Abstract
Humans have genetically based unique abilities making complex culture possible; an assemblage of traits which we term “cultural capacity”. The age of this capacity has for long been subject to controversy. We apply phylogenetic principles to date this capacity, integrating evidence from archaeology, genetics, paleoanthropology, and linguistics. We show that cultural capacity is older than the first split in the modern human lineage, and at least 170,000 years old, based on data on hyoid bone morphology, FOXP2 alleles, agreement between genetic and language trees, fire use, burials, and the early appearance of tools comparable to those of modern hunter-gatherers. We cannot exclude that Neanderthals had cultural capacity some 500,000 years ago. A capacity for complex culture, therefore, must have existed before complex culture itself. It may even originated long before. This seeming paradox is resolved by theoretical models suggesting that cultural evolution is exceedingly slow in its initial stages.
No other extant animal can acquire culture to the extent humans can1,2. For example, despite decades of intense animal language training, no animal has learned language3. In contrast, all humans (barring pathologies) can learn to speak, read, and write. Other abilities that are uniquely developed in humans include social learning, creative thinking, and planning for the future1. We term the set of genetically based cognitive abilities that, collectively, make human culture possible “cultural capacity”. Here we integrate data from genetics, paleoanthropology, archaeology, and linguistics to date this capacity.
Cultural capacity is customarily dated by identifying archaeological finds indicative of human-like culture, such as complex tools or ritual burials4,5,6. This method, however, underestimates the age of cultural capacity, because the capacity to create an artifact necessarily predates the artifact itself. For example, we cannot date the origin of the genetically based cognitive abilities necessary to solve Rubik's cube based on when Rubik's cube appeared. The data reviewed below support the hypothesis that human cultural capacity was present already at the time of the most recent common ancestor of modern humans, about 170 ka (thousands of years ago), and probably also in other Homo species. We reach this conclusion using phylogenetic principles: given several hypotheses that have the same or similar level of support, the one that assumes the fewest evolutionary events should be considered more likely7,8,9. Here, we test three hypotheses (Fig. 1A):
Early Origin: Cultural capacity was already present in the last common ancestor of all modern humans, living in East Africa about 170 ka10.
Multiple Origins: Elements of cultural capacity evolved at different times in different human lineages, and were later joined into full cultural capacity by gene flow between populations, later than 170 ka11.
Late Origin: Cultural capacity evolved once, later than 170 ka, and then spread by migration and gene flow12,13,14,15,16.
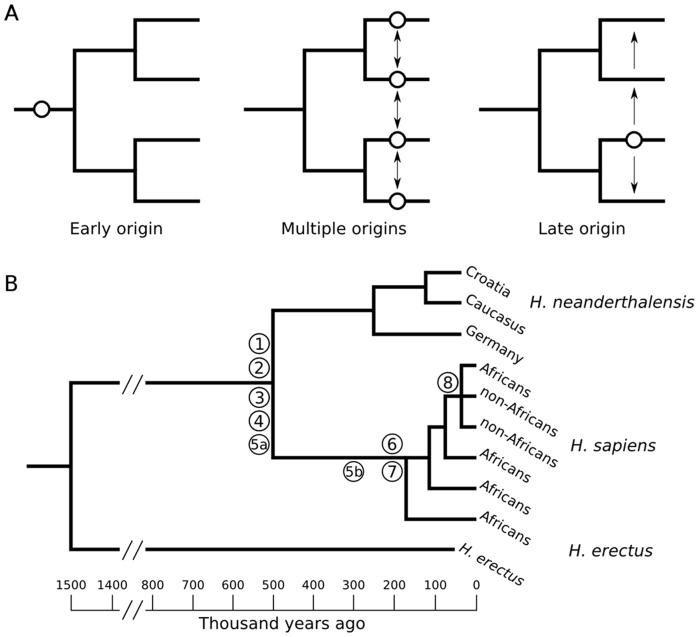
Figure 1. The figure shows the evolution of humans, their cultural capacity and culture.
