Abstract
The combined influence of (1) calving period (early or late) and (2) overwintering contamination by residual infective larvae (high or low) on subsequent exposure of suckler calves to gastrointestinal nematodes was investigated. We found that the effect of calving date was greater than the level of residual contamination. This was because the adult cows produced large quantities of manure containing small amounts of nematode eggs from turnout, which significantly contaminated the pasture, and thereby, reduced the effect of prior high-low contamination. Early born calves were found to be more heavily exposed to parasites, most likely due to ingesting more herbage than those born later. Late-born calves also had relatively high antibody levels at turnout, which first decreased and then increased again. We suggest that the high antibody levels at turnout reflect passive transfer of maternal antibodies through the milk. There was also a significant difference in animal performance, with the more heavily exposed early born calves having significantly lower daily weight gain than the late-born calves. However, this might not be entirely due to increased parasitism.
Keywords: Parasitology, Strongyles, ELISA, Cattle, Population dynamics, Diagnostics
Introduction
As in other temperate regions of the world, nematodes in the genera Ostertagia and Cooperia are the most common and economically damaging parasites in grazing cattle in Sweden (Höglund and others 2009). The necessity for intervention to control gastrointestinal nematodes (GIN) varies depending on a range of factors. It is known that growing dairy cattle turned out on contaminated pasture for the first time are highly susceptible to GIN, especially when they graze permanent pasture at high stocking rates (Eysker and others 2000, Höglund and others 2001, Vercruysse and Claerebout 2001).
It has been argued that the significance of GIN in suckler calves is of more limited importance, as they are mainly fed on milk in the beginning of their first grazing period, which in turn reduces intake of herbage and, thus, lowers their exposure to infective larvae on pasture (eg, Viring and others 2001). Furthermore, because the more immune cows ingest infective larvae that may have overwintered on pasture (Slocombe and Curtis 1989), the initial exposure of suckler calves to parasites is believed to be even more modest. Thus, suckler calves are, in general, less affected by GIN than their dairy equivalents (Hertzberg and others 2004), which are usually not grazed alongside their dams. In dairy calves, pasture contamination at turnout is the most important route of infection, whereas for suckler calves, the relative contribution of overwintering larvae and eggs excreted by dams to the epidemiology of these parasites is not clear.
Although suckler cows gradually acquire protective immunity to GIN, this is not complete. Infection levels are also likely to vary among individuals in relation to parturition, since for example, in ewes, faecal egg counts (FEC) are often increased due to suppression of immunity (Barger 1993). Similarly to ewes, dairy cows in early lactation have a negative energy balance around calving, which probably induces immune relaxation (Armour 1989). Although suckler cows are probably less vulnerable than ewes and dairy cows due to lower milk yield, this complex interplay clearly warrants further investigation.
The seminatural pastures of northern Europe are habitats with a wide diversity of valuable plant and animal species, but they are under threat because of the cessation of grazing (Luoto and others 2003). Therefore, farmers in many countries receive agrienvironmental payments for sustainably managing these pastures. In Swedish suckler calf production, these payments, mainly for managing seminatural pastures, represent as much income as that from the weaned calves (Deblitz 2011). The grazing management of suckler cows, therefore, aims both to rear weaned calves and to obtain grazing pressures high enough to preserve the biodiversity of the grasslands. At the same time, the added value of meat and other agrienvironmental payments have resulted in growing interest in organic beef production, where prophylactic anthelmintic treatment is prohibited (Salevid and Kumm 2011, Anonymous 2012a, b). The pastures currently grazed by organic animals are generally used for extended periods, both in response to the rules for organic livestock production and owing to ongoing climate change towards a longer growing season (Anonymous 2012b). Alterations in farm management in response to conversion to organic farming also often result in suckler cows calving earlier than previously. Although most (54 per cent) calvings in Sweden still occur in spring (March and April), the average calving date has been brought forward, and today, 28 per cent of calvings take place in January and February (Anonymous 2011). Taken together, these factors may have changed the risks of suckler calf exposure to GIN.
Knowledge about the GIN status in current Swedish suckler cow systems is limited. The aim of the present study was thus to determine the influence of earlier calving, and the importance of overwintered pasture parasite infection on infection dynamics and performance in beef cows and their suckling calves when exposed to different levels of overwintered larvae on pasture.
