Abstract
Insulin resistance is a characteristic feature of obesity and Type 2 diabetes and impacts the heart in various ways. Impaired insulin-mediated glucose uptake is a uniformly observed characteristic of the heart in these states, although changes in upstream kinase signaling are variable and dependent on the severity and duration of the associated obesity or diabetes. The understanding of the physiological and pathophysiological role of insulin resistance in the heart is evolving. To maintain its high energy demands, the heart is capable of utilizing many metabolic substrates. Although, insulin signaling may directly regulate cardiac metabolism, its main role is likely the regulation of substrate delivery from the periphery to the heart. In addition to promoting glucose uptake, insulin regulates long chain fatty acid uptake, protein synthesis, and vascular function in the normal cardiovascular system. Recent advances in understanding the role of metabolic, signaling, and inflammatory pathways in obesity have provided opportunities to better understand the pathophysiology of insulin resistance in the heart. This review will summarize our current understanding of metabolic mechanisms for and consequences of insulin resistance in the heart and discuss potential new areas for investigating novel mechanisms that contribute to insulin resistance in the heart.
Introduction
Under physiological circumstances, insulin regulates substrate utilization in multiple tissues including the heart, skeletal muscle, liver, and adipose tissue. In the heart, insulin stimulates glucose uptake and oxidation and although it increases FA uptake, it inhibits fatty acid utilization for energy. Generalized insulin resistance occurs primarily as a result of obesity, a consequence of caloric excess, physical inactivity, genetics, and age. Insulin resistance is associated with many serious medical conditions, such as type 2 diabetes, hypertension, atherosclerosis, and metabolic syndrome1, 2. In diabetes and insulin resistant states, metabolic, structural and functional changes in the heart and vasculature lead to diabetic cardiomyopathy, coronary artery disease and myocardial ischemia, and ultimately heart failure3, 4. There are many molecular mechanisms that contribute to the association between insulin resistance and increased cardiovascular disease. These include the impact of insulin resistance to induce impaired vascular function, which leads to impaired nitric oxide mediated vasorelaxation, which may contribute to hypertension and to increased risk of atherosclerosis5-8. Moreover, genetic manipulation of insulin action in the vasculature will increase atherosclerosis9-12. Insulin resistance via multiple mechanisms may contribute to macrophage accumulation in the vessel wall to increase atherosclerosis and instability of vulnerable plaques13. Finally, insulin resistance has been shown in many human and animal studies to increase the extent of myocardial injury in the context of myocardial ischemia, which may contribute to the increased risk of heart failure in affected individuals14. The interactions between insulin resistance and vascular disease will be the subject of other reviews in this series. The present review will focus on the mechanisms by which insulin resistance develops and contributes to structural heart disease. Although incompletely understood, these mechanisms involve the combination of changes insulin signal transduction pathways in the heart acting in concert with changes in mitochondrial function and metabolism glucose and free fatty acids 14.
Insulin Signaling in the Heart and the Molecular Changes in Insulin Resistance
Insulin release from pancreatic β-cells, induces glucose uptake into cardiomyocytes, skeletal muscle and adipose tissue upon binding by insulin to the cell surface insulin receptor (IR). The IR undergoes autophosphorylation after insulin binding, which initiates a signaling cascade initiated by tyrosine phosphorylation of insulin receptor substrates (IRS), followed by phosphorylation of phosphatidyl-inositol-3 kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1), Akt, and protein kinase C (PKC). These events result in glucose transporter type 1 and type 4 (GLUT1 and GLUT4) translocation to the membrane to facilitate glucose uptake into the cell3, 15. Although insulin mediated translocation of GLUT4 translocation is a major regulator of glucose utilization in glycolytic and oxidative skeletal muscle, in the heart it is likely that contractile mediated translocation of GLUT4 represents the major mechanism that regulates glucose entry in the beating heart, with GLUT1 playing a lesser role16. Thus insulin stimulation in isolated working hearts or in vivo increases myocardial glucose utilization by 40-60%17, 18, in contrast with a 3-8fold increase in insulin-treated skeletal muscle in vivo or in vitro19, 20. In addition to glucose uptake, insulin-mediated activation of PI3K and Akt regulates many other cellular processes such as cellular hypertrophy, protein translation, nitric oxide generation, apoptosis and autophagy by activating other intracellular signaling intermediates such as mTOR, S6K, forkhead transcription factors e.g. FOXO1/3, GSK3β and NOSIII21. Changes in many of these signaling pathways as develops in insulin resistant states could contribute to increasing the risk for cardiac hypertrophy, adverse left ventricular remodeling or heart failure.
