Abstract
Wnt pathways play an important role in pre-implantation embryo development, blastocyst implantation, and post-implantation uterine decidualisation. However, little is known about the potential role that Wnt signaling plays in patients with unexplained recurrent spontaneous miscarriage (URSM), and no single biomarker with a high predictive value of maternally caused URSM has been identified. We aim to study the molecular mechanisms by which the Wnt pathway controls the progression of early pregnancy by investigating the expression of Dickkopf-1 (DKK1), one of the Wnt agonists, in URSM patients. Plasma and fresh decidual tissues samples were collected from 59 subjects (29 patients with URSM and 30 patients with normal, early pregnancy). Time-resolved immunofluorometric assay system and quantitative real-time RT-PCR were used to determine the serum levels of DKK1 and DKK1 mRNA in the deciduas, respectively. Western blot and immunohistochemistry were used to measure DKK1 protein levels in the deciduas. Serum DKK1 levels were significantly higher in URSM patients compared to the control group (P < 0·001); the expression of DKK1 mRNA and protein in URSM patients were higher relative to healthy controls (P = 0·013). Glandular epithelium from decidual tissues demonstrated cytoplasmic signals for DKK1 in URSM patients, and DKK1 did not stain in healthy controls. Furthermore, serum DKK1 levels significantly correlated with those in the decidual tissues. Our study suggests that DKK1 may be a valuable biomarker of URSM; it can be reliably and conveniently detected in serum, thus obviating the need for decidual tissue analysis.
Keywords: Dickkopf-1, recurrent spontaneous miscarriage, wnt signaling pathway
Introduction
Recurrent spontaneous miscarriage (RSM), defined as two or more consecutive pregnancy losses before the 20th week of gestation, occurs in 1%–5% women of reproductive age, and has a negative effect on human reproductive health 1. The aetiology of RSM remains partially unknown, although chromosomal, anatomic, endocrinological, infectious, and auto-immunologic abnormalities have been implicated 2. Almost 50% of such cases, called unexplained recurrent spontaneous miscarriage (URSM), remain classified as ‘of unknown aetiology’, and usually occur during the first trimester 3. Although numerous signalling factors and pathways have been found to be important for a successful early pregnancy, the molecular basis of reciprocal interactions between the mother and foetus during pregnancy still remains largely unknown. Recent analyses regarding human endometrial gene expression within the window of implantation demonstrate the expression and regulation of select members of the Wnt signalling pathway, raising the question regarding the function of this pathway during human reproduction and suggest that it may be involved in this molecular network 4,5.
The Wnt signalling pathway is one of a handful of evolutionarily conserved signal transduction pathways used extensively during animal development, from Hydra to humans 6,7, and controls multiple aspects of development, including cell proliferation, fate specification, polarity, and migration. Wnt signals are transduced in at least two distinct ways: a well-established canonical or Wnt/β-catenin pathway, and a non-canonical β-catenin independent pathway or pathways 8. β-Catenin is an intracellular mediator of canonical Wnt signalling, which is phosphorylated and stabilized because of activation by Wnt ligand, and subsequently transported to the nucleus, where, as part of a transcriptional complex, it regulates gene expression. The Wnt signal is extracellularly regulated by two classes of antagonists. The first group consists of Wnt ligand-binding proteins, including Wnt-inhibitory factor (WIF-1), the secreted frizzled-related protein (sFRP) family, and cerberus. Dickkopf (DKK) family of proteins belong to the second group 9, and DKK1, the most well-characterised member of the family, promotes the internalisation of LRP5/6, making it unavailable for Wnt reception by forming a triplet complex with LRP5/6 and Kremens 10,11.
Since the intricate interplay between the embryo and uterus during implantation shares similar features with the reciprocal cell-cell communication involving Wnt signaling occurring during organogenesis, the same pathway may drive embryogenesis. Chen et al. 12 have briefly summarised recent progress on the patho-physiological significance of differential Wnt pathways during pre-implantation embryonic development, blastocyst implantation, and post-implantation uterine decidualisation.
To date, however, little is known about the potential role played by Wnt signaling in URSM patients, and no single biomarker with a high predictive value of maternally caused URSM has been identified. Non-genetic biomarkers in URSM may reflect conditions in the gravid uterus, and we rarely know whether they are the causes or consequences of miscarriage. Studies of specific biomarkers are probably the best way to reveal the pathogenesis underlying URSM 13. In this study, we examined DKK1 expression in both normal-pregnancy women and in women with URSM. Our results indicate abnormal expression of DKK1 in women with USRM, which suggests that interference with the Wnt signalling pathway may results in URSM.
