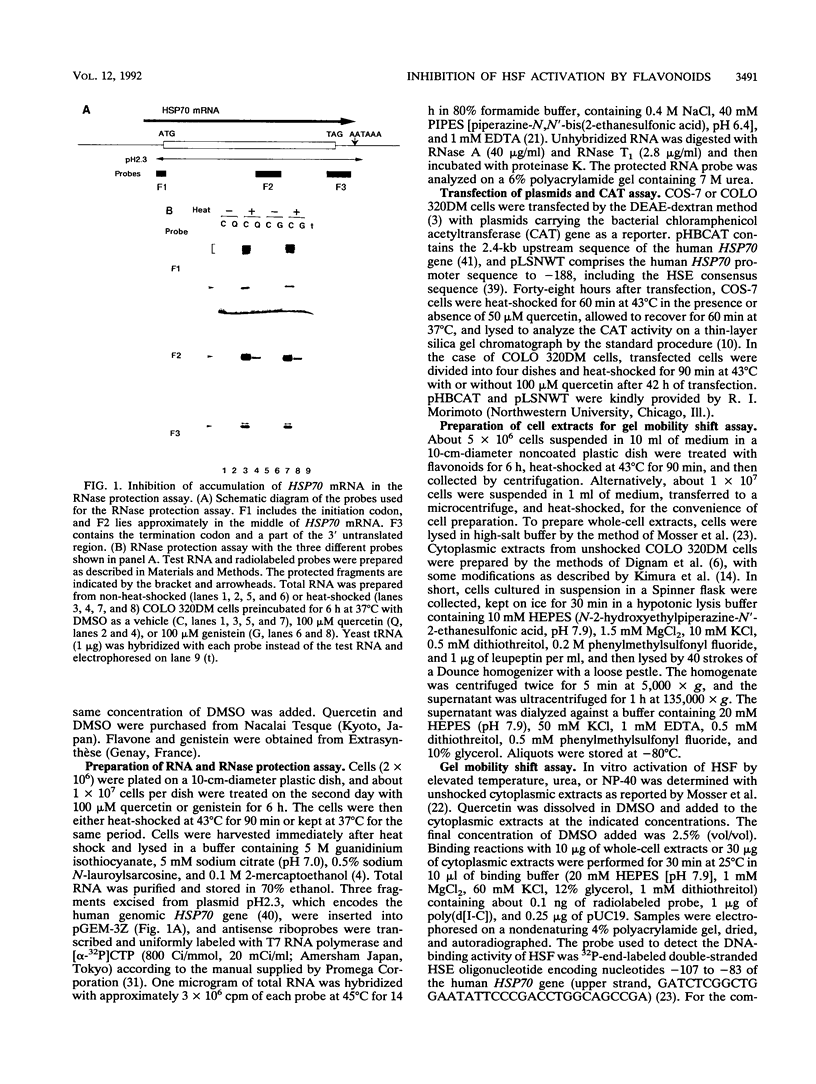
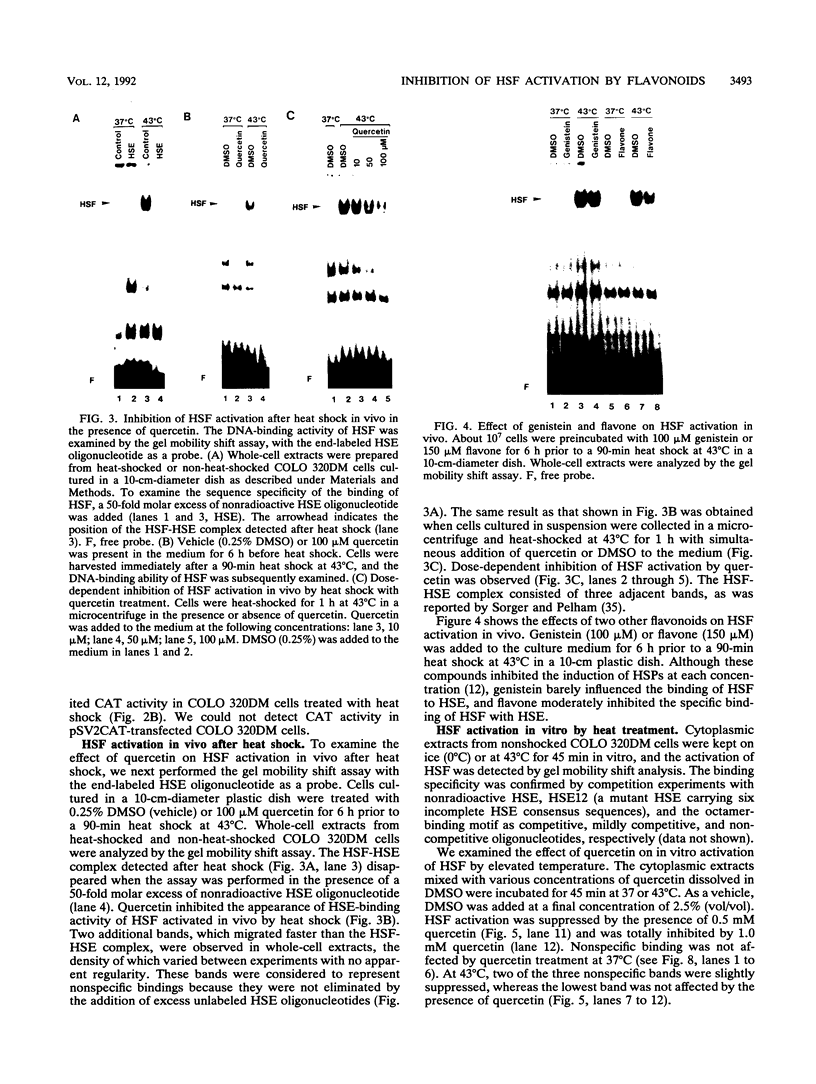
Abstract
Transcriptional activation of human heat shock protein (HSP) genes by heat shock or other stresses is regulated by the activation of a heat shock factor (HSF). Activated HSF posttranslationally acquires DNA-binding ability. We previously reported that quercetin and some other flavonoids inhibited the induction of HSPs in HeLa and COLO 320DM cells, derived from a human colon cancer, at the level of mRNA accumulation. In this study, we examined the effects of quercetin on the induction of HSP70 promoter-regulated chloramphenicol acetyltransferase (CAT) activity and on the binding of HSF to the heat shock element (HSE) by a gel mobility shift assay with extracts of COLO 320DM cells. Quercetin inhibited heat-induced CAT activity in COS-7 and COLO 320DM cells which were transfected with plasmids bearing the CAT gene under the control of the promoter region of the human HSP70 gene. Treatment with quercetin inhibited the binding of HSF to the HSE in whole-cell extracts activated in vivo by heat shock and in cytoplasmic extracts activated in vitro by elevated temperature or by urea. The binding of HSF activated in vitro by Nonidet P-40 was not suppressed by the addition of quercetin. The formation of the HSF-HSE complex was not inhibited when quercetin was added only during the binding reaction of HSF to the HSE after in vitro heat activation. Quercetin thus interacts with HSF and inhibits the induction of HSPs after heat shock through inhibition of HSF activation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Amin J., Ananthan J., Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Clos J., Westwood J. T., Becker P. B., Wilson S., Lambert K., Wu C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell. 1990 Nov 30;63(5):1085–1097. doi: 10.1016/0092-8674(90)90511-c. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabent B., Genthe A., Benecke B. J. In vitro transcription of a human hsp 70 heat shock gene by extracts prepared from heat-shocked and non-heat-shocked human cells. Nucleic Acids Res. 1986 Nov 25;14(22):8933–8948. doi: 10.1093/nar/14.22.8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- End D. W., Look R. A., Shaffer N. L., Balles E. A., Persico F. J. Non-selective inhibition of mammalian protein kinases by flavinoids in vitro. Res Commun Chem Pathol Pharmacol. 1987 Apr;56(1):75–86. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani Y., Chayoth R. Regulation of cyclic AMP level and synthesis of DNA, RNA and protein by quercetin in Ehrlich ascites tumor cells. Biochem Pharmacol. 1979;28(3):397–403. doi: 10.1016/0006-2952(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hirayoshi K., Nakai A., Hosokawa Y., Marui N., Yoshida M., Sakai T., Nishino H., Aoike A., Kawai K. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct. 1990 Dec;15(6):393–401. doi: 10.1247/csf.15.393. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Taniguchi T., Yahara I. An alteration in molecular form associated with activation of human heat shock factor. Cell Struct Funct. 1991 Jun;16(3):263–271. doi: 10.1247/csf.16.263. [DOI] [PubMed] [Google Scholar]