Materials and methods
Experimental design
The experiment was conducted in the grazing period of 2011 on 35 ha of permanent seminatural pasture at Götala Research Station, Skara, in southwestern Sweden (58°42′N, 13°21′E; elevation 150 m asl). The study started at turnout on 6 May and ended at weaning on 20 September.
The experiment had a split-plot design with repeated measures comprising, two periods of calving (early and late) and, within these, two levels of overwintered or residual parasite infection of the pasture (high and low). The two calving periods were obtained by allocating the cow-calf pairs into two groups, with calving season ranging from 15 December to 2 February (early), and 11 February to 5 April (late), respectively. The high level of pasture contamination was obtained by using pasture that had been grazed by untreated first-season grazing calves (FSGs) for the three previous years. The low level was obtained by using pasture that had been grazed for the three previous years by FSGs that were subcutaneously injected with doramectin (0.2 mg per kg body weight) at four-week intervals from turnout to housing. The different levels of pasture contamination were verified by conducting tracer counts with pairs of parasite-naive calves that were allowed to graze alongside those experimental animals in the year prior to the present study (data not shown).
Animals
The present study included 27 suckler cows with single heifer calves obtained from a commercial farm. The cows were mainly pure-bred Simmental cattle (16), but there were also eight crossbreeds of 50 per cent Simmental and 50 per cent Angus, and three purebred Angus. Sire breed of the calves was Angus (17), Limousin (8) or Simmental (2), and the birth date of the calves ranged from December 15 to April 5. During the previous grazing season, the cows had grazed permanent seminatural pastures, and during the indoor period they had been kept in the same free-stall house and fed grass/clover silage ad libitum. All calves were naive grazers at the start of the experiment. Both before and during the experiment, water, salt and minerals were supplemented to the animals.
At turnout to pasture, cow-calf pairs were randomly allocated in blocks of four, based on their age and breed, to one of the four experimental groups in four different enclosures A-D (Table 1). Within each calving season group, cow-calf pairs were allocated into one of the two levels of overwintering residual parasite infections, with as similar as possible breed composition in the high and low groups. No prophylactic anthelminthic treatments were given. During early summer, a breeding bull accompanied each of the cow-calf groups.
TABLE 1:
Description of the four enclosures of seminatural grassland used (A-D) for the treatments with high or low overwintered parasite infection of pasture and calving early (15 Dec–2 Feb) or late (11 Feb–5 Apr) in the year
| Enclosure | A | B | C | D |
|---|---|---|---|---|
| Treatments | ||||
| Overwintering contamination | High | High | Low | Low |
| Calving season | Early | Late | Early | Late |
| Animals | ||||
| Cow-calf pairs (n) | 8 | 8 | 6 | 5 |
| Initial cow weight (kg) | 739 (70) | 697 (86) | 733 (118) | 725 (107) |
| Initial calf weight (kg) | 150 (29) | 88 (16) | 137 (32) | 87 (15) |
| Pre-exp. calf weight gain (kg day−1) | 1.01 (0.15) | 0.86 (0.21) | 0.85 (0.16) | 0.91 (0.35) |
| Pasture | ||||
| Acreage (ha) | 9 | 10 | 9 | 7 |
| Dry matter (%) | 28 | 27 | 29 | 30 |
| Crude protein (g kg DM−1) | 116 | 113 | 118 | 109 |
| Neutral detergent fibre (g kg DM−1) | 511 | 540 | 536 | 523 |
| Metabolisable energy (g kg DM−1) | 9.6 | 9.8 | 9.6 | 10.0 |
The table also shows number and average initial body weight of the animals and pre-experimental live weight gain of the calves (sd); pasture acreage, dry matter content and chemical composition of herbage
The Ethical Committee on Animal Experiments in Gothenburg approved the protocol and execution of this study (registration number 78-2011).
Weighing, sampling and parasitological examinations
All animals were weighed at turnout and weaning. In addition, calves were weighed every four weeks during the grazing period, and their daily weight gain (DWG) was calculated. The body condition score (BCS) of the cows was established at turnout and weaning, according to Edmonson and others (1989), and was found to range from 1 (thin) to 5 (fat) (max difference 0.5).
Rectal faecal samples were collected at turnout and then at four-week intervals until housing. The faecal samples were used for quantitative analysis of gastrointestinal nematode eggs per gram faeces (epg), according to a modified McMaster technique based on 5 g of faeces, and using saturated salt as the flotation medium with a minimum detection level of 20 epg.