Materials and methods
Patients
All participants enrolled were outpatients at the Department of Gynaecology, Shanghai First Maternity and Infant Hospital. Twenty-nine patients who had experienced at least two consecutive first-trimester losses (9·40 ± 2·25 weeks of gestation) of unexplained aetiology, with an age (mean ± standard deviation) of 31·13 ± 1·07 years (age range, 26–38 years) were recruited as participants. ‘Unexplained’ RSM was diagnosed as per previously described guidelines, to exclude any verifiable causes 14.
For a relevant control group, we selected 30 healthy women who were undergoing elective termination of apparently normal pregnancies. The elective terminations were conducted during the first trimester (9·23 ± 1·89 weeks of gestation). All these women had at least one living child, and no history of spontaneous miscarriage, ectopic pregnancy, preterm delivery, or stillbirth. In all 30 cases, foetal heart activity had been identified within two weeks of sample collection.
The fresh decidual tissues collected from all patients under aseptic conditions, after receiving their consent, was immediately delivered to the laboratory.
This study was approved by the ethics review board of Shanghai First Maternity and Infant Hospital, and informed consent was obtained after the study was explained in detail to all the participants.
Serum DKK1 and progesterone measurement
Serum levels of DKK1 were measured by time-resolved immunofluorometric assay system 15. A monoclonal anti-DKK1 antibody and an anti-DKK1 antibody labelled with Eu3+ as a tracer were used to develop a non-competitive ‘sandwich-type’ assay. Fluorescence can be measured using a time-resolved fluorometer after the immunoreaction. The sensitivity of the assay was 0·7 μg/l. Accuracy studies, specificity, parallelism, and precision data were found to be satisfactory. As progesterone plays a pivotal role in ovulation, implantation, and the establishment and maintenance of pregnancy, we measured progesterone levels in the same set of serum samples from all subjects. Serum progesterone was detected using electrochemiluminescent assays by using Beckman Coulter, Inc., reagent sets and UniCel DxI 800 Immunoassay System.
Quantitative real-time RT-PCR
Total RNA from patient decidual tissue samples was prepared using Rneasy Mini or Micro Kits (QIAGEN, Valencia, USA) according to the manufacturer's instructions. Total RNA was quantified with a BioPhotometer plus (Eppendorf AG, Hamburg, Germany), and 2 μg of each sample was reverse transcribed using Random Hexamer Primers (Promega Corporation, Madison, WI, USA) and M-MLV Reverse Transcriptase (Promega Corporation) in a final volume of 25 μl. Subsequently, cDNA samples were used as PCR templates, along with specific primers specifically designed for real-time PCR.
For quantitative real-time RT-PCR, amplification was performed in LightCyclert system (Roche, Mannheim, Germany), and visualized with SYBR Green Kit (Quantitectt SYBR Green PCR, QIAGEN). Standard curves for each gene were established to quantify the copy number for each sample, and expression was considered only when the sample Ct was within the limit of each standard curve. Following primer sequences were used for real-time PCR: β-actin, 5': AAGGCCAACCGCGAG; 3': TAATGTCACGCACGATTCCCG; DKK1 5': CTCGGTTCTCAATTCCAACG; 3': GCACTCCTCGTCCTCTG. The cycling conditions were 15 min at 95°C, 40 cycles of 15 s at 94°C, 30 s at 55°C, 30 s at 72°C and 5 s at 82°C (β-actin) or 84°C (DKK1). Gene expression was presented as the ratio between the target gene and β-actin. Each experiment was done in triplicate and the mean value was calculated.
Western blotting
Western blotting using total tissue lysates was performed as previously described 16. Briefly, we subjected 30 μg of crude decidual tissue protein extract to 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and then transferred the proteins to a polyvinylidenedifluoride (PVDF) membrane (Bio-Rad, USA). Membranes were incubated overnight at 4°C in 1:1000 dilution of anti-human DKK1 antibody (Perkin Elmer, USA) or 1:1000 dilution of anti-β-actin antibody (Perkin Elmer, USA) then for 3 h with the corresponding secondary antibody. We visually estimated the band intensities for both proteins.