(A) Three hypotheses about the age of cultural capacity. Circles indicate origin and arrows indicate transmission of the capacity by gene flow. (B) Phylogenetic tracing of evidence shows that most characters linked to cultural capacity are dated before the first split between H. sapiens lineages (1: modern hyoid bone morphology (32–35), 2: human FOXP2 allele (38, 39), 3: fire (44, 45), 4: ritual burials and examples of symbolization (52–54), 5a: advanced stone tools (see text) present in both H. sapiens (3,16) and H. neanderthalensis (47–52), 5b: archaeological dating (3), 6: gene flow from Asia to Africa that did not reach South Africa (26), 7: agreement of language and genetic trees (41), 8: European cultural explosion (18–20, 42)). Phylogeny is based on figure 19.21 in (46).
Results
The Multiple Origins hypothesis assumes many evolutionary events and, lacking specific support, we reject it on the grounds of parsimony. The Early Origin and Late Origin hypotheses assume, respectively, one evolutionary event (origin of the capacity) or two events (origin of the capacity and subsequent diffusion to other populations). At first glance, support exists for both. Early Origin is supported by a multitude of archaeological finds demonstrating modern tool making during the African Middle Stone Age, at 300 ka4. A recent observation even indicates advanced stone tool production at 500 ka17. Late Origin is supported by the marked increase in population density, migration and material culture that changed human prehistory about 40 ka18,19,20,21, which have been taken as indications of a dramatic evolutionary event22. We summarize below data bearing on these hypotheses.
Migration and gene flow
The Early Origin hypothesis could be established by finding a population that has been genetically isolated since the first split in the human lineage. Mbuti pygmies have been considered sufficiently isolated23, but gene flow to this population probably occurred within the last 170 ka24,25,26. On the other hand, the Late Origin hypothesis would be supported if gene flow from one population to all others could be demonstrated later than 170 ka. Y-chromosome haplotypes27 and the β-globin locus28 show gene flow from Asia to Sub-Saharan East Africa around 20 to 4 ka and during the last 100 ka respectively. But the gene flow does not seem to have reached South Africa27, and seems hard to link to cultural capacity. In conclusion, there are no observations of alleles spreading from a population, for example from Eurasia, to all African populations after the split some 170 ka. This supports an early origin of cultural capacity.
Speech and language
Some researchers argue that language did not develop fully until about 40 ka29,30,31, while others maintain that archaic H. sapiens may have had language 200 ka32,33. Central parts of the human speech anatomy (e.g. the human hyoid bone) existed well before the first split in the human lineage33,34,35,36, but this evidence is not conclusive because the speech apparatus alone is not sufficient for language.
Some Southern African click-speaking populations share rare genetic elements with East African click-speaking populations, suggesting these populations split around 35-50 ka37. This date is compatible with both the Early and Late Origin hypotheses. Specific genes are hard to link to language, but mutations in the FOXP2 gene cause language deficits38. Humans have a unique FOXP2 allele that evolved after the split from our common ancestor with chimpanzees (Pan troglodytes)39. This allele was recently found in Neanderthals40, and thus predates the split between modern humans.
The age of divergence between languages is difficult to estimate earlier than 5–10 ka41, but the close match found between language trees and genetic trees would be highly unlikely if language had evolved after modern humans split into different populations42.
Archaeology
The strongest support for the Late Origin hypothesis comes from the demographic and cultural explosion that took place in Europe around 40 ka19,20,21,43. Stone tools of similar complexity, however, appeared 200–500 ka in Southern Africa4,17. Conservatively, we are not considering earlier hominid stone tools as evidence of human-like cultural capacity15. Another uniquely human trait is the ability to control fire44. There is strong evidence that hominids controlled fire 1,000 ka45, and certainly before 200 ka46.