- Kuriki Y., Racker E. Inhibition of (Na+, K+)adenosine triphosphatase and its partial reactions by quercetin. Biochemistry. 1976 Nov 16;15(23):4951–4956. doi: 10.1021/bi00668a001. [DOI] [PubMed] [Google Scholar]
- Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Alejandro M. A., Johnson G. A., Middleditch B. S., Clark J. H. Bioflavonoid interaction with rat uterine type II binding sites and cell growth inhibition. J Steroid Biochem. 1988;30(1-6):71–78. doi: 10.1016/0022-4731(88)90078-7. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Kotzbauer P. T., Sarge K. D., Morimoto R. I. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990 May;87(10):3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose K. Inhibition by flavonoids of RNA synthesis in permeable WI-38 cells and of transcription by RNA polymerase II. Biochem Pharmacol. 1984 Dec 1;33(23):3823–3827. doi: 10.1016/0006-2952(84)90046-7. [DOI] [PubMed] [Google Scholar]
- Ondek B., Gloss L., Herr W. The SV40 enhancer contains two distinct levels of organization. Nature. 1988 May 5;333(6168):40–45. doi: 10.1038/333040a0. [DOI] [PubMed] [Google Scholar]
- Ono K., Nakane H., Fukushima M., Chermann J. C., Barré-Sinoussi F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur J Biochem. 1990 Jul 5;190(3):469–476. doi: 10.1111/j.1432-1033.1990.tb15597.x. [DOI] [PubMed] [Google Scholar]
- Osada H., Magae J., Watanabe C., Isono K. Rapid screening method for inhibitors of protein kinase C. J Antibiot (Tokyo) 1988 Jul;41(7):925–931. doi: 10.7164/antibiotics.41.925. [DOI] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Price B. D., Calderwood S. K. Ca2+ is essential for multistep activation of the heat shock factor in permeabilized cells. Mol Cell Biol. 1991 Jun;11(6):3365–3368. doi: 10.1128/mcb.11.6.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987 Oct;6(10):3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamano Y., Kioka N., Kakehi Y., Yoshida O., Gottesman M. M., Pastan I., Komano T. Detection of multidrug resistance (MDR1) gene RNA expression in human tumors by a sensitive ribonuclease protection assay. Jpn J Cancer Res. 1989 Nov;80(11):1127–1132. doi: 10.1111/j.1349-7006.1989.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G., Seto D., Parker C. S. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988 Sep 9;54(6):841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- Williams G. T., McClanahan T. K., Morimoto R. I. E1a transactivation of the human HSP70 promoter is mediated through the basal transcriptional complex. Mol Cell Biol. 1989 Jun;9(6):2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]