Abstract
Marijuana (cannabis) is the most widely used illicit substance globally, and cannabis use is associated with a range of adverse consequences. Currently, no medications have been proven to be effective for the treatment of cannabis addiction. The goals of this study were to examine the safety and efficacy of a potential treatment medication, modafinil, in combination with oral Δ9-tetrahydrocannabinol (THC). Twelve male and female occasional cannabis users participated in an outpatient double-blind, placebo-controlled, crossover study. Across four sessions, participants were randomly assigned to a sequence of four oral treatments: (1) 400 mg modafinil + placebo, (2) 15 mg THC + placebo, (3) 400 mg modafinil + 15 mg THC, or (4) placebo + placebo. Outcome measures included heart rate, blood pressure, performance on the Rapid Visual Information Processing (RVIP), and the Hopkins Verbal Learning Test (HVLT), and subjective measures. Oral THC increased heart rate, and produced increased subjective ratings of feeling “high” and “sedated,” as well as increased ratings of euphoria. Modafinil alone increased the Profiles of Mood States (POMS) subscales of vigor and tension. These findings support the safety of modafinil in combination with THC. The effects of modafinil in combination with a range of doses of THC need to be determined in future studies.
Keywords: cannabis, modafinil, marijuana, THC, addiction
1. Introduction
Marijuana (cannabis) is the most widely used illicit substance globally and in the United States (Compton et al. 2004; ONDCP 2008; SAMHSA 2008). It is estimated that one out of 12 cannabis users will eventually become dependent (Wagner and Anthony 2002). Increased treatment-seeking has been observed among cannabis users (Stephens et al. 2002), making cannabis one of the most common illicit drugs of use among admissions to treatment programs in the US (SAMHSA 2008). Marijuana smoking is associated with a range of adverse consequences, including respiratory ailments, and impairments in memory, concentration, motivation, self-esteem, employment, and interpersonal relationships (Stephens et al. 2002). Currently, there are no effective medications for the treatment of cannabis addiction and available behavioral treatments are only modestly effective (Nordstrom and Levin 2007). For example, behavioral treatments including cognitive-behavioral therapy (CBT), motivational enhancement therapy (MET), and contingency management (CM) have shown promise in the short-term, but one year abstinence rates with a combination of these behavioral treatments have ranged from 9 to 29 % (Budney et al. 2007). Thus, development of effective treatment strategies, specifically for cannabis use disorders (dependence or abuse), is urgently needed.
Evidence shows that chronic exposure to cannabis is associated with dose-related cognitive impairments, most consistently in attention, working memory, verbal learning and memory functions (Bolla et al. 2002; Pope et al. 1995; Pope and Yurgelun-Todd 1996; Solowij 1995; Solowij et al. 1995; Solowij et al. 2002). Recently, we proposed that targeting cognitive impairment associated with chronic cannabis use by cognitive-enhancing medications may be a promising novel strategy for the treatment of cannabis addiction (Sofuoglu et al. 2010). We suggested that medications enhancing cognitive function may be effective either alone or in combination with behavioral treatments for cannabis addiction (Sofuoglu et al. 2010). One cognitive enhancer that has been evaluated for other addictive disorders is modafinil. Modafinil is a wakefulness-promoting medication, approved for the treatment of narcolepsy, sleep-apnea and shift-work induced sleep disorder (Ballon and Feifel 2006). Modafinil has been shown to improve cognitive performance in sleep deprived individuals, as well as in those with attention deficit hyperactivity disorder (ADHD) and schizophrenia (Muller et al. 2004; Turner et al. 2003; Wesensten et al. 2002). Modafinil's mechanism of action is complex and may include enhancement of glutamate release and inhibition of GABA release in various brain regions (Ferraro et al. 1998; Ferraro et al. 1999), as well as dopamine and norepinephrine transporter inhibition (Madras et al. 2006), which results in increased synaptic levels of dopamine and norepinephrine (Murillo-Rodriguez et al. 2007; Volkow et al. 2009). Modafinil is currently being examined for the treatment of cocaine and methamphetamine addiction (Dackis et al. 2005a; Hart et al. 2007; Morgan et al. 2010; Shearer et al. 2009a).