Discussion
The findings summarized above (see also Table 1 in Supplementary Information) point clearly to an Early Origin of the genetically based human cultural capacity. Figure 1B maps these findings onto a simplified hominid phylogeny, for which there is a broad consensus among anthropologists and evolutionary biologists13,47. The conclusion that cultural capacity is at least 170 ka old rests essentially on two lines of evidence. First, the genetically based cultural capacity is present in all human populations today. If it appeared later than 170 ka it must have, subsequently, spread to all corners of the world, but we are not aware of any evidence for such a worldwide genetic sweep. Secondly, most evidence came early, as it either appears in H. sapiens before 170 ka, or appears in both H. neanderthalensis and H. sapiens.
Indeed, Fig. 1B suggests that Neanderthals may have had cultural capacity comparable to H. sapiens. Archaeologists agree that Neanderthal stone tools are of similar complexity to contemporary H. sapiens tools48,49,50, but some hold that Neanderthals learned their most advanced technology from H. sapiens51,52. In either case, Neanderthals were able to at least acquire and maintain an advanced tool industry, rivaling the skill of H. sapiens flintknappers in producing Llevallois cores, flakes and points53.
Further, the find of a complete hyoid bone from a Neanderthal skeleton at Kebara Cave shows that Neanderthal vocal tracts were very similar to ours34,35. A recent review of Neanderthal culture cites 12 certain cases of burials54, and other studies indicate shelter building, regional cultural differences, rituals, and symbolization53,55. Lastly, recent genetic studies show that admixture took place between Neanderthals and early Eurasian H. sapiens56, which is suggestive of how similar the two species were. In conclusion, we find no grounds to reject the possibility that Neanderthals had cultural capacity comparable to ours53.
One could argue that it is difficult to date cultural capacity unless it is clear from archaeological observations of material culture that this capacity is present. And from a behaviour theoretical point of view; we do not know if a burial is a more cognitively demanding task than for example controlling fire for food production. Our solution to this problem was to include first appearances of traits (cultural and genetic) relevant to a unique human cultural capacity. For example, we attempted to use the earliest finds of stone tools for which there is consensus that they are advanced and a product of uniquely human cultural practices4. And importantly, our conclusions do not solely rest upon observations from material culture. That increasingly more material culture appears later in the archaeological record does not conflict with our conclusions; this is a straightforward prediction from theory of cultural evolution.
Further, our analyses cannot produce an exact date for the origin of human cultural capacity, but it can provide a latest date (terminus ante quem) for when it has to have appeared. Another point of concern may be whether it is correct to use phylogenetic principles for dating cultural capacity when that capacity may have existed only in one extant species. Importantly, when judging between competing hypotheses, one should select the hypothesis that makes the fewest assumptions. The conclusion is straightforward, if a trait is present on all branches (i.e. in all populations) of a phylogeny, this trait will be reconstructed as ancestral no matter what the branching pattern is and pretty much independently of phylogenetic reconstruction method. Since cultural capacity is present in all human populations it thus follows that this capacity has to be older than the oldest split in the human lineage, unless substantial gene-flow has occurred, as discussed above.
If cultural capacity is at least 170 ka old, how do we explain the gap of more than 100,000 years before we find traces of art, agriculture, and of the dramatic demographic and cultural growth that is still ongoing57? We suggest that the answer lies in the mechanisms of cultural evolution. Despite the potential of culture to grow exponentially, theory suggests that the initial stages of cultural evolution may be exceedingly slow2,58. Cultural innovations build upon already existing culture59, hence they appear rarely when little culture is present (Fig. 2A). Moreover, theory predicts that advanced culture is more likely to emerge with increasing population size and interactions between groups58,60,61,62. Thus ancestral populations may have been too small and isolated to foster rapid cultural growth (Fig. 2B). This observation has been used to explain historical cultural extinctions, such as the loss of culture in Tasmanian aborigines62, and could also help explain isolated archaeological observations such as the 125 ka stone industry in Jebel Faya, UAE63.
Figure 2. Examples of factors determining the rate of cultural evolution.