Immunohistochemistry
To detect DKK1 protein in decidual tissue samples embedded in paraffin blocks, we stained the sections as previously described 16. Briefly, 3·3 μg/ml of rabbit polyclonal anti-hDKK1 antibody (Perkin Elmer, USA) was added to each slide after blocking endogenous peroxidase and proteins, and the sections were incubated with HRP-labelled Anti-Rabbit IgG (Perkin Elmer, USA) as the secondary antibody. Substrate chromogen was added, and the specimens were counterstained with haematoxylin. Normal rabbit IgG was used as a substitute for the primary antibody as negative controls. All sections were observed and photographed with an Axioskop 2 microscope (Carl Zeiss, Oberkochen, Germany). Quantitative analysis of immunohistochemistry was done as follows: 1, weak; 2, moderate; and 3, strong. Positive cells were quantified as a percentage of the total number of decidual cells, and were assigned to one of the five categories: 0, <5%; 1, 5%–25%; 2, 26%–50%; 3, 51%–75%; and 4, >75%. The percentage of positive decidual cells and the staining intensity were then multiplied to generate the immunoreactive score (IS) for each decidual specimen. A sample was defined as DKK1+ if it had an IS of ≥1. An IS value of ≥2 was regarded as high level of expression. Two independent pathologists (Liu J and Huang WM) were involved in assessing protein expression.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). The data regarding serum DKK1 levels are presented as the mean ± standard deviation values unpaired student t-tests and the rank-sum test (Wilcoxon) were used to evaluate differences among unpaired observations within the two groups. A P-value of <0·05 was considered significant.
Results
Patient characteristics
Twenty-nine patients with URSM were recruited in the study and divided into two groups: 17 patients with two occurrences of miscarriages, and 12 patients with more than two occurrences of miscarriages. Thirty healthy women undergoing elective termination of apparently normal pregnancies were selected to form the control group (age, 30·0 ± 8·59; range, 25–36 years). No significant differences were observed in the age and gestational period between the two groups (Table 1).
Table 1.
Characteristics of URSM and control subjects (mean + SD and range of values)
| Group | n | Age (year) | No. of miscarriage | Gestational age(weeks) |
|---|---|---|---|---|
| URSM | 29 | 31·13 ± 1·07 (26–38) | 4·47 ± 0·21 (2–8) | 9·40 ± 2·25 (7–12) |
| Control | 30 | 30·0 ± 8·59 (25–36) | 0 | 9·23 ± 1·89 (7–12) |
URSM versus Control: Age, P = 0·14; Gestational age, P = 0·62.
Comparison of serum DKK1 and progesterone levels in URSM patients and control group
Serum DKK1 levels had a normal distribution in control subjects with a mean value of 11·98 μg/l, and they ranged from 5·43 to 18·5 μg/l. The 95th percentile of DKK1 levels for the control group, i.e. 14·43 μg/l, was used as the cut-off value for differentiating between patients and controls. For USRM patients, the mean value was 24·99 μg/l, and the range was 15·39–34·59 μg/l. Serum DKK1 levels were greater (P < 0·005; Fig. 1a) for USRM patients. Moreover, no significant difference were observed in the serum DKK1 levels between women with two or more than two incidences of USRM (P = 0·45; Fig. 1b). We found no significant differences between the serum progesterone levels in the USRM and the control subjects (P = 0·36, Fig. 1c). Moreover, no correlations were observed between the levels of DKK1 and progesterone, with a Pearson's coefficient of 0·05 (P = 0·699, Fig. 1d).
Fig. 1.
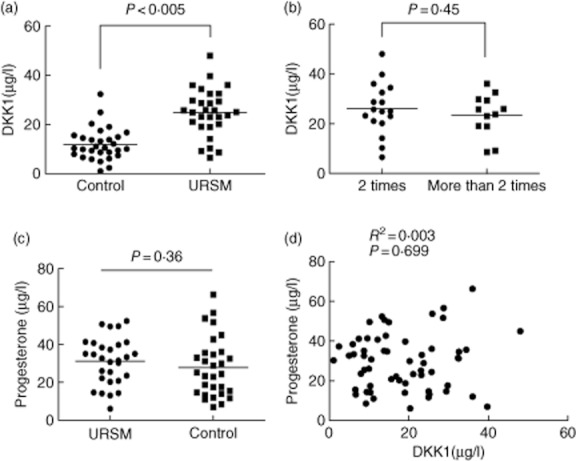
A, DKK1 levels in serum samples from patients with URSM and healthy pregnancies determined by using time-resolved immunofluorometric assay system. B, Point plot of serum DKK1 levels in URSM patients with two or more occurrences of miscarriages. C, Serum progesterone levels in URSM patients and control subjects. No difference was noted in the serum progesterone levels between the two groups. D, Correlation between serum DKK1 and progesterone levels in patients with URSM and control subjects.