Modafinil's potential utility for cannabis dependence has not yet been evaluated. The cognitive enhancing effects of modafinil may alleviate cognitive deficits associated with cannabis use, and its mood-elevating effects could potentially help to alleviate cannabis withdrawal, which include depressed mood, irritability, anxiety, and anger (Budney and Hughes 2006). In addition, modafinil has been shown to attenuate some of the positive subjective effects from cocaine, methamphetamine and nicotine in humans (Dackis et al. 2003b; De La Garza et al. 2010; Malcolm et al. 2006; Sofuoglu et al. 2008). Since increasing dopamine release in the nucleus accumbens is a shared mechanism for the rewarding effects of stimulants (Pich et al. 1997) as well as cannabis (Cheer et al. 2004; Robledo et al. 2007), it is plausible that modafinil may also attenuate the rewarding effects of cannabis.
The goals of this study were twofold. First, we examined the safety and tolerability of modafinil treatment in combination with oral Δ9-tetrahydrocannabinol (THC), as a potential treatment medication in cannabis users. Second, we examined modafinil's effects on the THC-induced physiological, subjective, and cognitive performance responses. We hypothesized that modafinil would attenuate the subjective and cognitive effects of oral THC.
2. Materials and methods
2.1. Participants
A total of 18 occasional cannabis users between the ages of 18 to 55 were recruited from the New Haven area by newspaper advertisements and flyers. The inclusion criteria included: (a) cannabis use at least once in last two months and at least 10 times in lifetime, and (b) a urine sample positive for cannabis. Participants were excluded if they met DSM-IV criteria for cannabis abuse or dependence, or were seeking treatment for substance abuse or dependence. The sample was limited to occasional cannabis users because heavy cannabis use is associated with tolerance to the acute effects of cannabis (D'Souza et al. 2008; Lichtman and Martin 2005; Ramaekers et al. 2009). Data from the first three participants were used to determine the duration and medication effects for safety, which led to a revised protocol. Data from these three participants were not used since the revisions included changes to the schedule of assessments. In addition, three participants did not complete the study due to drug use (n = 2), and personal reasons (n = 1). Of the 12 participants who completed the study (eight African-Americans, three Caucasian, and one Hispanic), 11 were male and one was female, with an average age (SD) of 33.7 years (7.7). All participants had normal physical, laboratory, and psychiatric examinations, and none were dependent on alcohol or other drugs except nicotine (n = 7). All participants provided informed consent prior to study entry and were paid for participation. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System, West Haven campus. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
2.2. Design and Procedures
In this double-blind, placebo-controlled, crossover study, subjects had four separate outpatient experimental sessions. Across four sessions, subjects were randomly assigned to a sequence of four treatment conditions which included placebo + placebo, modafinil (400mg) + placebo, THC (15 mg) + placebo, or modafinil (400 mg) + THC (15 mg). In each session, after baseline measures were obtained, subjects received the study medication followed by a light meal. The sessions started at 8:00 am and lasted for approximately 7 hours. Sessions were separated by a minimum of four days to minimize the carryover effects from modafinil and THC.
2.3. THC and modafinil administration
The oral form of THC (Marionol®) was obtained from Unimed Pharmaceuticals, Inc. (Buffalo Grove, IL). Oral THC is used in the treatment of anorexia associated with weight loss, in patients with AIDS, and for nausea and vomiting associated with chemotherapy. The typical dose associated with these treatments is in the 2.5 to 20mg/day range. We used 15 mg of THC, which has been shown to be well-tolerated by cannabis users (Chesher et al. 1990; Haney et al. 2003; Hart et al. 2005). After oral administration, THC has an onset of action of approximately 0.5 to 1 hour with peak effects at 2 to 4 hours. The duration of action for any psychoactive effects is 4 to 6 hours, with appetite stimulation continuing for up to 24 hours. Modafinil (Provigil®) was obtained from Cephalon (145 Brandywine Parkway, West Chester, PA 19380). Although a single daily 200 mg dose of modafinil is recommended for the treatment of narcolepsy or sleep apnea (Murray 2004), doses between 200 – 400 mg have been examined in combination with cocaine (Dackis et al. 2005a; Dackis et al. 2003a; Hart et al. 2008; Vosburg et al. 2010). Doses between 200 – 600 mg have been safely tolerated by cocaine users (Dackis et al. 2005b; Vosburg et al. 2010), and doses between 200 – 400 mg have been used for the treatment of methamphetamine dependence (Heinzerling et al. 2010; McGaugh et al. 2009). Previous laboratory studies examining the combination of cocaine and modafinil found that both 200 mg and 400 mg doses of modafinil were equivalent in attenuating the cardiovascular effects of cocaine (Hart et al. 2008), and both doses were not associated with any medical risk in combination with cocaine (Dackis et al. 2005a; Hart et al. 2008). The one study that examined 200 mg of modafinil for methamphetamine dependence did not find an effect for modafinil over placebo for methamphetamine use (Shearer et al. 2009b). However, at higher doses, modafinil can produce hypertension and tachycardia (Wong et al. 1999). Since this is the first study to assess modafinil in combination with THC, we wanted to maximize the possibility of having an effective dose, while minimizing side effects. Therefore, we chose the 400 mg dose of modafinil. Following oral administration, modafinil is rapidly absorbed, reaching peak plasma levels within 2–4 hours. The half-life of modafinil is 7–15 hours. Modafinil and THC were administered at the same time.