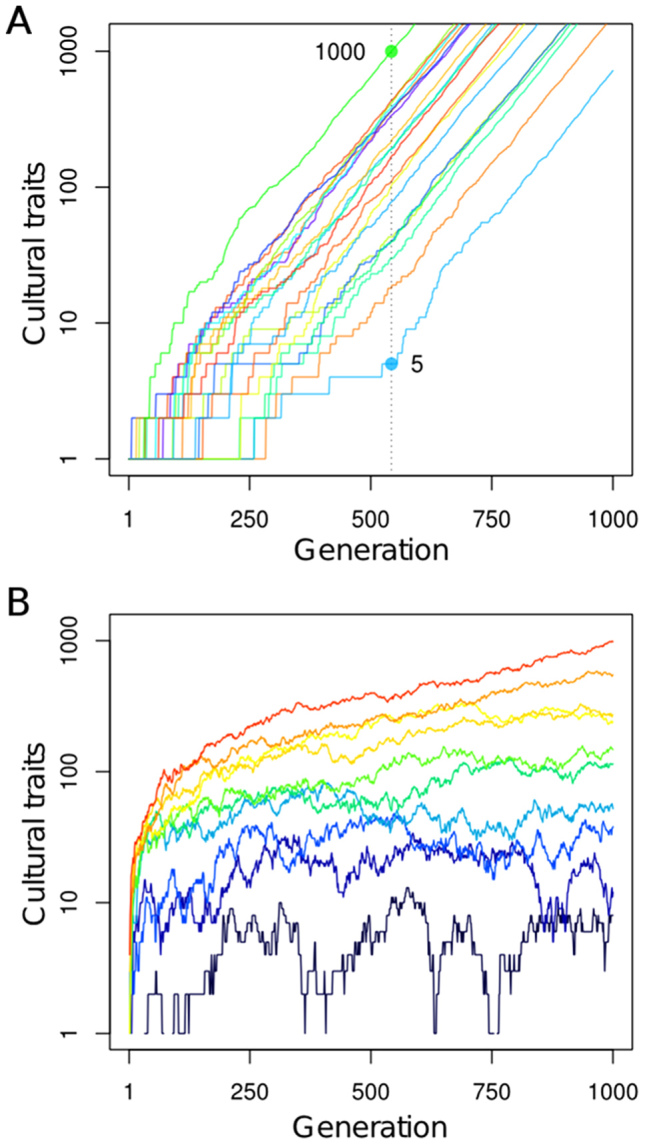
(A) Chance causes great variation in the onset of cultural growth in a model in which each existing cultural trait can give rise to a new one with probability 0.01 per generation. Twenty identical populations are simulated, each starting with one cultural trait. When the fastest growing population reaches 1000 traits, the slowest one has developed only 5. (B) Larger populations accumulate culture more easily. Here the probability that a cultural trait gives rise to a new one is 6 × 10−4 per individual per generation, and a cultural trait can be lost with probability 0.05 per generation. With these parameters, a critical population size of about 100 individuals is necessary for gains to consistently outnumber losses and cause sustained cultural growth. Population size varies between 10 (dark blue line, slowest growing) and 100 (red line, fastest growing).
In conclusion, cultural evolutionary theory suggests an extremely slow initial development of human culture even in the presence of a fully developed cultural capacity. Absence of evidence is thus not evidence of absence, and not sufficient to reject the hypothesis that humans have had cultural capacity since at least 170 ka.
Methods
The simulations in Fig. 2A were performed as follows. The population is assumed to start with 1 cultural element. At each generation, there is a probability of r = 0.01 that an existing cultural element gives rise to a new element, modeling innovation depending on existing culture. This stochastic process is implemented by the following recursion:
where C(t) is the number of cultural elements at generation t, and B(n, p) is a random number drawn from the binomial distribution corresponding to the total number of successes in n Bernoulli trials, each of which has a probability of success of p. This model leads to an exponential increase in the number of cultural traits, as observed in empirical data58, but the onset of such increase is highly variable because stochastic effects dominate. Once a number of cultural traits have been established, however, accumulation of further elements proceeds reliably and a regular increase in cultural elements is observed.
