Abstract
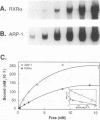
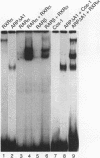
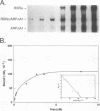
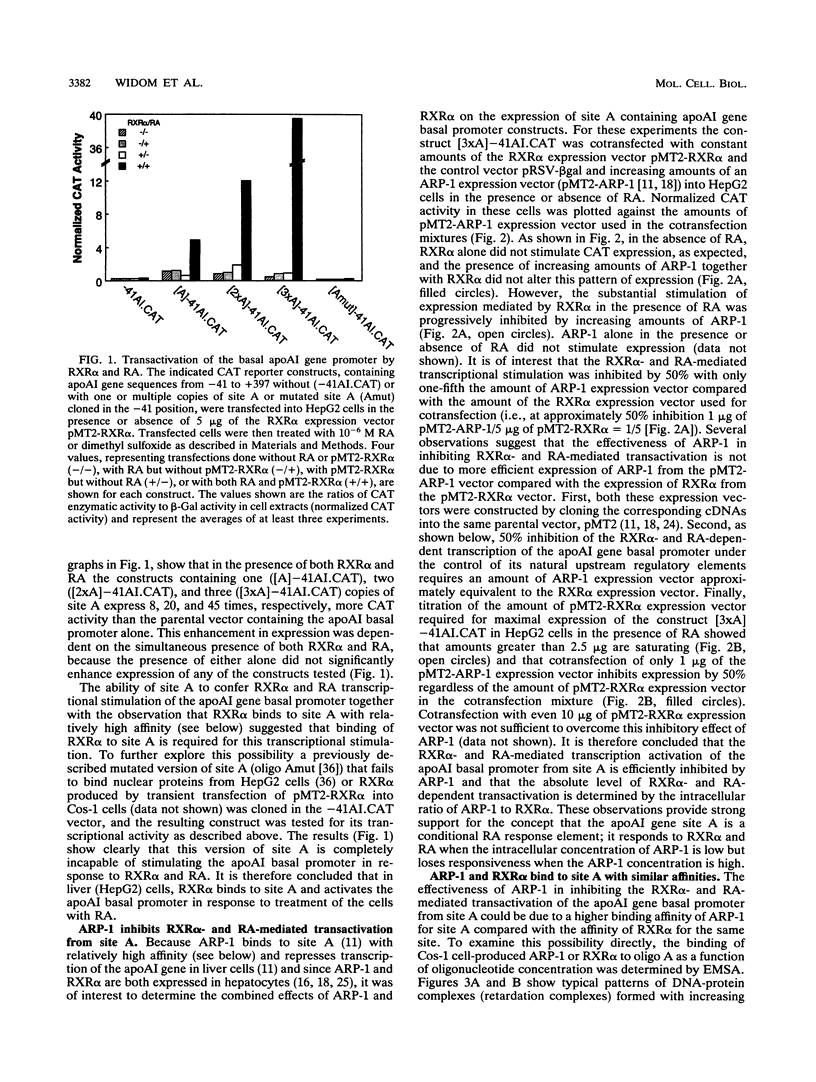
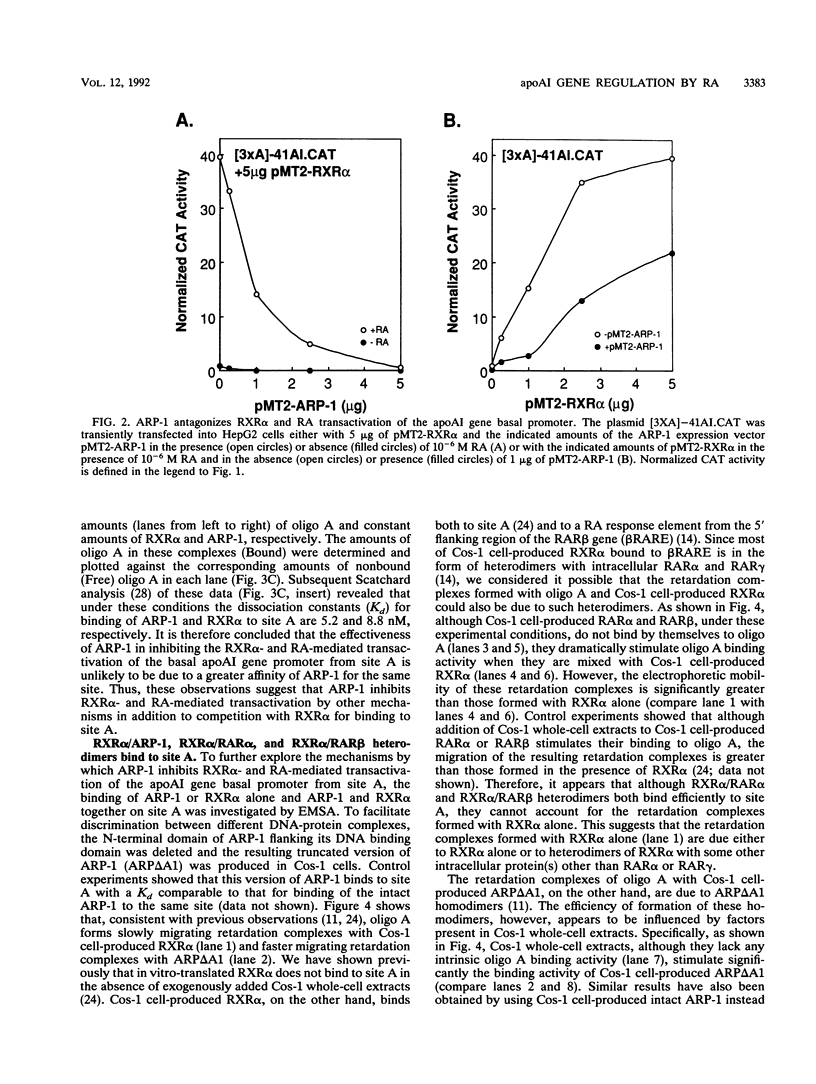
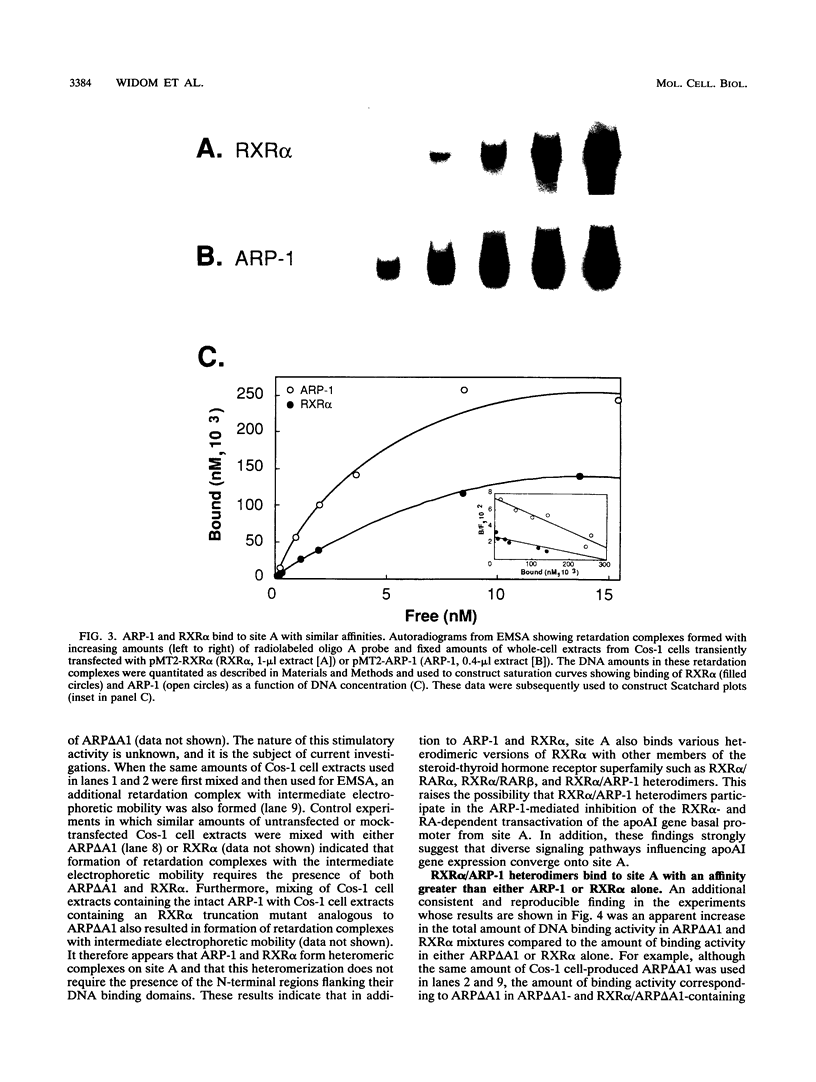
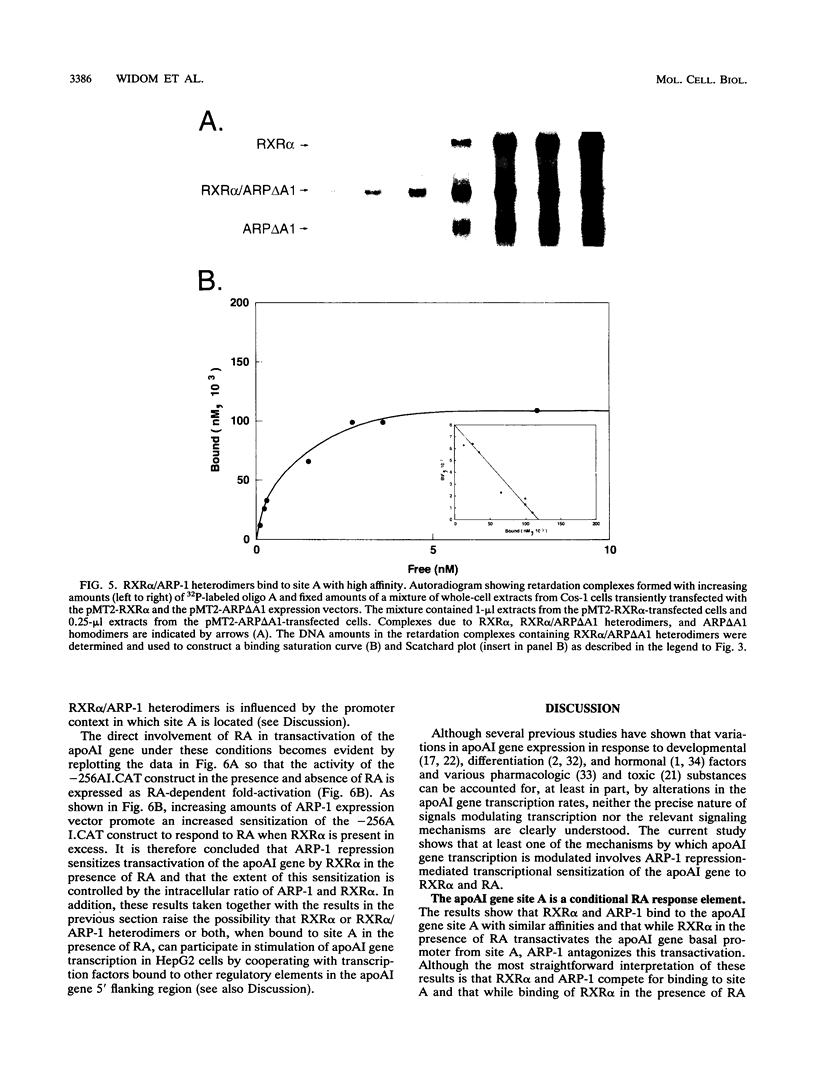
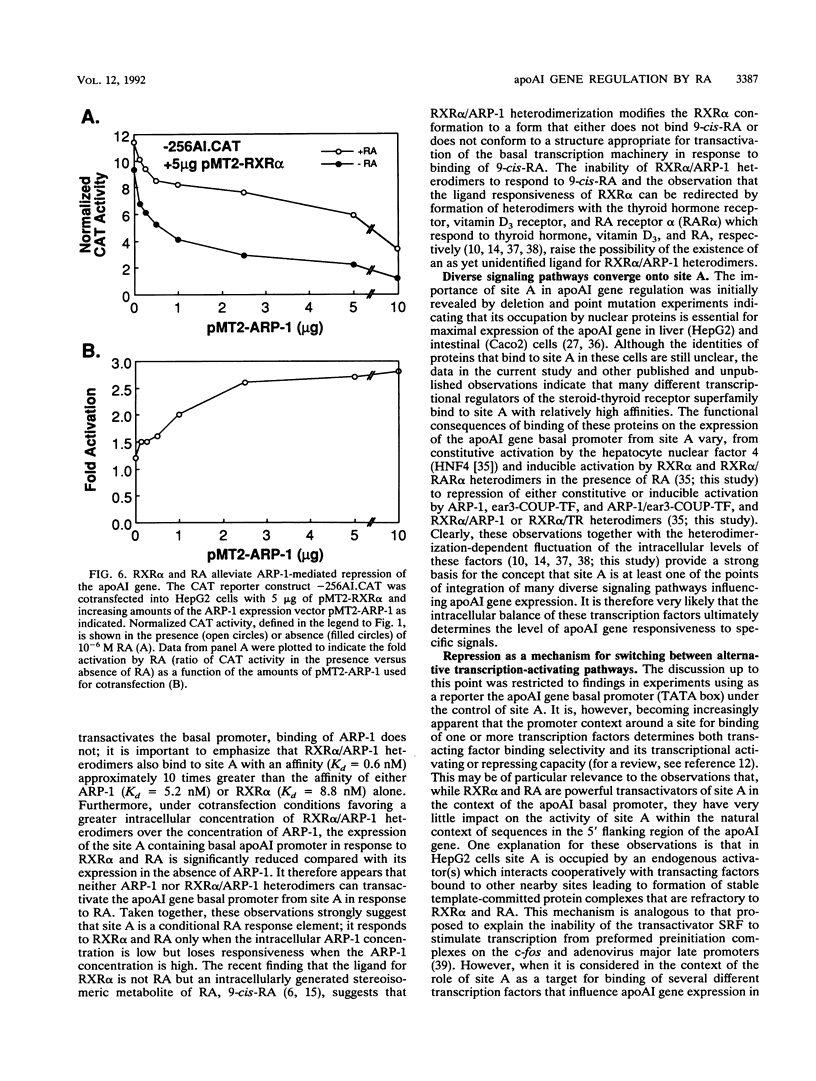
The gene coding for apolipoprotein AI (apoAI), a lipid binding protein involved in the transport of cholesterol and other lipids in the plasma, is expressed in mammals predominantly in the liver and the intestine. Liver-specific expression is controlled by synergistic interactions between transcription factors bound to three separate sites, sites A (-214 to -192), B (-169 to -146), and C (-134 to -119), within a powerful liver-specific enhancer located between nucleotides -222 and -110 upstream of the apoAI gene transcription start site (+1). Previous studies in our laboratory have shown that ARP-1, a member of the nuclear receptor superfamily whose ligand is unknown (orphan receptor), binds to site A and represses transcription of the apoAI gene in liver cells. In a more recent series of experiments, we found that site A is a retinoic acid (RA) response element that responds preferentially to the recently identified RA-responsive receptor RXR alpha over the previously characterized RA receptors RAR alpha and RAR beta. In this study we investigated the combined effects of ARP-1 and RXR alpha on apoAI gene expression in liver cells. Transient transfection assays showed that site A is necessary and sufficient for RXR alpha-mediated transactivation of