Every four weeks, 2×5 ml blood samples were taken from the coccygeal vein or artery of all animals, using tubes equipped with a cannula (Vacutainer, Becton Dickinson). Serum was separated to determine the pepsinogen concentration (SPC) according to a micromethod (Dorny and Vercruysse 1998, Charlier and others 2011), as a measure of parasitic lesions on the mucous membrane in the abomasum. The antibody levels to Ostertagia ostertagi were measured using the SVANOVIR Ostertagia-Ab ELISA kit (Svanova Biotech, Uppsala, Sweden), which is based on a crude adult worm capture antigen. The optical density (OD) of each sample was expressed as a ratio calculated according to the equation:
where NC and PC are the ODs of a negative and a positive test control sample included on each plate.
Pasture
The pasture was divided into four enclosures, A-D, one for each experimental group. When dividing the animals, similar stocking rates among the pasture enclosures were achieved, resulting in 0.8 (sd, 0.1) cow-calf pairs ha−1. All enclosures consisted of approximately 20 per cent dry, 60 per cent mesic and 20 per cent wet areas. The pasture was mainly open, but included small areas of mixed deciduous trees. In general, the dominant plant species were Deschampsia cespitosa (tufted hairgrass) and Festuca rubra (red fescue). In dry areas, Festuca ovina (sheep's fescue), Deschampsia flexuosa (wavy hairgrass), Nardus stricta (matgrass) and several herb species were abundant. Besides D cespitosa and F rubra, other herbs were prevalent in mesic areas, while D cespitosa and Cyperaceae (sedges/rushes) were dominant in wet areas.
Sward height and chemical composition of herbage were measured every four weeks from turnout to weaning, to further ensure similar conditions in the four enclosures. In each enclosure, sward height measurement followed a W-shaped route according to Frame (1993) with 42–53 recordings performed with a rising plate metre (0.3×0.3 m, weight 430 g). To estimate chemical composition, 12–15 herbage samples were hand picked in 3–m diameter circles along the route. For each enclosure, samples were pooled over the experiment and analysed for concentrations of dry matter (DM), crude protein (CP), neutral detergent fibre (NDF) and in vitro organic matter digestibility (Lindgren 1979). The DM concentration was determined at 105°C for 24 hours, CP was determined in a Tecator Kjeltec Auto Sampler 1035 Analyser (Tecator, Höganäs, Sweden) and NDF was determined according to Goering and van Soest (1970). Metabolisable energy concentration was calculated from in vitro organic matter digestibility (Lindgren 1979).
Statistical analyses
Data were summarised and, if necessary normalised (log-transformed), and then exported to software for statistical analyses and graphical illustrations in both JMP version 6.00 (SAS Institute, Cary, North Carolina, USA) and GraphPad Prism version 4.0c (San Diego, California, USA). Mean values of FEC, SPC, Ostertagia optical density ratio (ODR) and DWG of the calves were calculated and differences in the temporal trends of the four response variables were tested separately for cows and suckler calves (before and/or after normalisation of the data if necessary). These calculations were carried out in relation to early versus late calving and low versus high residual pasture contamination with parasite larvae in the fit-model platform of JMP. A random-effects split-plot repeated measures analysis of variance design was used, with one between-subject factor (calving season) and two within-subject factors (residual pasture contamination and sampling date). The significance level was set to P<0.05.
Results
Nematode egg counts
The FEC showed a highly significant (P<0.0001) seasonal pattern, with the highest values observed in calves after they had been on pasture for eight weeks. In the calves from groups A-D (see Table 1), the average cumulative results of FECs at housing were 311, 234, 128 and 124 epg, respectively. Although the highest numerical values were observed in those calves from the enclosures with the high overwintering contamination of larvae (ie, groups A and B), differences were not significant.
Low numbers of parasite eggs were observed on every sampling occasion in faeces from the dams. However, unlike the situation for the calves, there was no seasonal pattern or any other difference between the groups. In the cows from group A (high-early), average FEC ranged from 4 to 14 epg on the different sampling occasions, in group B (high-late) from 8 to 21 epg, in group C (low-early) from 3 to 17 epg and in group D (low-late) from 6 to 40 epg.