In the simulations in Fig. 2B, we allowed cultural elements to be forgotten at a rate d = 0.05 per element per generation, and we let the probability that an existing cultural element gives rise to a new element be an increasing function of population size, p(N) = 1 − (1 − r)N, where r = 6 × 10−4 and N is the population size. Such an increase reflects the fact that larger populations have more potential inventors. Thus, the only situation in which a given cultural element fails to give rise to new ones is when every individual fails to derive a new element from it. If the individual success rate is r, such a probability is 1 − (1 − r)N. The recursion determining the number of cultural elements at generation t is thus
Parameter values are not meant to model specific historical processes, but to illustrate the fact that stochastic factors can influence greatly the process of accumulation of cultural elements.
Author Contributions
J.L., P.L., S.G., K.L. and M.E. wrote the manuscript. J.L. prepared figure 1 and S.G. prepared figure 2. All authors reviewed the manuscript.
Supplementary Material
Table 1
Acknowledgments
We thank Anna-Carin Stymne, Sven Isaksson and several anonymous reviewers for comments and discussions.
References
- Tomasello M. The Cultural Origins of Human Cognition. (Harvard University Press, Cambridge) (2000). [Google Scholar]
- Boyd R. & Richerson P. J. The Origin and Evolution of Culture (Oxford University Press, New York, 2005). [Google Scholar]
- Wynne C. D. L. Do Animals Think? (Princeton University Press, Princeton) (2004). [Google Scholar]
- McBrearty S. & Brooks A. The revolution that wasn't: a new interpretation of the origin of modern human behavior. J. Hum. Evol. 39, 453–563 (2000). [DOI] [PubMed] [Google Scholar]
- Henshilwood C., d'Errico F., Marean C., Milo R. & Yates R. An early bone tool industry from the Middle Stone Age at Blombos Cave, South Africa: implications for the origins of modern human behaviour, symbolism and language. J. Hum. Evol. 41, 631–678 (2001). [DOI] [PubMed] [Google Scholar]
- Wadley L., Hodgskiss T. & Grant M. Implications for complex cognition from the hafting of tools with compound adhesives in the Middle Stone Age, South Africa. Proc. Natl. Acad. Sci. USA 106, 9590–9594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanntorp H. E. et al. Phylogenetic approaches in ecology. Oikos 57, 119–132 (1990). [Google Scholar]
- Futuyma D. J. Evolutionary Biology (Sinauer, Sunderland) (1998). [Google Scholar]
- Mace R. & Holden C. J. A phylogenetic approach to cultural evolution. Trends. Ecol. Evol. 20, 116–121 (2005). [DOI] [PubMed] [Google Scholar]
- Henshilwood C. S. & Marean S. W. The origin of modern human behaviour. Curr. Anthropol. 44, 627–637 (2003). [DOI] [PubMed] [Google Scholar]
- Wolpoff M. H., Hawks J. & Caspari R. Multiregional, not multiple origins. Am. J. Phys. Anthropol. 112, 129–136 (2000). [DOI] [PubMed] [Google Scholar]
- Klein R. G. Southern Africa and modern human origins. J. Anthropol. Res. 57, 1–16 (2001). [Google Scholar]
- Klein R. G. Out of Africa and the evolution of human behavior. Evol. Anthropol. 17, 267–281 (2008). [Google Scholar]
- Ambrose S. H. Chronology of the Later Stone Age and food production in East Africa. J. Archaeol. Sci. 25, 377–392 (1998). [Google Scholar]
- Ambrose S. H. Paleolithic technology and human evolution. Science 291, 1748–1753 (2001). [DOI] [PubMed] [Google Scholar]
- Tattersall I. The Fossil Record: How We Know What We Know About Human Evolution. (Oxford University Press, New York) (1995). [Google Scholar]
- Balter M. New light on revolutions that weren't. Science 336, 530–531 (2012). [DOI] [PubMed] [Google Scholar]
- White R. K. In Handbook of Human Symbolic Evolution. edited by Lock A., and Peters C. R. pp. 239–262. Clarendon Press, Oxford (1996).
