects for the next anti-Pseudomonas drug. Curr Opin Pharmacol. 2009;9(5):558–565. doi: 10.1016/j.coph.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect. 2006;36(2):78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Lee DG, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7(10):R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA. 2005;102(31):11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visca P, Imperi F, Lamont IL. Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 2007;15(1):22–30. doi: 10.1016/j.tim.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonasaeruginosa. Proc Natl Acad Sci USA. 2002;99(10):7072–7077. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol. 2010;12(12):1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 12.Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: Identification of novel pyoverdine biosynthesis genes. Mol Microbiol. 2002;45(5):1277–1287. doi: 10.1046/j.1365-2958.2002.03084.x. [DOI] [PubMed] [Google Scholar]
- 13.Drake EJ, Gulick AM. Structural characterization and high-throughput screening of inhibitors of PvdQ, an NTN hydrolase involved in pyoverdine synthesis. ACS Chem Biol. 2011;6(11):1277–1286. doi: 10.1021/cb2002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodilis J, et al. Distribution and evolution of ferripyoverdine receptors in Pseudomonas aeruginosa. Environ Microbiol. 2009;11(8):2123–2135. doi: 10.1111/j.1462-2920.2009.01932.x. [DOI] [PubMed] [Google Scholar]
- 15.Bragonzi A, et al. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med. 2009;180(2):138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 16.Tiburzi F, Imperi F, Visca P. Intracellular levels and activity of PvdS, the major iron starvation sigma factor of Pseudomonas aeruginosa. Mol Microbiol. 2008;67(1):213–227. doi: 10.1111/j.1365-2958.2007.06051.x. [DOI] [PubMed] [Google Scholar]
- 17.Michel L, González N, Jagdeep S, Nguyen-Ngoc T, Reimmann C. PchR-box recognition by the AraC-type regulator PchR of Pseudomonas aeruginosa requires the siderophore pyochelin as an effector. Mol Microbiol. 2005;58(2):495–509. doi: 10.1111/j.1365-2958.2005.04837.x. [DOI] [PubMed] [Google Scholar]
- 18.Edlind TD, Katiyar SK. Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob Agents Chemother. 2010;54(11):4733–4738. doi: 10.1128/AAC.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan X, et al. Development of 5-Fluorouracil derivatives as anticancer agents. Curr Med Chem. 2011;18(29):4538–4556. doi: 10.2174/092986711797287584. [DOI] [PubMed] [Google Scholar]
- 20.Danielsen S, Kilstrup M, Barilla K, Jochimsen B, Neuhard J. Characterization of the Escherichia coli codBA operon encoding cytosine permease and cytosine deaminase. Mol Microbiol. 1992;6(10):1335–1344. doi: 10.1111/j.1365-2958.1992.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 21.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46(2):171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Yamada H, Kotaki H, Takahashi T. Recommendations for the treatment of fungal pneumonias. Expert Opin Pharmacother. 2003;4(8):1241–1258. doi: 10.1517/14656566.4.8.1241. [DOI] [PubMed] [Google Scholar]
- 23.Stamm AM, et al. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med. 1987;83(2):236–242. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- 24.Jenner BM, Landau LI, Phelan PD. Pulmonary candidiasis in cystic fibrosis. Arch Dis Child. 1979;54(7):555–556. doi: 10.1136/adc.54.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radike K, Kunzmann S, Abele-Horn M, Beer M, Hebestreit H. Osteoarticular infection by Candida albicans in an infant with cystic fibrosis. J Med Microbiol. 2011;60(Pt 10):1542–1545. doi: 10.1099/jmm.0.031757-0. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 27.Ohman DE, Sadoff JC, Iglewski BH. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: Isolation and characterization. Infect Immun. 1980;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becher A, Schweizer HP. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques. 2000;29(5):948–950, 952. doi: 10.2144/00295bm04. [DOI] [PubMed] [Google Scholar]
- 29.Cunliffe HE, Merriman TR, Lamont IL. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J Bacteriol. 1995;177(10):2744–2750. doi: 10.1128/jb.177.10.2744-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massai F, et al. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens Bioelectron. 2011;26(8):3444–3449. doi: 10.1016/j.bios.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Cipollone R, et al. Involvement of Pseudomonas aeruginosa rhodanese in protection from cyanide toxicity. Appl Environ Microbiol. 2007;73(2):390–398. doi: 10.1128/AEM.02143-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampioni G, et al. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol. 2010;12(6):1659–1673. doi: 10.1111/j.1462-2920.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imperi F, Tiburzi F, Visca P. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2009;106(48):20440–20445. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imperi F, Tiburzi F, Fimia GM, Visca P. Transcriptional control of the pvdS iron starvation sigma factor gene by the master regulator of sulfur metabolism CysB in Pseudomonas aeruginosa. Environ Microbiol. 2010;12(6):1630–1642. doi: 10.1111/j.1462-2920.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller JH. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab Press; 1972. [Google Scholar]
- 36.Zhang S, et al. Comparative signature-tagged mutagenesis identifies Pseudomonas factors conferring resistance to the pulmonary collectin SP-A. PLoS Pathog. 2005;1(3):259–268. doi: 10.1371/journal.ppat.0010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox CD, Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imperi F, et al. Membrane-association determinants of the omega-amino acid monooxygenase PvdA, a pyoverdine biosynthetic enzyme from Pseudomonas aeruginosa. Microbiology. 2008;154(Pt 9):2804–2813. doi: 10.1099/mic.0.2008/018804-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.