2.4. Measures
The outcome measures included physiological, subjective, and cognitive performance measures. The physiological measures consisted of systolic and diastolic blood pressure and heart rate. The subjective measures were comprised of the Drug Effects Questionnaire (DEQ), the Addiction Research Center Inventory-Short Form (ARCI), and the Profile of Mood States (POMS). The DEQ assessed the acute subjective effects of THC, and asked subjects to rate “stimulated”, “high”, “anxious”, “sedated”, “down”, “feeling the drug strength”, “feel good drug effects”, “feel bad drug effects”, “want more drug”, and “like the drug” on a scale from 0 (“not at all”) to 10 (“extremely”). The ARCI (Martin et al. 1971) 49-item version consists of true-or-false questions with five subscales: drug-induced euphoria (Morphine-Benzedrine Group; MBG), stimulant-like effects (Amphetamine; A), intellectual efficiency and energy (Benzedrine Group; BG), dysphoria (Lysergic Acid; LSD), and sedation (Pentobarbital-Chlorpromazine; PCAG) (Martin et al., 1971). We also included the ARCI `M' scale, which consists of four questions specific to marijuana (Chait et al. 1985). The POMS is a 65-item rating scale used to measure the effects of medication treatments on mood using six subscales: Tension, Depression, Anger, Vigor, Fatigue, and Confusion (McNair et al. 1971). The POMS is widely used as a research tool in behavioral pharmacology (Fischman and Foltin 1991). It has been found to be sensitive to the mood-altering effects of drugs including cannabis and modafinil.
Cognitive performance was assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R) and a module from the Cambridge Neuropsychological Test Automated Battery (CANTAB): the Rapid Visual Information Processing (RVIP). The HVLT-R is a brief verbal learning and memory test with six alternate forms, making it ideal for repeated neuropsychological examinations such as this study. The revised version also includes a delayed recall trial (Benedict et al. 1998), which is the type of recall that appears most sensitive to the effects of cannabis (Hooker and Jones 1987). For the HVLT-R we used total recall, delayed recall, and the Recognition Discrimination Index as the main outcome measures. The Total Recall is the sum of correct responses for Trial 1, 2, and 3. Delayed Recall is the number of correct responses for Trial 4 (after 20–25 min). The Recognition Discrimination Index is the total number of true positives minus the total number of false positives on delayed recognition trial (given right after trial 4 recall). The RVIP is widely used as a measure of sustained attention with a working memory component. In this task, subjects are asked to respond to any of three digit sequences in a continuous stream of digits lasting for seven minutes. A white box appears in the center of the computer screen, inside which digits from 2 to 9 appear in a pseudo-random order at the rate of 100 digits per minute. Subjects are instructed to detect consecutive odd or even sequences of digits (e.g., 2-4-6, 3-5-7, 4-6-8, 5-7-9, etc.) and to register responses using a press-pad. Impairments in attention have long been recognized in cannabis users (Harvey et al. 2007; Ranganathan and D'Souza 2006). For the RVIP key measures were mean latency, A' (target sensitivity, a measure of the ability to detect sequences), and B” (response bias, a measure of the tendency to respond regardless of whether a target is present). Subjective assessments were completed at baseline, then at 30, 60, 90, 150, 180, 210, 240, 270, and 300 minutes after the study medications administration. Heart rate and blood pressure measurements were obtained at the same time points, as well as at 120 and 360 minutes after medication administration. Cognitive assessments were given to participants two hours after medication administration.