Pepsinogen
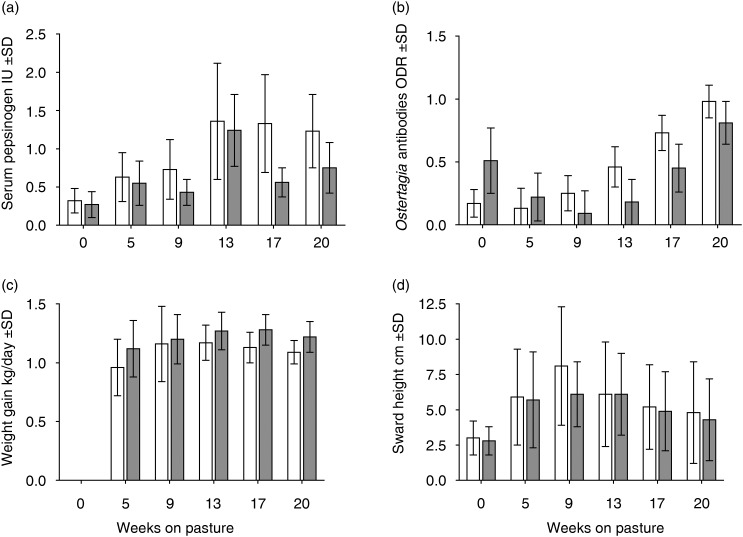
Measurements of SPC in the calves revealed a similar and highly significant (P<0.0001) seasonal pattern in all groups (Fig 1a), with gradually increasing levels towards the middle of the grazing season. However, there was also a highly significant effect of calving season (P<0.0001), with slightly higher concentrations in early calves than in late. The average SPC in early calves (groups A and C) ranged from 0.32 to 1.36 IU tyrosin, whereas in late calves (groups C and D) it varied from 0.27 to 1.24 IU tyrosin. Although average SPC varied from 0.36 to 1.89 IU tyrosin in the cows, and was thus much higher than in calves, no statistically significant differences were found between the groups.
FIG 1.
(a) Mean level of serum pepsinogen, (b) Ostertagia antibody optical density ratio (ODR), (c) daily weight gain determined from 0 to 20 weeks in early born calves (open bars) and late-born calves (filled bars) on pasture and (d) pasture sward height during the same period (sd)
Optical density ratio
As for FEC and SPC in the calves, seasonal fluctuations were observed in the Ostertagia antibody levels, with highly significant differences (P<0.0001) depending on the number of weeks the animals had been on pasture. However, in contrast with FEC and SPC, there was also a highly significant interaction (P<0.0001) between sampling occasion and calving season, with antibody levels behaving differently depending on when the calves were born. The antibody levels in the calf sera based on the results from the SVANOVIR ELISA are shown in Fig 1b. The ODR gradually increased on average from 0.13 to 0.98 in early calves, whereas it initially decreased from 0.51 and then increased again to 0.81 in late calves. By contrast, no seasonal trends or significant differences between groups were observed in the cows. They also had much higher antibody levels (ODR 0.92-1.03), irrespective of when they were sampled.
Weight gain and BCS
The mean DWG of the suckler calves is shown in Fig 1c. The average DWG from turnout to housing in the early calves (groups A and C) ranged between 1.00 and 1.17 kg, whereas in late calves (groups B and D) it ranged between 1.16 and 1.25 kg. Testing of random effects showed significant differences for calving season (P=0.0005) when nested with overwintering pasture contamination (P=0.0005), but not in relation to the number of weeks on pasture. From turnout to weaning, the average weight change in the cows varied from −2.8 to +6.4 kg across groups A-D, whereas the change in BCS varied from 0.0 to 0.6. No differences between the groups were found.
Discussion
Nematode egg shedding patterns and exposure to GIN were examined in four grazing groups of suckler cows with early or late calves, turned out on pastures with a high or low level of residual contamination resulting from overwintering infective larvae from the previous grazing season. Interestingly, we found a significant influence due to calving date, while the contribution of the level of residual contamination of overwintering larvae was insignificant. The most likely explanation for the lack of effect of residual contamination level is that the dams excreted low levels of nematode eggs from turnout and throughout the grazing season, irrespective of the residual contamination present on the pasture at turnout. According to both the ODR and SPC data, early calves were more exposed to GIN and also had significantly lower DWG. This is interesting, as there is currently a tendency for early calving (Dec–Feb) in Swedish beef herds. No significant differences were observed between the cows in terms of FEC, SPC or ODR values.