Clinical significance of serum DKK1 as a serologic biomarker for URSM
The utility of serum DKK1 levels in the detection of URSM was evaluated using receiver-operator characteristic curve (ROC) analysis. At a cut-off level of 14·43 μg/l, the positive rate in URSM was 82·76% (24/29) and 30% (9/30) in the control. The sensitivity and specificity of serum DKK1 for URSM were 82·76% and 73·33%, respectively. The area under the ROC curves was 0·8609.
DKK1 expression in decidual tissues
To investigate whether high serum DKK1 levels coincided with DKK1 expression in the decidual tissues, 32 frozen tissues (18 from URSM group and 14 from control group) were randomly chosen from 59 cases for analysis by quantitative real-time RT-PCR. As shown in Fig. 2a, DKK1 mRNA expression in URSM patients was higher than that in the control group (P = 0·013). Significant correlations were noted between DKK1 mRNA expression in individual decidual tissue samples and DKK1 concentrations in serum samples (P < 0·001, Fig. 2b). Using Western bloting analysis, we confirmed that DKK1 protein expression in URSM patients was higher than that in the control group (P < 0·001, Fig. 2c and d). Among 59 study subjects, high expression of DKK1 protein was significantly correlated with the serum DKK1 levels (P < 0·001, Fig. 2e).
Fig. 2.
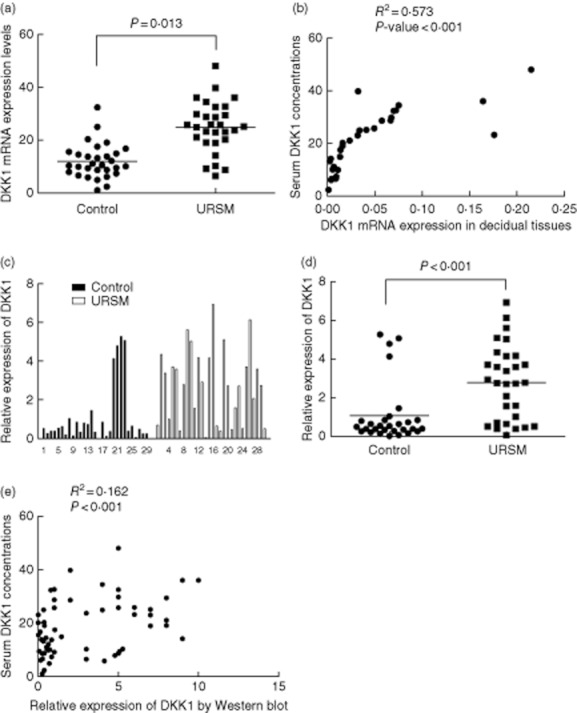
Expression of DKK1 in individual tissues from patients with URSM and healthy pregnancy. A, DKK1 mRNA expression as measured by quantitative real-time RT-PCR in patients with URSM and control group. B, Correlative analysis of DKK1 mRNA levels in individual decidual tissue samples and serum DKK1 levels by Spearman's correlation test (2-tailed). C, Each DKK1 intensity was divided by the corresponding intensity of β-actin from the same tissue sample to adjust for the sample variation. D, Increased DKK1 protein expression was present in URSM decidual tissues than the control. E, Among 59 study subjects, high expression of DKK1 protein was significantly correlated with the serum DKK1 levels.
Local expression of DKK1
To further characterise the identified markers and localise DKK1 in the decidual tissue sections, we examined its expression in several decidual tissue samples by immunohistochemistry using specific antibodies against DKK1. Negative controls without the primary antibody or with non-specific immunoglobulins had no signal. Decidual glandular epithelial tissues demonstrated cytoplasmic signals for DKK1 in URSM patients. However, DKK1 expression was lower, or even absent in the control deciduas. Two or more than two occurrences of miscarriages did not produce a significant difference (Fig. 3a). Overall, cytoplasmic DKK1 was present in 27 of 29 URSM cases (93·1%), and DKK1 cytoplasmic staining was present in 10 of 30 healthy pregenacies (33·3%). The intensity of staining and the representative tissues from all 59 cases are presented in Fig. 3b. The lack of DKK1 expression was more prevalent in the control group compared with the URSM group (Fig. 3c). A significant correlation was noted between elevated cytoplasmic expression of DKK1 in the decidual tissues and serum DKK1 levels (P < 0·001). Data were analysed using Spearman's correlation test (2-tailed).