2.5. Statistical Analysis
We conducted two types of analyses using mixed-effects repeated-measures analyses using SAS Proc Mixed, version 9.2. All models included fixed main-effect estimates for THC (0 mg or 15 mg), modafinil (0 mg or 400 mg) and their interaction, as well as a blocking factor for treatment sequence. For the first analyses, all data collected at each time point were included in the model. For the second analyses, change-from-baseline scores were used as outcome measures. Change scores, maximum post-dose measurement minus the pre-dose measurement at baseline, are commonly used summary measures that capture the magnitude of the response. Change scores were calculated for the physiological and subjective scales, where multiple measurements were obtained at different time points. Significant treatment or treatment-by-treatment interactions (p <0.05) were followed by post hoc group comparisons using Fisher's LSD.
3. Results
3.1. Physiological Responses
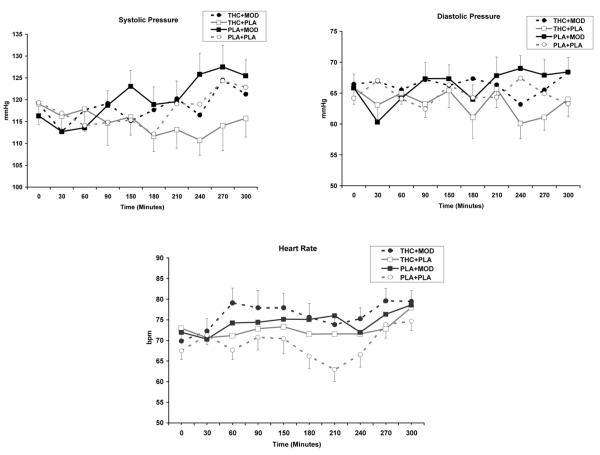
As shown in Figure 1, THC increased heart rate (THC main effect; [F (1, 461) = 18.6; p<0.0001]) and lowered systolic blood pressure (THC main effect; [F (1, 461) = 4.6; p<0.05]), compared to placebo. Modafinil, compared to placebo, increased heart rate (Modafinil main effect; [F (1, 461) = 48.8; p<0.0001]), increased systolic blood pressure (modafinil main effect; [F (1, 461) = 6.5; p<0.05]) and increased diastolic blood pressure, (modafinil main effect; [F (1, 461) = 12.2; p<0.001]). For change scores in heart rate, modafinil plus THC, had greater increases in heart rate than THC alone [THC by modafinil effect; F (1, 30) = 4.2; p<0.05].
Fig. 1.
Heart rate (a), systolic (b), and diastolic blood pressure (c) responses under 15 mg active THC plus 400 mg active modafinil (THC+MOD), 15 mg active THC plus placebo (THC+PLA), placebo plus 400 mg active modafinil (PLA+MOD), or placebo plus placebo (PLA+PLA) conditions. Heart rate was significantly greater for active THC and modafinil conditions compared to placebo. Systolic blood pressure was significantly lower for active THC and modafinil conditions compared to placebo. Diastolic blood pressure was a significantly greater for active modafinil conditions.
3.2. Subjective Responses
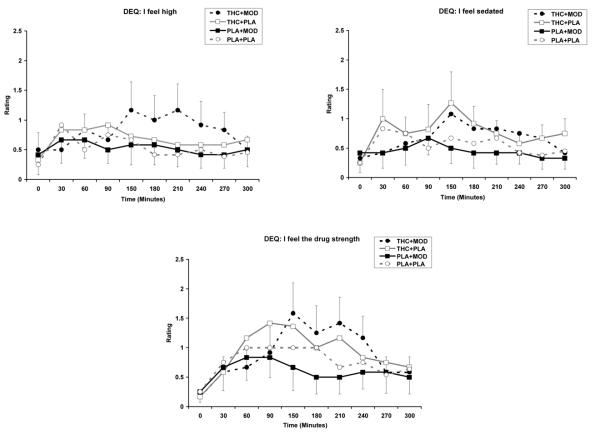
THC increased subjective ratings of “feel high” (THC main effect; [F (1, 460) = 6.7 p<0.05]), “feel sedated” (THC main effect; [F (1, 460) = 5.5; p<0.05]), and “feel the drug strength” (THC main effect; [F (1, 460) = 5.9; p<0.05]) scales of the DEQ, compared to placebo (see Figure 2). There were no significant modafinil-by-THC interactions for any of the DEQ scales. For the change score analyses, modafinil treatment reduced the rating of “feel sedated” (modafinil main effect; [F (1, 30) = 4.3; p<0.05]) and THC treatment increased the rating of “feel the bad effects” (THC main effect; [F (1, 30) = 4.3; p<0.05].