- Bar-Yosef O. The Upper Paleolithic revolution. Ann. Rev. Anthropol. 31, 363–393 (2002). [Google Scholar]
- Klein R. G. Archeology and the evolution of human behavior. Evol. Anthropol. 9, 17–36 (2000). [Google Scholar]
- Cochran G. & Harpending H. The 10,000 Year Explosion: How Civilization Accelerated Cultural Evolution. (Basic Books, New York) (2009). [Google Scholar]
- Wynn T. Hafted spears and the archaeology of mind. Proc. Natl. Acad. Sci. USA 106, 9544–9545 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. et al. Genetic structure of human populations. Science 298, 2381–2385 (2002). [DOI] [PubMed] [Google Scholar]
- Tishkoff S. A. et al. The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton A. R. Out of Africa again and again. Nature 416, 45–51 (2002). [DOI] [PubMed] [Google Scholar]
- Templeton A. R. Coherent and incoherent inference in phylogeography and human evolution. Proc. Natl. Acad. Sci. USA 14, 6376–6381 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani F. et al. A back migration from Asia to Sub-Saharan Africa is supported by high-resolution analysis of human y-chromosome haplotypes. Am. J. Hum. Gen. 70, 1197–1214 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. M., Fullerton S. M., Griffiths J. B. & Clegg A. Archaic African and Asian lineages in the genetic ancestry of modern humans. Am. J. Hum. Gen. 60, 772–789 (1997). [PMC free article] [PubMed] [Google Scholar]
- Chase P. G. & Dibble H. L. Middle Paleolithic symbolism: A review of current evidence and interpretations. J. Anthropol. Archaeol., 6, 263–296 (1987). [Google Scholar]
- Whallon R. In: Mellars P., & Stringer C. (Eds), The Human Revolution: Behavioral and Biological Perspectives on the Origins of Modern Humans. Princeton, Princeton University Press, pp. 433–454 (1989).
- Mellars P. Cognitive changes and the emergence of modern humans. Cam. Archaeol. J. 1, 63–76 (1991). [Google Scholar]
- Bradshaw J. L. Animal asymmetry and human heredity: Dextrality, tool use, and language in evolution, 10 years after Walker (1980). Br. J. Psychol. 82, 39–59 (1991). [DOI] [PubMed] [Google Scholar]
- Mithen S. Palaeolithic archaeology and the evolution of mind. J. Archaeol. Res. 3, 305–332 (1995). [Google Scholar]
- Arensburg B. et al. Y. A Middle Palaeolithic human hyoid bone. Nature 338, 758–760 (1989). [DOI] [PubMed] [Google Scholar]
- Arensburg B., Schepartz L. A., Tillier A. M., Vandermeersch B. & Rak Y. A reappraisal of the anatomical basis for speech in Middle Palaeolithic hominids. Am. J. Phys. Anthropol. 83, 137–146 (1990). [DOI] [PubMed] [Google Scholar]
- Martínez I. et al. Human hyoid bones from the middle Pleistocene site of the Sima de los Huesos (Sierra de Atapuerca, Spain). J. Hum. Evol. 54, 118–124 (2008). [DOI] [PubMed] [Google Scholar]
- Tishkoff S. A. et al. History of click-speaking populations of Africa inferred from mtDNA and Y chromosome genetic variation. Mol. Biol. Evol. 24, 2180–2195 (2007). [DOI] [PubMed] [Google Scholar]
- Lai C. S., Fisher S. E., Hurst J. A., Vargha-Khadem F. & Monaco A. P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523 (2001). [DOI] [PubMed] [Google Scholar]
- Enard W. et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418, 869–872 (2002). [DOI] [PubMed] [Google Scholar]
- Krause J. et al. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr. Biol. 17, 1908–1912 (2007). [DOI] [PubMed] [Google Scholar]
- Gray R. D., Atkinson Q. D. & Greenhill S. J. Language evolution and human history: what a difference a date makes. Phil. Trans. R. Soc. B. 366, 1090–1100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L. & Feldman M. W. The application of molecular genetic approaches to the study of Human evolution. Nature Gen. Supplement 33, 266–275 (2003). [DOI] [PubMed] [Google Scholar]