In discussing the concept of myocardial insulin resistance it is important to distinguish between effects that are secondary to the disturbed systemic milieu in insulin resistant states (hyperinsulinemia, hyperglycemia, hyperlipidemia), and changes that occur in insulin signaling pathways that are intrinsic to the cardiac tissue. The earliest and most consistent change that develops in the hearts in animal models, in the evolution of insulin resistance is impairment in the ability of insulin to increase glucose transport18. This early change occurs prior to any defect in the ability of insulin to increase PI3K and Akt signaling and occurs as a consequence of both reduced GLUT4 protein and impaired GLUT4 translocation. Similar changes have been reported in ventricular muscle biopsies obtained from subjects with type 2 diabetes22. Indeed in this human study diabetes was associated increased signaling to Akt and PI3K despite reduced GLUT4 translocation to the plasma membrane. A recent study in mice also revealed that the generalized insulin resistance and hyperinsulinemia that develops in the context of pressure overload cardiac hypertrophy drives excessive myocardial insulin signaling to Akt that contributes to accelerated LV remodeling and the transition to heart failure23. In support of this concept, decreased myocardial insulin signaling may represent a mechanism for the potential benefit of high-fat diets in ameliorating heart failure in rodent models of pressure overload or post-myocardial infarction left ventricular remodeling24. Thus in considering the impact of insulin resistance on the heart it is important to distinguish between effects that are secondary to hyperactivation of signaling pathways that may remain responsive to insulin, versus changes that are the consequence of an impaired ability of insulin to modulate glucose metabolism. In animal models with longer term exposure to high-fat diets or in genetic models of severe insulin resistance such as ob/ob and db/db mice, clear evidence exists for an impaired ability of insulin to activate intracellular signaling kinases such as Akt or FOXO1, which might also contribute to LV dysfunction25-27. Indeed genetic inactivation of insulin signaling in the heart as been shown to contribute to LV dysfunction by increasing mitochondrial dysfunction, decreasing angiogenesis and increasing fibrosis particularly in response to hemodynamic stressors17, 28-31. Thus given the broad spectrum of abnormalities that may characterize “cardiac insulin resistance” it is critical to dissect and distinguish between those mechanisms that are a consequence of increased or decreased signal transduction to intracellular kinases, changes that are secondary to intrinsic regulation of substrate metabolism or changes that are secondary to altered delivery of substrates to the heart.
Because of its high and tightly regulated energy demands changes in systemic insulin sensitivity or changes in myocardial insulin action can significantly impact cardiac metabolism and function. The constant demand for mechanical power in the heart is met by high rates of ATP production from fat and carbohydrate oxidation32, 33. The myocardium rapidly adjusts to fluctuations in circulating substrate concentrations34, 35, giving the heart the metabolic flexibility needed for feeding, fasting, and intense exercise. Insulin resistance impairs the ability of the heart to adjust to changing energy demands by increasing the delivery of fatty acids to the heart and by reducing the ability of the heart to use glucose, thereby shifting the heart towards a greater reliance on fatty acids for energy36, 37. As a result, the diabetic heart undergoes cellular stress, including elevated reactive oxygen species (ROS) production, mitochondrial dysfunction, and apoptosis. These changes in myocardial metabolism that occur as a result of insulin resistance may contribute to downstream structural and functional alterations in the heart that can lead to cardiomyopathy and heart failure3. While there are many aspects of insulin resistance that impact the heart, this review will focus on the mechanisms of insulin resistance in the heart related to glucose and fatty acid metabolism.
Glucose and Fatty Acid Metabolism in the Heart
Upon insulin-mediated uptake of glucose into the cell, glucose is converted to glucose-6-phosphate by hexokinase in heart, skeletal muscle, and adipose tissue. Glucose-6-phosphate has several fates in the cell, but the two primary fates are glycolysis for energy production and glycogen for storage, both of which are augmented by insulin signaling. Under ambient physiological conditions, a small percentage of glucose is shunted to the hexosamine biosynthesis pathway, pentose phosphate pathway, or the polyol pathway and glycolysis and subsequent glucose oxidation accounts for approximately 20% of total myocardial energy generation38. Short-term hyperglycemia can increase total myocardial glucose utilization to 60-70% of total energy generation38. However, in the context of diabetes, these changes are not long lasting because of downregulation of glucose transport and increased delivery of fatty acids to the heart39.
Circulating FFAs contribute to the development of insulin resistance via a number of mechanisms. Circulating concentrations of plasma FFAs are determined to a large extent by the release by lipolysis of adipocyte triglyceride stores by adipose triglyceride lipase (ATGL) and hormone-sensitive lipase40. (HSL)-stimulated release of FFAs from triglyceride stores in adipose tissue, is tightly controlled by hormones that are regulated by the metabolic status33. During conditions such as fasting, when blood glucose is low or when energy demands are increased, glucagon, glucocorticoids and catecholamines lead to activation of HSL to promote hydrolysis of triglycerides to FFAs. By contrast, in the fed state insulin inactivates HSL and inhibits lipolysis33. In vivo, the majority of FFAs that are delivered to tissues arise from hydrolysis of a triglycerides, which are transported in plasma in chylomicrons or very-lowdensity lipoproteins (VLDL) particles and the remainder exist in the non-esterified form bound to albumin. Plasma FFAs can increase in healthy individuals due to adrenergic stimulation brought on by exercise, stress, fasting, ischemia, or diabetes. The release of FFAs from chylomicrons or VLDL by lipoprotein lipases in these situations also increases plasma FFAs33.