Fig. 2.
Subjective responses to selected items on DEQ under 15 mg active THC plus 400 mg active modafinil (THC+MOD), 15 mg active THC plus placebo (THC+PLA), placebo plus 400 mg active modafinil (PLA+MOD), or placebo plus placebo (PLA+PLA) conditions. There were significantly greater ratings of “feel high,” “feel sedated,” and “feel the drug strength” for the two active THC conditions.
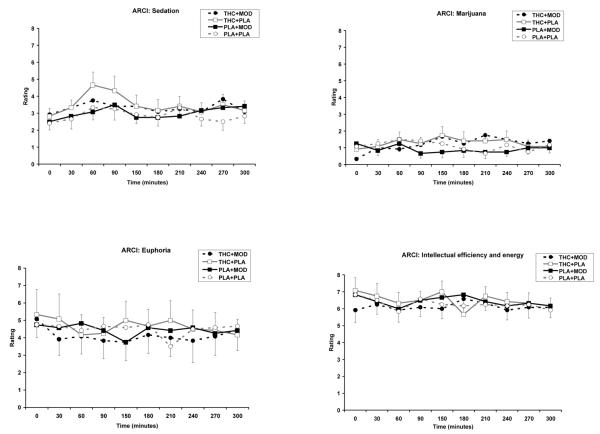
For ratings on the ARCI, THC increased ratings on the PCAG (sedation) subscale (THC main effect; [F (1, 461) = 14.0; p<0.001]), the LSD (dysphoria) subscale (THC main effect; [F (1, 461) = 7.2; p<0.001]), and the M (marijuana) subscale (THC main effect; [F (1, 461) = 5.4; p<0.001]), compared to placebo (see Figure 3). The THC plus modafinil condition had significantly lower ratings on the MBG (drug-induced euphoria) subscale (THC by modafinil effect; [F (1, 461) = 5.3; p<0.05]), compared to other conditions. In addition, the THC plus modafinil condition had significantly lower ratings on the BG (intellectual efficiency and energy) subscale (THC by modafinil effect; [F (1, 461) = 6.1; p<0.05]), compared to modafinil placebo or THC-placebo conditions (see Figure 3). For change score analysis of the ARCI, THC increased the marijuana M-scale [F (1, 30) = 6.2; p<0.05]. THC plus modafinil, compared to modafinil alone had greater score in the marijuana scale (THC by modafinil effect; [F (1, 30) = 4.4; p<0.05]).
Fig. 3.
Subjective responses to selected items on ARCI under 15 mg active THC plus 400 mg active modafinil (THC+MOD), 15 mg active THC plus placebo (THC+PLA), placebo plus 400 mg active modafinil (PLA+MOD), or placebo plus placebo (PLA+PLA) conditions. There were significantly greater ratings of sedation, dysphoria, and marijuana for active THC conditions. Compared to other conditions, the THC+MOD condition had significantly lower ratings of drug-induced euphoria. Compared to THC+PLA and PLA+MOD conditions, the THC+MOD condition has significantly lower ratings on the intellectual efficiency and energy subscale.
THC reduced scores for the depressed (THC main effect; [F (1, 461) = 4.1; p<0.05]) and vigor (THC main effect; [F (1, 461) = 30.7; p<0.001]), subscales of the POMS, compared to placebo. Modafinil increased ratings of tension (modafinil main effect; [F (1, 451) = 10.8; p<0.005]) and vigor (modafinil main effect; [F (1, 461) = 4.1; p<0.05]), and decreased depressed ratings (main effect modafinil; [F (1, 461) = 7.2; p<0.01]), on the POMS. There were no significant modafinil-by-THC interactions for any of the POMS scales. For change score analyses, no significant treatment differences were found.
3.3.Cognitive Performance Assessments
There were no significant treatment effects for the RVIP, and the HVLT. Figure 4 graphically represents the means for the three subscales of the RVIP and the three subscales of the HVLT, by condition.
Fig. 4.