the apoAI gene basal promoter in human hepatoma HepG2 cells in the presence of RA and that this transactivation is abolished by increasing amounts of cotransfected ARP-1. Electrophoretic mobility shift assays and subsequent Scatchard analysis of the data revealed that ARP-1 and RXR alpha bind to site A with similar affinities. These assays also revealed that ARP-1 and RXR alpha bind to site A as heterodimers with an affinity approximately 10 times greater than that of either ARP-1 or RXR alpha alone. Further transfection assays in HepG2 cells, using as a reporter a construct containing the apoAI gene basal promoter and its upstream regulatory elements (including site A) in their natural context, revealed that RXR alpha has very little effect on the levels of expression regardless of the presence or absence of RA. However, while ARP-1 alone or ARP-1 and RXR alpha together dramatically repress expression in the absence of RA, the repression by ARP-1 and RXR alpha together, but not ARP-1 alone, is almost completely alleviated in the presence of RA. These results indicate that transcriptional repression by ARP-1 sensitizes apoAI gene responsiveness to RXR alpha and RA and suggest that the magnitude of this responsiveness is regulated by the intracellular ratio of ARP-1 to RXR alpha. These observations raise the possibility that transcriptional repression is a general mechanism for switching gene transcription between alternative transcription activation pathways.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolopoulos J. J., La Scala M. J., Howlett G. J. The effect of triiodothyronine on rat apolipoprotein A-I and A-IV gene transcription. Biochem Biophys Res Commun. 1988 Aug 15;154(3):997–1002. doi: 10.1016/0006-291x(88)90238-0. [DOI] [PubMed] [Google Scholar]