After FAs are taken up by target tissues, they have three major fates in the cell. They can be esterified into triglycerides, diglycerides, or phospholipids; converted to sphingolipids; or oxidized for energy41. FFAs are transported across the sarcolemma and into the cardiomyocyte by either passive diffusion or transport proteins (fatty acid translocase or fatty acid binding proteins)33. Since the majority of FFAs that enter the heart are used for energy (70-90%)42, they must enter the mitochondrial matrix for β-oxidation. FFAs are transported across the outer and inner mitochondrial membrane by carnitine palmitoyl transferase 1 (CPT1), which is the rate-limiting step of fatty acid oxidation, and CPT2. The acetyl-CoA resulting from β-oxidation enters the tricarboxylic acid cycle, yielding NADH and FADH2, which enter the electron transport chain to produce ATP33. Persistent exposure of tissues to increased concentrations of fatty acids, and associated changes in the metabolic fate of fatty acids are an important cause of insulin resistance.
Obesity, Lipotoxicity, and Insulin Resistance
Obesity is the leading cause of insulin resistance, and obese individuals tend to have higher plasma FFAs as a result of decreased suppression of lipolysis by insulin resistance. It is also believed that an impaired ability of adipocytes to store excess calories as triglycerides also contributes to increased accumulation of lipids and their metabolites in other tissues that are not necessarily adapted to lipid storage such as muscle and liver. As a consequence, the accumulation of lipid metabolic intermediates incites a variety of cellular abnormalities such as apoptosis, oxidative stress, and ER stress, which impairs cellular function.
FFAs are the main substrate for ATP production in the heart under normal conditions. FFAs undergo β-oxidation to yield 60-70% of the energy needed to maintain cardiac work37. The level of circulating FFAs largely determines FFA uptake in the heart33, 42, 43. In situations where FFAs are elevated, myocardial lipid accumulation can occur, which is detrimental to left ventricular function. Myocardial lipid accumulation occurs as a result of a mismatch between FA uptake and oxidative metabolism, which increases the partitioning of lipids into other metabolic pathways that may contribute to impaired insulin action in the heart such as reduced insulin-stimulated glucose transport and impaired insulin signaling1. Increased availability and utilization of FFAs lead to the accretion of triglycerides and lipid metabolites, such as long chain acyl-CoAs and diacylglycerol (DAG) in the heart and other tissues, including liver and skeletal muscle44. Although triglyceride accumulation is often interpreted as a cause of lipotoxicity, it is likely that the triglycerides per se might not represent the toxic lipid moiety but may represent a mechanism by which the tissue is attempting to sequester the excess lipids into a relatively inert pool45. However lipid metabolites, such as DAG stimulates protein kinase C Φ (PKC)1, 46, a serine/threonine kinase that may inhibit insulin signaling by increasing the serine phosphorylation of IRS proteins47, 48. In mice fed a high fat diet for 10 weeks, cardiac insulin resistance as evidenced by a decrease in glucose oxidation was associated with an increase in DAG, but not triacylglycerol, ceramide, or long chain acyl-CoA49, suggesting that DAG accumulation may play a role in high fat diet-induced insulin resistance in the heart.
Lipid-induced cardiac dysfunction (cardiac lipotoxicity) may contribute to apoptosis50, impaired mitochondrial function51, and ultimately to cardiac dysfunction. The heart has a limited capacity to store triglycerides, thereby increasing its susceptibility to the consequence of accumulation of toxic lipid species52. An underappreciated mechanism that may contribute to lipotoxicity in the insulin resistant state is hyperinsulinemia itself. As discussed earlier, the ability of insulin to activate Akt, may be relatively preserved in the heart. Akt activation promotes the translocation of CD36 go the plasma membrane, which would increase the uptake of fatty acids53. However the concurrent inhibition of mitochondrial FA oxidation increases the flux of FAs into lipid storage pathways, thereby contributing to lipotoxicity. Transcription factors related to lipid metabolism have also been implicated in the pathogenesis of liptoxicity. Peroxisome proliferator activated receptors (PPARs), which are members of the nuclear receptor superfamily of transcription factors, are key regulators of fatty acid metabolism54. There are three major PPAR isoforms: PPARα, PPARβ/δ, and PPARγ, which have distinct but overlapping functions in regulating fatty acid metabolism and are differentially expressed in various tissues. Transgenic mice overexpressing cardiac-specific PPARγ display augmented expression of fatty acid oxidation genes, dilated cardiomyopathy, and enhanced lipid deposition in the heart55. Cardiac-specific overexpression of PPARα in mice leads to enhanced β-oxidation of fatty acids and reduced glucose oxidation and accumulation of triglycerides 56. Myocardial insulin resistance may also contribute to contractile dysfunction in these hearts57. Conversely, PPARα-null mice have reduced rates of β-oxidation of fatty acids, elevated rates of glucose oxidation, cardiac fibrosis, and they cannot maintain cardiac output during conditions of increased workload58, 59.
Another transcription factor involved in lipotoxicity is sterol regulatory element binding-protein (SREBP)-1c, which regulates hepatic lipogenesis and converts glucose to fatty acids and triglycerides during conditions of over-nutrition60, 61. SREBP1c is activated by insulin during insulin resistance62. Furthermore, there is a correlation between reduced ejection fraction and lipid accumulation within cardiomyocytes of patients with the metabolic syndrome and increased levels of SREBP-1c and PPARγ in the heart63. This suggests that SREBP-1c may promote lipid deposition in cardiomyocytes in the metabolic syndrome by upregulating PPARγ, which promotes lipotoxicity and contractile dysfunction.