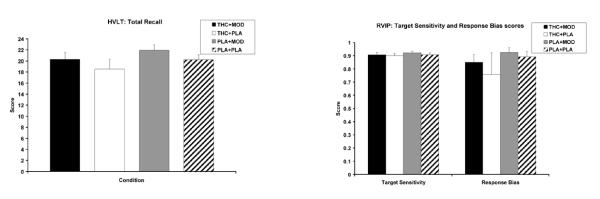
Total recall on the HVLT (a) and target sensitivity and response bias on the RVIP (b) under 15 mg active THC plus 400 mg active modafinil (THC+MOD), 15 mg active THC plus placebo (THC+PLA), placebo plus 400 mg active modafinil (PLA+MOD), or placebo plus placebo (PLA+PLA) conditions.
4. Discussion
In this study 15 mg oral THC treatment increased subjective ratings on the ARCI subscales of PCAG (sedation), LSD (dysphoria), and M (marijuana). These findings are consistent with previous research that has showed the oral THC increased ratings on the ARCI LSD, PCAG, and M subscales (Kirk and de Wit 1999; Makela et al. 2006; McDonald et al. 2003; Wachtel et al. 2002). Results also showed that THC increased DEQ item ratings of “feel high,” “feel sedated,” and “feel the drug strength,” and THC reduced ratings of vigor and depression on the POMS subscales. These findings are consistent with many previous studies that administered oral THC (Chesher et al. 1990; McDonald et al. 2003; Wachtel et al. 2002).
In contrast to oral THC, modafinil alone did not affect the ratings on the ARCI or DEQ scales. However, modafinil had significant effects on mood, assessed by the POMS. Participants had greater ratings for tension and vigor, and lower responses for depressed. In previous studies, modafinil treatment has been shown to decrease fatigue and enhance vigor especially in sleep-deprived individuals, while increased anxiety and aggression has also been reported as a result of modafinil treatment (MacDonald et al. 2002; Rush et al. 2002; Wesensten 2006). In a more recent study with healthy adults, modafinil increased both the negative and positive scales of the PANAS (Taneja et al. 2007). It has been suggested that the arousing effect of modafinil may be interpreted as negative by some individuals.
We also observed a significant modafinil-by-THC interaction, with lower attenuated ratings on the euphoria subscale of the ARCI, compared to other treatment conditions. Given that the single dose of THC in this study had very little effect, this minor interaction cannot be meaningfully interpreted. Moreover, these effects were not significant when the change scores, rather than the all the time points, were analyzed.
As expected, THC increased heart rate. The cardiovascular effects of THC are mediated by sympathetic activation and cholinergic inhibition (Jones 2002). In our study, 400 mg modafinil increased participants' heart rate and systolic and diastolic blood pressure. These findings are consistent with previous studies where modafinil increased the resting heart rate and blood pressure in healthy controls (Taneja et al. 2005) and abstinent smokers (Sofuoglu et al. 2008). The increased blood pressure and heart rate induced by modafinil have been attributed to noradrenergic activation (Taneja et al. 2005). In our study, modafinil did not enhance the heart rate and blood pressure induced by oral THC. These findings support the safety of modafinil in combination with THC.
In this study, the cognitive effects of oral THC or modafinil were not significant. There was a trend for a THC-induced impairment on the HVLT. This lack of treatment effect on cognitive outcomes could be due to several limitations of this study. First, we only used one dose (400 mg) of modafinil in this study. Since this is the first study to assess modafinil in combination with THC, we based our dosage on other studies in the literature that have examined modafinil in combination with illicit drug use (i.e. cocaine and methamphetamine). Future research should examine the combination of THC and modafinil in cannabis users using multiple doses in order to more fully understand modafinil's effects. Second, the treatment duration was brief, and only one relatively small dose of THC was administered. It is possible that higher doses of THC would show effects on cognitive performance. Third, the cognitive assessment in this study involved only two tasks, did not include a comprehensive assessment, and was limited by small sample sizes. Fourth, the exclusion of heavy cannabis users limits the ability to generalize these results to future examinations of modafinil for the treatment of cannabis dependence. Finally, although our sample was limited to occasional users, we did not collect data on specific cannabis use patterns of participants; therefore we cannot rule out the possibility that the effects of THC may differ by patterns of use.
In summary, these results show that modafinil can be safely combined with oral THC. Further studies with larger sample sizes and a range of cannabis doses are warranted to investigate the therapeutic efficacy of modafinil. It is also recommended that future studies examine the effects of modafinil with regular marijuana smokers, and include a more comprehensive cognitive assessment battery in order to detect possible effects of modafinil and THC on cognitive performance.