- Basheeruddin K., Stein P., Strickland S., Williams D. L. Expression of the murine apolipoprotein E gene is coupled to the differentiated state of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):709–713. doi: 10.1073/pnas.84.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Vectors used for expression in mammalian cells. Methods Enzymol. 1990;185:487–511. doi: 10.1016/0076-6879(90)85041-l. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lamb P., McKnight S. L. Diversity and specificity in transcriptional regulation: the benefits of heterotypic dimerization. Trends Biochem Sci. 1991 Nov;16(11):417–422. doi: 10.1016/0968-0004(91)90167-t. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietus-Snyder M., Sladek F. M., Ginsburg G. S., Kuo C. F., Ladias J. A., Darnell J. E., Jr, Karathanasis S. K. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992 Apr;12(4):1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. E. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J. 1987 Feb;113(2 Pt 2):589–597. doi: 10.1016/0002-8703(87)90638-7. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Race K. R. Biogenesis of retinoic acid from beta-carotene. Differences between the metabolism of beta-carotene and retinal. J Biol Chem. 1988 Nov 25;263(33):17372–17377. [PubMed] [Google Scholar]
- Panduro A., Shalaby F., Shafritz D. A. Changing patterns of transcriptional and post-transcriptional control of liver-specific gene expression during rat development. Genes Dev. 1987 Dec;1(10):1172–1182. doi: 10.1101/gad.1.10.1172. [DOI] [PubMed] [Google Scholar]
- Pandurò A., Lin-Lee Y. C., Chan L., Shafritz D. A. Transcriptional and posttranscriptional regulation of apolipoprotein E, A-I, and A-II gene expression in normal rat liver and during several pathophysiologic states. Biochemistry. 1990 Sep 11;29(36):8430–8435. doi: 10.1021/bi00488a033. [DOI] [PubMed] [Google Scholar]
- Ringer T. V., DeLoof M. J., Winterrowd G. E., Francom S. F., Gaylor S. K., Ryan J. A., Sanders M. E., Hughes G. S. Beta-carotene's effects on serum lipoproteins and immunologic indices in humans. Am J Clin Nutr. 1991 Mar;53(3):688–694. doi: 10.1093/ajcn/53.3.688. [DOI] [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A., Eager N. S., Brickell P. M. A member of the RXR nuclear receptor family is expressed in neural-crest-derived cells of the developing chick peripheral nervous system. Development. 1991 Mar;111(3):771–778. doi: 10.1242/dev.111.3.771. [DOI] [PubMed] [Google Scholar]
- Sastry K. N., Seedorf U., Karathanasis S. K. Different cis-acting DNA elements control expression of the human apolipoprotein AI gene in different cell types. Mol Cell Biol. 1988 Feb;8(2):605–614. doi: 10.1128/mcb.8.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Sorci-Thomas M., Prack M. M., Dashti N., Johnson F., Rudel L. L., Williams D. L. Apolipoprotein (apo) A-I production and mRNA abundance explain plasma apoA-I and high density lipoprotein differences between two nonhuman primate species with high and low susceptibilities to diet-induced hypercholesterolemia. J Biol Chem. 1988 Apr 15;263(11):5183–5189. [PubMed] [Google Scholar]