Ceramide is a sphingolipid that is a key mediator of cellular stress pathways that induce apoptosis and mitochondrial dysfunction. In normal physiology, ceramide is derived from de novo synthesis or can be derived from sphingomyelin hydrolysis. Ceramide acts as a lipotoxic intermediate when it builds up as a result of elevated circulating FFAs. The role of ceramide in insulin resistance in skeletal muscle has been studied more closely than in the heart. Ceramide and ceramide metabolites interfere with insulin signaling by activating PKCζ64, blocking Akt activation and subsequently reducing glucose uptake65. In skeletal muscle, ceramide decreases GLUT4 translocation to the membrane66, 67 and inhibition of serine palmitoyl transferase 1 (SPT1) reverses insulin resistance68. We recently showed that ceramide also plays an important role in the pathogenesis of obesity-mediated vascular dysfunction via a mechanism that involves PP2A mediated dephosphorylation of NOSIII5. Moreover, treatment of mice with lipotoxic cardiomyopathy with the inhibitor of ceramide synthesis myriocin, reversed contractile dysfunction in a mouse model of lipotoxic cardiomyopathy69. Taken together, it is therefore likely that ceramide accumulation may contribute to the pathogenesis of cardiac dysfunction in insulin resistant states.
Other Mediators of the Interaction of Insulin Resistance and Cardiac Dysfunction
Recent studies have implicated novel mechanisms that may directly contribute to the pathophysiology of insulin resistance and its cardiovascular complications48 such as changes in AMPK signaling70, oxidative stress71, inflammation72, advanced glycation end products (AGEs)73, endoplasmic reticulum (ER) stress74, autophagy75, and changes in adipokines76. Some of these mechanisms are discussed in more detail below.
AMP-activated Protein Kinase
AMP-activated protein kinase (AMPK) is an important mediator of energy balance in various tissues, including the heart77. Under conditions of inhibited ATP production or elevated ATP consumption, such as ischemia or exercise, the AMP/ATP ratio is elevated and AMPK is stimulated. As a result, AMPK acts to maintain ATP production and contractile function by increasing glucose and fatty acid uptake and oxidation in the heart78-80. AMPK directly impacts fatty acid metabolism through inhibition of acetyl-CoA carboxylase (ACC), which is responsible for the synthesis of malonyl CoA, a potent inhibitor of carnitine palmitoyl transferase 1. AMPK directly enhances insulin signaling in endothelial cells, protecting from insulin resistance81. It is possible that obesity-related impairment of AMPK function may contribute to insulin resistance in the heart. Moreover, a reduced circulating level of adiponectin, which is a characteristic of obesity, is associated with impaired AMPK signaling and mitochondrial biogenesis82. These observations raise the possibility that reduced activity of AMPK might contribute to mitochondrial dysfunction in the heart in obesity and insulin resistant states.
Reactive Oxygen Species and Insulin Resistance
Obesity is associated with increased oxidative stress in the heart and vasculature14. The sources of ROS are mitochondrial and extramitochondrial. For example, hyperglycemia may contribute to ROS production by activation of NADPH oxidase in cardiomyocytes 83. Ceramide may also contribute to ROS by reducing the mitochondrial ubiquinone pool of complex III84, 85 and increasing NADPH oxidase activity in endothelial cells86. Although the mitochondria are largely responsible for generating ROS, they can also be damaged by ROS. Oxidative stress has also been implicated in the pathophysiology of insulin resistance both in animals as well as in cultured cells48. It is not yet known if oxidative stress will impair myocardial insulin action. However there is strong evidence that oxidative stress may contribute to mitochondrial dysfunction in obesity and insulin resistant states87. ROS in the heart can act as a second messenger, initiating hypertrophic signaling, extracellular matrix remodeling, and apoptosis88.
Inflammation and Insulin Resistance
It is generally accepted that systemic inflammation contributes to insulin resistance 89. Proinflammatory cytokines induce insulin resistance, which may influence the fate of glucose and fatty acid utilization via direct and indirect mechanisms. By inducing insulin resistance, inflammation will increased the reliance of the heart on triglycerides from the liver and free fatty acids from adipose tissue for energy90. Obesity is accompanied by increased in circulating concentrations of inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α. These are believed to be derived in large part from macrophage infiltration of adipose tissue60. Given the well-documented associations between inflammation, obesity, and insulin resistance in other tissues91, inflammation in the heart may be a contributor to myocardial insulin resistance. Inflammatory cytokines impair insulin signaling by activating intracellular signaling kinases such as Jun N terminal kinase (JNK) that impairs insulin signaling by increasing the serine phosphorylation of IRS proteins48. It is possible that this mechanism may potentially occur in cardiomyocytes. Ko et al. reported that high fat feeding increased inflammation in the obese mouse heart, as evidenced by interleukin 6-mediated increases in macrophage and cytokine infiltration into the heart92. In addition, glucose oxidation was reduced as a result of cardiac inflammation in an IL-6-dependent manner92. It remains to be demonstrated if the local increase in myocardial inflammation directly contributes to impaired myocardial insulin action or if it the metabolic changes are secondary to systemic changes.