Fig. 3.
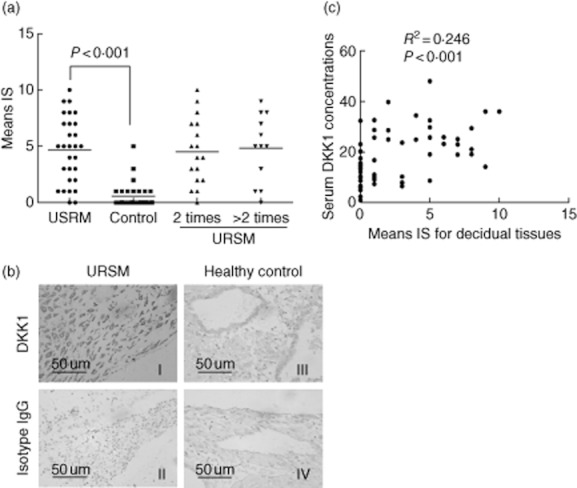
Immunohistochemical analysis of DKK1 in decidual tissues. A, The average immunoreactive score (IS) was markedly enhanced in URSM subjects compared with control group. However, no significant difference was noted between 2 and >2 occurrences of miscarriages. B, DKK1 expression in representative decidual tissues showing staining primarily in the cytoplasm of the glandular epithelium (original magnification, ×400). C, Significant correlations among DKK1 levels (immunoreactive scores) in individual decidual tissues and those in serum. Data were analysed by Spearman's correlation test (2-tailed).
Discussion
It has recently been clarified that secreted Wnt antagonists play important roles in regulating Wnt signalling 9. DKK proteins are secreted antagonists of the Wnt cell- signalling molecules that possess a novel mode of action. The DKK family consists of four main members in vertebrates (DKK1, 2, 3, 4), and DKK1, the founding member of the family, was originally identified as the embryonic head inducer and Wnt antagonist in Xenopus species 17. DKK1 binds to the LRP5/6 Wnt co-receptor and prevents the formation of active Wnt-Frizzled-LRP5/6 receptor complexes, thus blocking the canonical Wnt/β-catenin pathway 18,19.
In this study, we found that serum DKK1- levels increase in URSM, and this increase appears to be independent of instances of miscarriage, which suggests that the Wnt/β-catenin pathway may be down-regulated by the induction of DKK1 expression. Concurrently, the sensitivity and specificity of serum DKK1 for URSM were found to be 82·76% and 73·33%, repectively, by using ROC analysis; thus, DKK1 may be a useful indicator for URSM. However, future studies are necessary to identify the molecular mechanisms underlying DKK1 actions in URSM.
In mice, the expression of several Wnt genes fluctuates in adult mouse uteri during the oestrous cycle 20,21. In addition, some antagonists of the Wnt signalling pathway, such as sFRP family members, showed dynamic changes during this process 22,23. During the human endometrial menstrual cycle, although the expression of Wnt ligands, receptors, or downstream effectors does not vary significantly, dramatic changes in the expression of DKK1 and FrpHE(frizzled-related protein, an inhibitor of Wnt signalling) were evident 5,24. Some researchers suggested that DKK1 mRNA regulation is dependent on progesterone, and its expression increases during the early-secretory phase, peaks during the mid-secretory phrase, and decreases during the late-secretory phase 25. However, there is insufficient evidence of DKK1 staining during pregnancy. Our data showed that the expression of DKK1 mRNA and protein in the deciduas in URSM was higher relative to the control group. To investigate whether high serum DKK1 levels, coincides with DKK1 expression in the decidual tissues, we used Spearman correlation analysis. Significant correlations were noted between decidual DKK1 mRNA expression and serum concentrations.