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and the National Institute on Drug Abuse (NIDA) grant K02-DA021304 (MS). We would like to thank Ellen Mitchell, R.N. and Stacy Minnix for technical assistance.
References
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Benedict R, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–8. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. 'Hangover' effects the morning after marijuana smoking. Drug and Alcohol Dependence. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-x. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesher GB, Bird KD, Jackson DM, Perrignon A. The effects of orally administered Δ9-tetrahydrocannabinol in man on mood and performance measures: A dose-response study. Pharmacology, Biochemistry and Behavior. 1990;35:861–864. doi: 10.1016/0091-3057(90)90371-n. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–16. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005a;30:205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A Double-Blind, Placebo-Controlled Trial of Modafinil for Cocaine Dependence. Neuropsychopharmacology. 2005b;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: A double-blind, placebo-controlled drug interaction study. Drug and Alcohol Dependence. 2003a;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003b;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–80. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–8. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O'Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–56. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–70. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology. 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Δ9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked Cocaine Self-Administration is Decreased by Modafinil. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–19. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson A-N, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology (Berl) 1987;91:20–4. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral Δ9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacology, Biochemistry and Behavior. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- MacDonald JR, Hill JD, Tarnopolsky MA. Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy. Neurology. 2002;59:1876–80. doi: 10.1212/01.wnl.0000037481.08283.51. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of Δ9-tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–87. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- Martin W, Sloan J, Sapira J, Jasinski D. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical Pharmacological Therapy. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsitivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra ME, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. Journal of Clinical Psychopharmacology. 2009;29:488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Manual for Profile of Mood States. Educational and Industrial Testing Services, Educational and Industrial Testing Services. 1971 [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2020;167:331–40. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology (Berl) 2004;177:161–9. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Haro R, Palomero-Rivero M, Millan-Aldaco D, Drucker-Colin R. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res. 2007;176:353–7. doi: 10.1016/j.bbr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Murray L. Physician's Desk Reference. Thomson PDR; 2004. Thomson PDR. [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16:331–42. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- ONDCP. OoNDCP . Marijuana: The Greatest Cause of Illegal Drug Abuse The Marijuana Factbook. Executive Office of the President; Washington, DC: 2008. 20503. [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–6. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr., Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: the current status of research. Drug & Alcohol Dependence. 1995;38:25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Jama. 1996;275:521–7. [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–77. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–44. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Robledo P, Trigo JM, Panayi F, de la Torre R, Maldonado R. Behavioural and neurochemical effects of combined MDMA and THC administration in mice. Psychopharmacology (Berl) 2007;195:255–64. doi: 10.1007/s00213-007-0879-8. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–22. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- SAMHSA Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: national findings. 2008 [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009a;104:224–33. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009b;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, Carroll KM. Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol. 2010;18:109–19. doi: 10.1037/a0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M. Modafinil and nicotine interactions in abstinent smokers. Hum Psychopharmacol. 2008;23:21–30. doi: 10.1002/hup.878. [DOI] [PubMed] [Google Scholar]
- Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sciences. 1995;56:2119–26. doi: 10.1016/0024-3205(95)00197-e. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biological Psychiatry. 1995;37:731–9. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J, Marijuana Treatment Project Research G Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–31. doi: 10.1001/jama.287.9.1123. [comment][erratum appears in JAMA2002 Apr 3;287(13):1651] [DOI] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M, MTP Research Group The Marijuana Treatment Project: Rationale, design, and participant characteristics. Addiction. 2002;97:109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Taneja I, Diedrich A, Black BK, Byrne DW, Paranjape SY, Robertson D. Modafinil elicits sympathomedullary activation. Hypertension. 2005;45:612–8. doi: 10.1161/01.HYP.0000158267.66763.63. [DOI] [PubMed] [Google Scholar]
- Taneja I, Haman K, Shelton RC, Robertson D. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol. 2007;27:76–9. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug and Alcohol Dependence. 2010;106:233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Δ9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–88. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12:2457–71. doi: 10.2174/138161206777698819. [DOI] [PubMed] [Google Scholar]
- Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl) 2002;159:238–47. doi: 10.1007/s002130100916. [DOI] [PubMed] [Google Scholar]
- Wong Y, Simcoe D, Hartman L, Laughton W, King S, McCormick G, PE G. A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. J Clin Pharmacol. 1999;39:30–40. doi: 10.1177/00912709922007534. [DOI] [PubMed] [Google Scholar]