Advanced Glycation End Products and Insulin Resistance
Several studies have reported interesting associations between advanced glycation end product (AGE) levels and insulin resistance, even in the absence of diabetes. Studies by Tan and colleagues, in healthy non-diabetic subjects, showed an association between AGE levels, inflammatory markers and insulin resistance (the latter by the homeostatic model assessment index or HOMA-IR)93. Tahara and colleagues reported correlation between serum AGE levels and HOMA-IR in Japanese subjects94. Sarkar and colleagues found a highly significant correlation between the degree of insulin resistance and pre-AGE carbonyl levels in type 2 diabetic subjects95. Recent animal studies demonstrated that flux via the aldose reductase (AR) pathway in hyperglycemia contributes to driving formation of pre-AGE methylglyoxal and oxidative stress. In an AGE-enriched environment of aging, treatment with AR inhibitors reduce levels of methylglyoxal and AGEs96. Interaction of AGEs with its receptor RAGE has been linked to poor outcome after ischemic stress in diabetic and non-diabetic hearts97-99. AGE precursor generating AR pathway has also been demonstrated to play a key role in mediating ischemic injury and cardiovascular complications in diabetes100-102. These data suggest that examination of AR and RAGE is likely to be useful in determining potential relationships to impaired myocardial insulin action.
Therapeutic Implications
Therapies that modulate generalized insulin resistance such as PPARγ or PPARα agonists have been shown in animal models to improve myocardial function in part by decreasing circulating levels of fatty acids and switching myocardial substrate metabolism towards glucose14, 103. However, thiazolidinediones also induce cardiac hypertrophy via mechanisms that might be independent of effects on myocardial insulin signaling, which might independently contribute to increased heart failure risk104. Moreover, recent analyses of this therapeutic class in humans have indicated an independent increase in cardiovascular mortality resulting from increased coronary events105. Fewer studies have directly examined the impact of insulin sensitizers on cardiac structure, function and metabolism in humans with diabetes. In a study of well-controlled subjects with type 2 diabetes, pioglitazone modestly improved diastolic dysfunction in association with increased glucose utilization, while metformin treatment was without effect106. However, metformin on the other hand may have a potentially beneficial impact on cardiovascular outcomes via mechanisms that might not only be related to its impact to improve systemic metabolic homeostasis, but via mechanisms that may include beneficial impact of increasing AMPK signaling and the repression of autophagy107-109. The notion that excessive insulin signaling and hyperinsulinemia may accelerate left ventricular remodeling on the basis of hyperactivation of Akt22, 23 raises a therapeutic conundrum in that one consequence of achieving metabolic control in subjects with type 2 diabetes is the use of increased doses of insulin that will increase hyperinsulinemia and increase Akt signaling in the heart. Indeed analyses of the impact of tight metabolic control on the outcomes on cardiovascular outcomes or heart failure have been disappointingly neutral110 or in the case of heart failure, could potentially worsen heart failure risk111. Thus it is imperative to explore the impact of novel therapeutic strategies for treating diabetes such as agents that modulate GLP1 signaling on the interactions between insulin resistance and cardiac structure, metabolism and function. Moreover, additional studies in humans are required to investigate the impact of therapies that modulate inflammation, ER stress, autophagy and other novel mediators of insulin resistance on cardiovascular outcomes in insulin resistant states.
Concluding Remarks
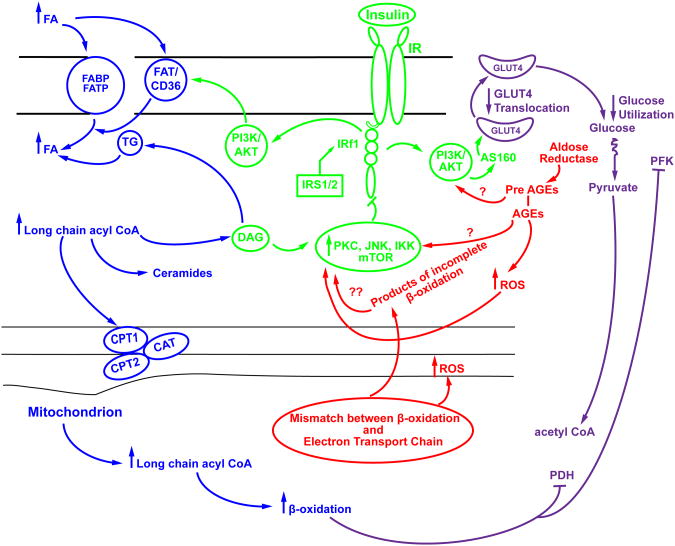
The mechanisms for and consequences of insulin resistance in the heart are complex and multifactorial. Whereas, impaired insulin stimulated glucose uptake is a common observation, changes in upstream signaling kinases are variable, and may be increased or decreased, with varied impact on cardiac structure and function. The heart requires a constant, tightly regulated supply of energy, relying primarily on fatty acid oxidation to meet this demand. Glucose oxidation also contributes to the energy demand of the heart; however, in insulin resistant states the contribution of glucose is decreased, while that of FAs is proportionately increased. The main cause of insulin resistance is obesity and the associated increase in FFA delivery to the heart precipitates many problems in the cardiomyocyte, including lipotoxicity, ROS production, oxidative stress, and changes in insulin signaling. One unexplored territory in the field of cardiac insulin signaling is the role of advanced glycation end products, its receptor RAGE and the pre-AGE generating AR pathway. The potential interplay between known metabolic mediators of insulin resistance and the unexplored pathways are summarized in Figure 1. In conclusion, the development of new therapeutic targets that may normalize impaired myocardial insulin action may contribute to novel strategies for treatment of diabetic heart disease.