- Klein R. G. Anatomy, behavior, and modern human origins. J. World. Prehist. 9, 167–198 (1995). [Google Scholar]
- Goudsblom J. The human monopoly on the use of fire: its origins and conditions. Hum. Evol. 1, 517–523 (1986). [Google Scholar]
- Berna F. et al. Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc. Natl. Acad. Sci. USA 109, E1215–E1220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkanas P. et al. Evidence for habitual use of fire at the end of the Lower Paleolithic: Site-formation processes at Qesem Cave, Israel. J. Hum. Evol. 53, 197–212 (2007). [DOI] [PubMed] [Google Scholar]
- Freeman S. & Herron J. C. Evolutionary Analysis. (Benjamin Cummings, New York) (2003). [Google Scholar]
- Zilhão J. et al. Analysis of Aurignacian interstratification at the Chatelperronian-type site and implications for the behavioral modernity of Neandertals. Proc. Natl. Acad. Sci. USA 103, 12643–12648 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riel-Salvatore J., Miller A. E. & Clark G. A. An empirical evaluation of the case for a Châtelperronian-Aurignacian inter-stratification at Grotto des fées de Châtelperron. World. Archaeol. 40, 280–492 (2008). [Google Scholar]
- Semal P. et al. New data on the late Neandertals: direct dating of the Belgian spy fossils. Am. J. Phys. Anthropol. 138, 421–428 (2009). [DOI] [PubMed] [Google Scholar]
- Mellars P., Gravina B. & Ramse C. B. Confirmation of Neanderthal/Modern human interstratification at the Chatelperronian type-site. Proc. Natl. Acad. Sci. USA 104, 3657–3662 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellars P. & Gravina B. Châtelperron: theoretical agendas, archaeological facts, and diversionary smoke-screens. Paleoanthropology 43–64(2008). [Google Scholar]
- Hayden B. The cultural capacities of Neanderthals: a review and re-evaluation. J. Hum. Evol. 24, 113–146 (1993). [Google Scholar]
- Langley M. C. & Clarkson C. U. S. Behavioural complexity in Eurasian Neanderthal populations: a chronological examination of the archaeological evidence. Cam. Archaeol. J. 18, 289–307 (2008). [Google Scholar]
- Morin E. & Laroulandie V. Presumed Symbolic Use of Diurnal Raptors by Neanderthals. PLoS ONE 7, e32856 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. E. et al. A Draft Sequence of the Neandertal Genome. Science 328, 710–722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew C. Neuroscience, evolution and the sapient paradox: the factuality of value and of the sacred. Phil. Trans. R. Soc. Lond. B. 363, 2041–2047 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist M., Ghirlanda S., Jarrick A. & Wachtmeister C.-A. Why does human culture increase exponentially? Theor. Pop. Biol. 74, 46–55 (2008). [DOI] [PubMed] [Google Scholar]
- Ogburn W. F. Social Change. (Viking, New York) (1950). [Google Scholar]
- Powell A., Shennan S. & Thomas M. G. Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301 (2009). [DOI] [PubMed] [Google Scholar]
- Ghirlanda S. & Enquist M. Cumulative culture and explosive demographic transitions. Qual Quant 41, 581–600 (2007). [Google Scholar]
- Henrich J. Demography and cultural evolution: How adaptive cultural processes can produce maladaptive losses - The Tasmanian case. Am. Antiq. 69, 197–214 (2004). [Google Scholar]
- Armitage S. J. et al. The Southern Route “Out of Africa”: Evidence for an Early Expansion of Modern Humans into Arabia. Science 331, 453–456 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1