In previous studies, epg levels in dairy cattle have been reported to be much lower in cows than in calves. For example, in The Netherlands (Borgsteede and others 2000) and Belgium (Agneessens and others 2000), FEC in dairy cows is, on average, 5–10 epg. Experiences from cows in beef herds are more limited, but where data are available, epg are generally low (Bairden and Armour 1981, Forbes and others 2002), but comparable with those reported in dairy cows. In the present study, the FEC results ranged from 10 to 25 epg and, surprisingly, the level was similar in all calves, suggesting moderate infection levels in all groups. This was probably related to the cows in all groups shedding parasite eggs onto pasture, and these quickly transforming into larvae, creating pasture infectivity.
The anti-Ostertagia antibody levels in this study were more than twice as high in the cows (ODR 0.98±0.20) as in the calves (0.42±0.33), but cows did not exhibit the seasonal differences seen in calves. In a study using the same ELISA test in dairy cows, it has been shown that an ODR≥0.8 reflects heavy parasite exposure, which is associated with decreased milk yield (Forbes and others 2008). This may not be the case in beef cows, but the antibody levels observed here were similar to, or somewhat higher than, those reported earlier in dairy cows in Sweden (Höglund and others 2010) and elsewhere in northern Europe (Agneessens and others 2000, Borgsteede and others 2000, Forbes and others 2008). A very interesting result in the present study was the seasonal variation in antibody levels in the calves, with relatively high levels in the late calves (average ODR 0.51±0.26) at turnout, but subsequently declining and then increasing again in the late grazing period. The most feasible explanation is that the higher levels observed at the very beginning of the study reflected passive transfer of maternal antibodies via intake of colostrum by the calves. To our knowledge, this is a novel finding for nematode infections in cattle, but it has been reported for other helminths such as the trematode Fasciola hepatica (Mezo and others 2010).
It is well known that pepsinogen concentration (SPC) reflects damage to the abomasal mucosa caused by the late larval stages of O ostertagia. Thus, SPC is a reliable diagnostic marker, but mainly in first-season grazing cattle (Charlier and others 2011). In general, low levels were observed in the present study, with only one sample having a SPC above the threshold indicating subclinical damage (Dorny and Vercruysse 1998). However, the average SPC was slightly higher in the early than in the late calf group, indicating somewhat higher parasite exposure in the former.
Daily weight gain was approximately 100 g lower in early calves. The possibility cannot be excluded that this reflected slightly higher parasite exposure than in the late group. However, information about the effects of subclinical parasitism in spring-born beef suckler calves is lacking, and thus, there are few data with which to compare. According to Forbes and others (2002), who conducted a three-year study in four beef herds in southern England where suckling calves were excreting comparable numbers of eggs to those observed in the present study, the average DWG was about 100 g higher in calves treated with a long-acting anthelmintic in mid-summer. Although the parasitological analyses in the present study point in the same direction, it must be acknowledged that DWG in early and late-born calves may differ due to factors other than parasites. For example, when formulating diets for ad libitum feeding (Spörndly 2003), growing cattle weighing between 200 and 300 kg are expected to have higher DWG when those weighing between 100 and 200 kg. This indicates that for natural reasons, our early calves would have been able to attain higher DWG than the late calves. Still, both groups were dependent on the milk production of their dams based on a low-nutrient herbage with a rather low sward height, and the early calves had a higher total nutrient requirement for maintenance and production at a specific DWG (Spörndly 2003). Furthermore, the milk yield in the early calving cows might have been lower than in the late-calving cows, due to their later stage of lactation at turnout (Manninen 2007). Taken together, the early calves might not have been able to exploit their growth potential just by intake of milk, suggesting that the lower DWG is not necessarily due to parasites at all. In any case, we cannot neglect these factors other than parasites.
In conclusion, despite relatively low epg but with faeces excretion rates of up to 30 kg per animal and day, the cows in the present experiment were able to contaminate the pastures in such an efficient manner that it affected the calves. Suckling by later-born calves appeared to partly protect this group, and they were also less exposed to parasites because they ingested less grass than the early born calves, especially at the beginning of season. Higher ODR and SPC levels in the early born group confirmed this. However, the epg distribution followed a similar pattern in both groups, and was comparable with previous findings. By contrast, DWG was lower in early born calves than in those born later, for reasons that are still unclear and warrant further investigation. Overall, it seems that egg excretion from cows plays a larger role in cow-calf transmission than previously thought. Therefore, the focus should perhaps be on deworming the cows around turnout in order to control parasites even in the growing calves. This should be examined in further studies with early born suckler calves on pasture.