Figure 1.
Proposed scheme linking metabolic and signaling pathways to insulin resistance in the heart. Enhanced supply of fatty acids results in enhanced fatty acid uptake into the heart, which in turn, leads to an increase in mitochondrial uptake of long chain fatty acyl CoA . β-oxidation of long chain acyl CoA in the heart is increased in insulin resistance, and may exceed the capacity of the TCA cycle and the electron transport chain to utilize β-oxidation products, leading to increased oxidative stress and ROS production. A mismatch between β-oxidation and the TCA cycle and electron transport chain leads to a buildup of products of incomplete β-oxidation. ROS and products of incomplete oxidation impact signaling cascade such as PKC, JNK and IKK, as well as that of IRS-1 and downstream signaling mediators in the insulin signaling pathway, such as PI3 kinase, Akt and AS160, resulting in reduced GLUT4 translocation and consequently a decrease in glucose uptake in to the heart. However, GLUT4 translocation has also been shown to be impaired in the heart in insulin resistant states in the absence of defects in PI3K and Akt signaling. In these circumstances, hyperactivation of Akt may further exacerbate lipotoxicity by increasing the translocation of CD36 to the plasma membrane. Increased β-oxidation leads to increased production of acetyl-CoA, NADH, AND FADH2, and citrate, which inhibit PDH and PFK respectively. Generation of Advanced glycation end product (AGE) precursors and AGEs due to increased flux of glucose via the aldose reductase pathway, are an unexplored pathway in insulin resistance. These pathways have been shown to impact signaling via PKC and PI3K/Akt and ROS generation.
Acknowledgments
Work in the authors laboratories are supported by the National Institutes of Health, the American Heart Association and the Juvenile Diabetes Research Foundation.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boden G. Obesity, insulin resistance and free fatty acids. Current opinion in endocrinology, diabetes, and obesity. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Gray S, Kim JK. New insights into insulin resistance in the diabetic heart. Trends in endocrinology and metabolism: TEM. 2011;22:394–403. doi: 10.1016/j.tem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell DS. Heart failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 5.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by pp2a-mediated dephosphorylation of the enos-akt complex. Diabetes. 2012 doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circulation research. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed c57bl/6j mice. Circulation research. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 8.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinology and metabolism clinics of North America. 2008;37:685–711. ix–x. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rask-Madsen C, Buonomo E, Li Q, Park K, Clermont AC, Yerokun O, Rekhter M, King GL. Hyperinsulinemia does not change atherosclerosis development in apolipoprotein e null mice. Arterioscler Thromb Vasc Biol. 2012;32:1124–1131. doi: 10.1161/ATVBAHA.111.239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell metabolism. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. Foxos integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell metabolism. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiang L, Tsuchiya K, Kim-Muller JY, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of foxo1 gene. The Journal of biological chemistry. 2012;287:13944–13951. doi: 10.1074/jbc.M111.332767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell metabolism. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MF, Kahn CR. The insulin signaling system. The Journal of biological chemistry. 1994;269:1–4. [PubMed] [Google Scholar]
- 16.Abel ED. Glucose transport in the heart. Frontiers in bioscience: a journal and virtual library. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 17.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. The Journal of clinical investigation. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovascular research. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoda T, An D, Witczak CA, Koh HJ, Hirshman MF, Fujii N, Goodyear LJ. Myo1c regulates glucose uptake in mouse skeletal muscle. The Journal of biological chemistry. 2011;286:4133–4140. doi: 10.1074/jbc.M110.174938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryder JW, Kawano Y, Chibalin AV, Rincon J, Tsao TS, Stenbit AE, Combatsiaris T, Yang J, Holman GD, Charron MJ, Zierath JR. In vitro analysis of the glucose-transport system in glut4-null skeletal muscle. The Biochemical journal. 1999;342(Pt 2):321–328. [PMC free article] [PubMed] [Google Scholar]
- 21.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocrine reviews. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 22.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. European heart journal. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. The Journal of clinical investigation. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher BA, Huang HM, Berthiaume JM, McElfresh TA, Chen X, Croniger CM, Muzic RF, Jr, Chandler MP. Myocardial insulin resistance induced by high fat feeding in heart failure is associated with preserved contractile function. American journal of physiology Heart and circulatory physiology. 2010;299:H1917–1927. doi: 10.1152/ajpheart.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 26.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of foxo1 triggers diabetic cardiomyopathy in mice. The Journal of clinical investigation. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Xu Y, Lu L, Bergman B, Leitner JW, Greyson C, Draznin B, Schwartz GG. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. American journal of physiology Heart and circulatory physiology. 2010;298:H310–319. doi: 10.1152/ajpheart.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sena S, Hu P, Zhang D, Wang X, Wayment B, Olsen C, Avelar E, Abel ED, Litwin SE. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. Journal of molecular and cellular cardiology. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, Hoffman JL, Yun UJ, Chakrabarti G, Wang Z, Albertine KH, Abel ED, Litwin SE. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: Possible role of reduced capillary density. Journal of molecular and cellular cardiology. 2005;39:882–892. doi: 10.1016/j.yjmcc.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: Effects of altered cardiomyocyte insulin signaling during pressure overload. American journal of physiology Heart and circulatory physiology. 2003;285:H1261–1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 32.Bing RJ. The metabolism of the heart. Acta cardiologica. 1955;10:1–14. [PubMed] [Google Scholar]