- Sorci-Thomas M., Prack M. M., Dashti N., Johnson F., Rudel L. L., Williams D. L. Differential effects of dietary fat on the tissue-specific expression of the apolipoprotein A-I gene: relationship to plasma concentration of high density lipoproteins. J Lipid Res. 1989 Sep;30(9):1397–1403. [PubMed] [Google Scholar]
- Spiegelberg T., Bishop J. O. Tissue-specific gene expression in mouse hepatocytes cultured in growth-restricting medium. Mol Cell Biol. 1988 Aug;8(8):3338–3344. doi: 10.1128/mcb.8.8.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B., van Tol A., Andreu T., Auwerx J. Fibrates influence the expression of genes involved in lipoprotein metabolism in a tissue-selective manner in the rat. Arterioscler Thromb. 1992 Mar;12(3):286–294. doi: 10.1161/01.atv.12.3.286. [DOI] [PubMed] [Google Scholar]
- Strobl W., Gorder N. L., Lin-Lee Y. C., Gotto A. M., Jr, Patsch W. Role of thyroid hormones in apolipoprotein A-I gene expression in rat liver. J Clin Invest. 1990 Mar;85(3):659–667. doi: 10.1172/JCI114489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom R. L., Ladias J. A., Kouidou S., Karathanasis S. K. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991 Feb;11(2):677–687. doi: 10.1128/mcb.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhu H., Roy A. L., Roeder R. G., Prywes R. Serum response factor affects preinitiation complex formation by TFIID in vitro. New Biol. 1991 May;3(5):455–464. [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]