Acknowledgments
The Swedish University of Agricultural Sciences, Agroväst and the 7th framework program of the EU (GLOWORM, Project FP7-KBBE-2012-288975) funded the study. We thank Stenhammars Gods AB for kindly placing the animals at our disposal, Jonas Dahl, David Johansson and Karin Wallin at Götala Research Station for carrying out the daily work with the animals, and Sofia Sollenberg and Annie Engström at BVF for technical assistance with the parasitological analyses.
Footnotes
Provenance: not commissioned; externally peer reviewed
Correction notice: This paper has been corrected since it was published Online First. The figure 1 legend has been corrected to show that the filled bars on the graphs represent late-born calves, and open bars represent early born calves.
Open Access: This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
References
- 1.Agneessens J., Claerebout E., Dorny P., Borgsteede F. H. M., Vercruysse J. (2000) Nematode parasitism in adult dairy cows in Belgium. Veterinary Parasitology 90, 83–92 [DOI] [PubMed] [Google Scholar]
- 2.ANONYMOUS (2011) Seasonal Distribution of Calvings (percent) 2010. Cattle statistics 2011. Swedish Dairy Association p 29 [Google Scholar]
- 3.ANONYMOUS (2012a) European forum on nature conservation and pastoralism. www.efncp.org/. Accessed 07 03, 2012 [Google Scholar]
- 4.ANONYMOUS (2012b) The world of organic agriculture 2012. Statistics and emerging trends 2012. www.organic-world.net/index.html. Accessed 07 03, 2012 [Google Scholar]
- 5.Armour J. (1989) The influence of host immunity on the epidemiology of trichostrongyle infections in cattle. Veterinary Parasitology 32, 5–19 [DOI] [PubMed] [Google Scholar]
- 6.Bairden K., Armour J. (1981) A survey of abomasal parasitism in dairy and beef cows in southwest Scotland. Veterinary Record 109, 153–155 [DOI] [PubMed] [Google Scholar]
- 7.Barger I. (1993) Influence of sex and reproductive status on susceptibility of ruminants to nematode parasitism. International Journal of Parasitology 23, 463–469 [DOI] [PubMed] [Google Scholar]
- 8.Borgsteede F. H. M., Tibbena J., Cornelissen J. B. W. J., Agneessens J., Gaasenbeek C. P. H. (2000) Nematode parasites of adult dairy cattle in the Netherlands. Veterinary Parasitology 89, 287–296 [DOI] [PubMed] [Google Scholar]
- 9.Charlier J., Dorny P., Levecke B., Demeler J., Von samson-himmelstjerna G., Höglund J., Vercruyssem J. (2011) Serum pepsinogen levels to monitor gastrointestinal nematode infections in cattle revisited. Research in Veterinary Science 90, 451–456 [DOI] [PubMed] [Google Scholar]
- 10.Deblitz C. (2011) Beef and Sheep Report Understanding Agriculture Worldwide. Agri benchmark. Braunschwieg, German: Johann Heinrich von Thünen-Institut; 41 [Google Scholar]
- 11.Dorny P., Vercruysse J. (1998) Evaluation of a micro method for the routine determination of serum pepsinogen in cattle. Research in Veterinary Science 65, 259–262 [DOI] [PubMed] [Google Scholar]
- 12.Edmonson A. J., Lean I. J., Weaver L. D., Farver T., Webster G. (1989) A body condition scoring chart for Holstein dairy cows. Journal of Dairy Science 72, 68–78 [Google Scholar]
- 13.Eysker M., Boersema J. H., Kooyman F. N., Ploeger H. W. (2000) Resilience of second year grazing cattle to parasitic gastroenteritis following negligible to moderate exposure to gastrointestinal nematode infections in their first year. Veterinary Parasitology 89, 37–50 [DOI] [PubMed] [Google Scholar]
- 14.Forbes A. B., Cutler K. L., Rice B. J. (2002) Sub-clinical parasitism in spring-born, beef suckler calves: epidemiology and impact on growth performance during the first grazing season. Veterinary Parasitology 104, 339–344 [DOI] [PubMed] [Google Scholar]
- 15.Forbes A. B., Vercruysse J., Charlier J. (2008) A survey of the exposure to Ostertagia ostertagi in dairy cow herds in Europe through the measurement of antibodies in milk samples from the bulk tank. Veterinary Parasitology 20, 100–107 [DOI] [PubMed] [Google Scholar]