- 33.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 34.Wisneski JA, Stanley WC, Neese RA, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. The Journal of clinical investigation. 1990;85:1648–1656. doi: 10.1172/JCI114616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. The Journal of clinical investigation. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochimica et biophysica acta. 2005;1734:112–126. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Dirkx E, Schwenk RW, Glatz JF, Luiken JJ, van Eys GJ. High fat diet induced diabetic cardiomyopathy. Prostaglandins, leukotrienes, and essential fatty acids. 2011;85:219–225. doi: 10.1016/j.plefa.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovascular research. 2008;79:238–248. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- 39.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 40.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in lipid research. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: Molecular mechanisms. Biochimica et biophysica acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochimica et biophysica acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 43.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14c-labeled substrates in humans. The Journal of clinical investigation. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell metabolism. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase c, and ikappab-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 47.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (irs-1)-associated phosphatidylinositol 3-kinase activity in muscle. The Journal of biological chemistry. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 48.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovascular research. 2011;89:148–156. doi: 10.1093/cvr/cvq266. [DOI] [PubMed] [Google Scholar]
- 50.Unger RH, Orci L. Lipoapoptosis: Its mechanism and its diseases. Biochimica et biophysica acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 51.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 52.Wende AR, Abel ED. Lipotoxicity in the heart. Biochimica et biophysica acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiological reviews. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 54.Duncan JG. Peroxisome proliferator activated receptor-alpha (pparalpha) and ppar gamma coactivator-1alpha (pgc-1alpha) regulation of cardiac metabolism in diabetes. Pediatric cardiology. 2011;32:323–328. doi: 10.1007/s00246-011-9889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of ppargamma leads to cardiac dysfunction in mice. The Journal of clinical investigation. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by pparalpha overexpression mimics that caused by diabetes mellitus. The Journal of clinical investigation. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, Lee MK, Danton C, Deshmukh S, Cline GW, Wu JJ, Bennett AM, Rothermel B, Kalinowski A, Russell KS, Kim YB, Kelly DP, Kim JK. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 58.Campbell FM, Kozak R, Wagner A, Altarejos JY, Dyck JR, Belke DD, Severson DL, Kelly DP, Lopaschuk GD. A role for peroxisome proliferator-activated receptor alpha (pparalpha) in the control of cardiac malonyl-coa levels: Reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking pparalpha are associated with higher concentrations of malonyl-coa and reduced expression of malonyl-coa decarboxylase. The Journal of biological chemistry. 2002;277:4098–4103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 59.Panagia M, Gibbons GF, Radda GK, Clarke K. Ppar-alpha activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. American journal of physiology Heart and circulatory physiology. 2005;288:H2677–2683. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher EJ, Leroith D, Karnieli E. The Mount Sinai journal of medicine. Vol. 77. New York: 2010. Insulin resistance in obesity as the underlying cause for the metabolic syndrome; pp. 511–523. [DOI] [PubMed] [Google Scholar]
- 61.Vallim T, Salter AM. Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2010;82:211–218. doi: 10.1016/j.plefa.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong RH, Sul HS. Insulin signaling in fatty acid and fat synthesis: A transcriptional perspective. Current opinion in pharmacology. 2010;10:684–691. doi: 10.1016/j.coph.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D'Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. Journal of lipid research. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase czeta activation play an essential role in palmitate-induced insulin resistance in rat l6 skeletal muscle cells. The Biochemical journal. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: Dual mechanisms linking ceramide accumulation to the inhibition of akt/protein kinase b. The Journal of biological chemistry. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 66.JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent rac-activation and actin remodeling in muscle cells. Diabetes. 2007;56:394–403. doi: 10.2337/db06-0823. [DOI] [PubMed] [Google Scholar]
- 67.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. Irs1-independent defects define major nodes of insulin resistance. Cell metabolism. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. Journal of lipid research. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: Is it a consequence of ampk downregulation? Cell Cycle. 2011;10:3447–3451. doi: 10.4161/cc.10.20.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Current diabetes reviews. 2011;7:313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 72.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Current opinion in endocrinology, diabetes, and obesity. 2012;19:81–87. doi: 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puddu A, Viviani GL. Advanced glycation endproducts and diabetes. Beyond vascular complications. Endocrine, metabolic & immune disorders drug targets. 2011;11:132–140. doi: 10.2174/187153011795564115. [DOI] [PubMed] [Google Scholar]