Consistent with these findings, DKK1 showed positive immunostaining in the maternal decidual compartment in ‘unexplained’ URSM. A significant correlation was noted between elevated cytoplasmic expression decidual DKK1 and the serum DKK1 levels. However, evidence for DKK1 staining in the decidual tissue during pregnancy is inadequate. Our results indicate higher expression of DKK1 mRNA and protein in the decidua from the USRM patients compared to the control patients. These results are not in agreement with other findings 26 regarding progesterone-regulated DKK1 expression, which could be regulated by other factors, since there were no differences in the concentrations of progesterone in the serum samples of USRM and control subjects in the present study. Macdonald et al. 27 reported on DKK1 expression in normal early pregnancy which appears to be in contrast to our findings, maybe all participants in our study were ‘Unexplained’ RSM, and were different from patients having luteal phase defection (LPD), one of the major causes of RSM. Different molecular mechanism exists in RSM due to endocrinological disorder and ‘Unexplained’ RSM.
Moreover, some investigators have found that the activation of the canonical Wnt pathway was essential in regulatory T (Treg) homeostasis, and may play a key role in anergy induction in mature T cells 28.The vital role of Treg cell in maintaining self-tolerance is quite clear 29. Defects in Treg cell development lead to systemic autoimmunity and early death in both mice and humans. Many researchers suggest that CD4+CD25bright Treg cells might play a role in the maintaining pregnancy, and a decreased number of CD4+CD25bright Treg cells in URSM women may induce maternal lymphocyte activation in the foetal allograft 30. Thus, we speculate that increased DKK1 levels in the deciduas may affect paracrine as well as autocrine Wnt signalling by inhibiting the Wnt pathway, and thereby regulating signalling at a specific time in URSM patients by reducing the number of CD4+CD25bright Treg cells. However, further work is needed to explore this possibility.
Our study suggests that DKK1 may be a valuable biomarker of URSM; it can be reliably and conveniently detected in the serum, thus obviating the need for decidual tissue analysis. Our findings may shed light on a new mechanism that exists in URSM. However, the precise mechanisms of how DKK1 regulates decidual and trophoblast function, as well as the mechanisms underlying Wnt regulation and action during early pregnancy in humans, are not well understood. Our future studies will address in detail the molecular mechanisms by which the Wnt pathway controls the progression of early pregnancy.
Funding
This study was supported by research grants as follows: National Natural Science Foundation of China (No. 81100424), Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20100072120066), Natural Science Foundation of Shanghai (No. 11ZR1428800), Research Funds of Shanghai Health Bureau (No.2009153), and the Fundamental Research Funds for the Central Universities (No.1515-219-013).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90:S60. doi: 10.1016/j.fertnstert.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 2.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 3.Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–317. doi: 10.1016/j.molmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Carson DD, Lagow E, Thathiah A, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 5.Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 6.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 7.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis – a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 8.Eisenmann DM. Wnt signaling. WormBook. 2005;25:1–17. doi: 10.1895/wormbook.1.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 10.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signaling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 11.Rothbacher U, Lemaire P. Creme de la. Kremen of Wnt signalling inhibition. Nat Cell Biol. 2002;4:E172–173. doi: 10.1038/ncb0702-e172. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Zhang Y, Lu J, et al. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15:215–221. doi: 10.1093/molehr/gap009. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen OB, Steffensen R, Nielsen HS, Varming K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol Obstet Invest. 2008;66:257–267. doi: 10.1159/000149575. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Qiu L, Chen G, Ye Z, Lü C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous miscarriage patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656–1664. doi: 10.1373/clinchem.2009.125641. [DOI] [PubMed] [Google Scholar]
- 16.Bao SH, Sheng SL, Peng YF, Lin QD. Effects of letrozole and clomiphene citrate on the expression of HOXA10 and integrin alpha v beta 3 in uterine epithelium of rats. Fertil Steril. 2009;91:244–248. doi: 10.1016/j.fertnstert.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Davidson G, Mao B, del Barco Barrantes I, Niehrs C. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development. 2002;129:5587–5596. doi: 10.1242/dev.00154. [DOI] [PubMed] [Google Scholar]
- 18.Cselenyi CS, Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1:pe10. doi: 10.1126/stke.18pe10. [DOI] [PubMed] [Google Scholar]
- 19.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 20.Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. 2005;62:1964–1973. doi: 10.1007/s00018-005-5230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou X, TanY LM, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daikoku T, Song H, Guo Y, et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 24.Tulac S, Nayak NR, Kao LC, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 25.Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 26.Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91:1453–1461. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald LJ, Sales KJ, Grant V, Brown P, Jabbour HN, Catalano RD. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol Hum Reprod. 2011;17:626–636. doi: 10.1093/molehr/gar031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous miscarriage cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]