- 16.Frame J. (1993) Herbage mass. In Sward Measurement Handbook. Eds Davis A., Baker R. D., Grant S. A., Laidlaw A. S. British Grassland Society; pp 39–67 [Google Scholar]
- 17.Goering H. K., Van soest P. J. (1970) Forage Fibre Analysis (apparatus, reagents, procedures and some applications). Agricultural Handbook No. 379. USDA Department pp 20 [Google Scholar]
- 18.Hertzberg H., Figi R., Noto F., Heckendorn F. (2004) Control of gastrointestinal nematodes in organic beef cattle through grazing management. Proceedings of the 2nd SAFO Workshop: Organic livestock farming: potential and limitations of husbandry practice to secure animal health and welfare and food quality. Eds Hovi M, et al. Witzenhausen, Germany, March 25 to 27 2004 pp 129–135 [Google Scholar]
- 19.Höglund J., Dahlström F., Engström A., Hessle A., Jakubek E. B., Schnieder T., Strube C., Sollenberg S. (2010). Antibodies to major pasture borne helminth infections in bulk-tank milk samples from organic an nearby conventional dairy herds in south-central Sweden. Veterinary Parasitology 171, 293–29 [DOI] [PubMed] [Google Scholar]
- 20.Höglund J., Morrison D. A., Charlier J., Dimander S. O., Larsson A. (2009) Assessing the feasibility of targeted selective treatments for gastrointestinal nematodes in first-season grazing cattle based on mid-season daily weight gains. Veterinary Parasitology 164, 80–86 [DOI] [PubMed] [Google Scholar]
- 21.Höglund J., Svensson C., Hessle A. (2001) A field study on the status of internal parasites in calves on organic dairy farms in south-western Sweden. Veterinary Parasitology 99, 113–128 [DOI] [PubMed] [Google Scholar]
- 22.Lindgren E. (1979) The nutritional value of roughages determined in vivo and by laboratory methods. Report No. 45. Uppsala, Sweden (in Swedish): Department of Animal Nutrition, Swedish University of Agricultural Sciences [Google Scholar]
- 23.Luoto M., Rekolainen S., Aaukula J., Pykalä J. (2003) Loss of plant species richness and habitat connectivity in grasslands associated with agricultural change in Finland. Ambio 32, 447–452 [DOI] [PubMed] [Google Scholar]
- 24.Manninen M. (2007) Winter feeding strategies for suckler cows in cold climatic conditions. Doctoral thesis, publication N:o 91 Finland: Department of Animal Science, University of Helsinki, pp 60–68 [Google Scholar]
- 25.Mezo M., Gonzá lez–Warleta M., Castro-Hermida J. A., Carro C., Ubeira F. M. (2010) Kinetics of anti-Fasciola IgG antibodies in serum and milk from dairy cows during lactation, and in serum from calves after feeding colostrum from infected dams. Veterinary Parasitology 168, 36–44 [DOI] [PubMed] [Google Scholar]
- 26.Salevid P., Kumm K. -I. (2011) Searching for economically sustainable Swedish beef production systems after decoupling of EU-income support. Outlook on Agriculture 40, 131–138 [Google Scholar]
- 27.Slocombe J. O., Curtis R. A. (1989) Aspects of the epidemiology of nematode infections in a cow-calf herd in Ontario. Canadian Journal of Veterinary Research 53, 336–339 [PMC free article] [PubMed] [Google Scholar]
- 28.Spörndly R. (2003) Fodertabeller för idisslare 2003. Report No. 257. Uppsala, Sweden (in Swedish): Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences [Google Scholar]
- 29.Vercruysse J., Claerebout E. (2001) Treatment vs non-treatment of helminth infections in cattle: defining the threshold. Veterinary Parasitology 12, 195–214 [DOI] [PubMed] [Google Scholar]
- 30.Viring S., Bengtsson B., Christensson D. (2001) Förekomst av mag-tarmparasiter och lungmask hos dikalvar (Occurence of gastro-intestinal parasites and lungworm in suckler calves). Svensk Veterinärtidning 53, 441–445 (in Swedish with an English summary) [Google Scholar]