- 74.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends in molecular medicine. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 75.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced bcl2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong AK, Howie J, Petrie JR, Lang CC. Amp-activated protein kinase pathway: A potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116:607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial glut-4 and increased glucose uptake through activation of ampk by aicar. The American journal of physiology. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 79.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/cd36 translocation in rat cardiac myocytes is mediated through amp-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 80.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart pfk-2 by ampk has a role in the stimulation of glycolysis during ischaemia. Current biology: CB. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 81.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-coa and amp-activated protein kinase (ampk): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochemical Society transactions. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 82.Kadowaki T, Yamauchi T, Waki H, Iwabu M, Okada-Iwabu M, Nakamura M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harbor symposia on quantitative biology. 2012 doi: 10.1101/sqb.2012.76.010587. [DOI] [PubMed] [Google Scholar]
- 83.Balteau M, Tajeddine N, de Meester C, Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S, Vanoverschelde JL, Gailly P, Hue L, Bertrand L, Beauloye C. Nadph oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires sglt1. Cardiovascular research. 2011;92:237–246. doi: 10.1093/cvr/cvr230. [DOI] [PubMed] [Google Scholar]
- 84.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. The Journal of biological chemistry. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 85.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex iii by cell-permeable ceramide. The Journal of biological chemistry. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Forstermann U. Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 87.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovascular research. 2010;88:229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. American journal of physiology Heart and circulatory physiology. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 89.Straub RH. Concepts of evolutionary medicine and energy regulation contribute to the etiology of systemic chronic inflammatory diseases. Brain, behavior, and immunity. 2011;25:1–5. doi: 10.1016/j.bbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Kuipers RS, de Graaf DJ, Luxwolda MF, Muskiet MH, Dijck-Brouwer DA, Muskiet FA. Saturated fat, carbohydrates and cardiovascular disease. The Netherlands journal of medicine. 2011;69:372–378. [PubMed] [Google Scholar]
- 91.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. The Journal of clinical investigation. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing amp-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan KC, Shiu SW, Wong Y, Tam X. Serum advanced glycation end products (ages) are associated with insulin resistance. Diabetes/metabolism research and reviews. 2011;27:488–492. doi: 10.1002/dmrr.1188. [DOI] [PubMed] [Google Scholar]
- 94.Tahara N, Yamagishi S, Matsui T, Takeuchi M, Nitta Y, Kodama N, Mizoguchi M, Imaizumi T. Serum levels of advanced glycation end products (ages) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovascular therapeutics. 2012;30:42–48. doi: 10.1111/j.1755-5922.2010.00177.x. [DOI] [PubMed] [Google Scholar]
- 95.Sarkar P, Kar K, Mondal MC, Chakraborty I, Kar M. Elevated level of carbonyl compounds correlates with insulin resistance in type 2 diabetes. Annals of the Academy of Medicine, Singapore. 2010;39:909–904. [PubMed] [Google Scholar]
- 96.Hallam KM, Li Q, Ananthakrishnan R, Kalea A, Zou YS, Vedantham S, Schmidt AM, Yan SF, Ramasamy R. Aldose reductase and age-rage pathways: Central roles in the pathogenesis of vascular dysfunction in aging rats. Aging cell. 2010;9:776–784. doi: 10.1111/j.1474-9726.2010.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bucciarelli LG, Ananthakrishnan R, Hwang YC, Kaneko M, Song F, Sell DR, Strauch C, Monnier VM, Yan SF, Schmidt AM, Ramasamy R. Rage and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, Zou YS, Yan SF, Schmidt AM, Ramasamy R. Receptor for advanced-glycation end products: Key modulator of myocardial ischemic injury. Circulation. 2006;113:1226–1234. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 99.Ramasamy R, Yan SF, Schmidt AM. Receptor for age (rage): Signaling mechanisms in the pathogenesis of diabetes and its complications. Annals of the New York Academy of Sciences. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 101.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circulation research. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes. 1997;46:292–300. doi: 10.2337/diab.46.2.292. [DOI] [PubMed] [Google Scholar]
- 103.Larsen TS, Aasum E. Metabolic (in)flexibility of the diabetic heart. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 2008;22:91–95. doi: 10.1007/s10557-008-6083-1. [DOI] [PubMed] [Google Scholar]
- 104.Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED. Cardiac hypertrophy caused by peroxisome proliferator- activated receptor-gamma agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology. 2007;148:6047–6053. doi: 10.1210/en.2006-1559. [DOI] [PubMed] [Google Scholar]
- 105.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: Systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. doi: 10.1136/bmj.d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 107.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Current opinion in lipidology. 2011;22:445–453. doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 108.Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, Lange T, Abildstrom SZ, Schramm TK, Vaag A, Kober L, Torp-Pedersen C, Gislason GH. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: A retrospective nationwide cohort study. Diabetologia. 2010;53:2546–2553. doi: 10.1007/s00125-010-1906-6. [DOI] [PubMed] [Google Scholar]
- 109.Xie Z, He C, Zou MH. Amp-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy. 2011;7:1254–1255. doi: 10.4161/auto.7.10.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castagno D, Baird-Gunning J, Jhund PS, Biondi-Zoccai G, MacDonald MR, Petrie MC, Gaita F, McMurray JJ. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: Evidence from a 37,229 patient meta-analysis. American heart journal. 2011;162:938–948. e932. doi: 10.1016/j.ahj.2011.07.030. [DOI] [PubMed] [Google Scholar]