Abstract
Granulocyte–macrophage colony-stimulating factor (GM-CSF) autoantibodies are associated with stricturing behaviour in Crohn disease (CD). We hypothesized that CD ileal lamina propria mononuclear cells (LPMC) would produce GM-CSF autoantibodies and peripheral blood (PB) samples would contain GM-CSF neutralizing capacity (NC). Paediatric CD and control PBMC and ileal biopsies or LPMC were isolated and cultured and GM-CSF, immunoglobulin (Ig)G and GM-CSF autoantibodies production were measured by enzyme-linked immunosorbent assay (ELISA). Basal and GM-CSF-primed neutrophil bacterial killing and signal transducer and activator of transcription 5 (STAT5) tyrosine phosphorylation (pSTAT5) were measured by flow cytometry. GM-CSF autoantibodies were enriched within total IgG for LPMC isolated from CD ileal strictures and proximal margins compared to control ileum. Neutrophil bacterial killing was reduced in CD patients compared to controls. Within CD, neutrophil GM-CSF-dependent STAT5 activation and bacterial killing were reduced as GM-CSF autoantibodies increased. GM-CSF stimulation of pSTAT5 did not vary between controls and CD patients in washed PB granulocytes in which serum was removed. However, GM-CSF stimulation of pSTAT5 was reduced in whole PB samples from CD patients. These data were used to calculate the GM-CSF NC. CD patients with GM-CSF NC greater than 25% exhibited a fourfold higher rate of stricturing behaviour and surgery. The likelihood ratio (95% confidence interval) for stricturing behaviour for patients with elevation in both GM-CSF autoantibodies and GM-CSF NC was equal to 5 (2, 11). GM-CSF autoantibodies are produced by LPMC isolated from CD ileal resection specimens and are associated with reduced neutrophil bacterial killing. CD peripheral blood contains GM-CSF NC, which is associated with increased rates of stricturing behaviour.
Keywords: bacterial killing, cytokine autoantibody, granulocyte–macrophage colony-stimulating factor, neutrophil, paediatric Crohn disease, stricture, surgery
Introduction
The therapeutic use of cytokines and anti-cytokine monoclonal antibodies has been the focus of intense investigation for inflammatory bowel disease (IBD) over the past decade 1. However, fewer than 50% of patients achieve a sustained clinical remission with this approach, suggesting heterogeneity in pathogenic mechanisms 2. This may include endogenous cytokine autoantibodies which have been described in healthy individuals and those with chronic inflammatory disorders 3. For example, interleukin (IL)-8 autoantibodies which enhance IL-8 action and neutrophil recruitment have been associated with a more severe disease course in patients with adult respiratory distress syndrome (ARDS), while flares of lupus have been associated with a decrease in the titres of tumour necrosis factor (TNF)-α autoantibodies 3. High titres of neutralizing granulocyte–macrophage colony-stimulating factor (GM-CSF) autoantibodies reduce neutrophil functions, including bacterial killing, and cause the rare lung disease primary alveolar proteinosis (PAP) 4.
The role of cytokine autoantibodies in IBD pathogenesis has only recently been investigated, with IL-10 and TNF-α autoantibodies identified in a subset of patients 5,6. IBD patients with higher endogenous TNF-α neutralizing capacity (NC) due to TNF-α autoantibodies exhibited a lower rate of clinical response to therapeutic anti-TNF monoclonal antibody administration 5,6. We have reported recently that GM-CSF autoantibodies are detectable in adult and paediatric Crohn disease (CD) patients, and that circulating levels above 1·6 μg/ml are associated with higher rates of stricturing/penetrating disease behaviour and surgery 7. We found that GM-CSF induction of CD11B on circulating neutrophils was reduced in CD patients with high levels of GM-CSF autoantibodies, suggesting a blocking effect upon GM-CSF action 7.
In order for a cytokine autoantibody to have an effect, it would be anticipated that the autoantibody would be produced in high levels locally in the affected tissue, where direct regulation of cytokine bioactivity could occur. In this regard, studies from our group and others have identified pleotropic effects of GM-CSF in the gut relevant to CD pathogenesis 8. These include both direct effects upon intestinal epithelial cell (IEC) survival and proliferation in response to injury, and priming of neutrophil anti-microbial function 8. Mice with genetic loss of GM-CSF exhibit more severe small bowel and colon injury following non-steroidal anti-inflammatory drugs (NSAID) or dextran sulphate sodium (DSS) administration, respectively, and clinical trials in CD have demonstrated a reduction in disease activity in some patients 7,9–13. However, it was not known whether GM-CSF autoantibody production would be enriched in the affected ileum in CD, or whether this would be associated with differences in neutrophil anti-microbial function or peripheral blood GM-CSF neutralizing capacity. To test this, we measured GM-CSF autoantibody production by cultured ileal lamina propria mononuclear cells (LPMC) and examined the relationship between GM-CSF autoantibodies and neutrophil functions, including bacterial killing and signal transducer and activator of transcription 5 (STAT5) activation, in a cohort of paediatric CD patients and healthy controls.
Materials and methods
Peripheral blood mononuclear cells (PBMC) and ileal biopsy/LPMC isolation and culture
PBMC were isolated from heparinized blood by Ficoll-Hypaque centrifugation 14. Ileal surgical tissue was cut into small pieces and washed in Hanks's balanced salt solution (HBSS) and incubated with 1 mM dithiothreitol (DTT) at room temperature (RT). The tissue was then washed in HBSS and incubated with 1 mM ethylenediamine tetraacetic acid (EDTA)/HBSS and washed with HBSS at RT. In the final step, tissue was incubated in HBSS containing 1 mg/ml collagenase D and 0·1 mg/ml DNAse for 20 min at 37°C. LP cells were harvested over a 70 μm filter and then washed and centrifuged over Ficoll-Hypaque. PBMC and ileal biopsies or LPMC were cultured in 10% fetal bovine serum (FBS)/RPMI-1640 for 14 days at four biopsies or 400 000 cells per well. Ileal biopsies were cultured in a single well. PBMC and LPMC were cultured in 5–10 wells per sample and at the end of culture the wells were pooled together.
Enzyme-linked immunosorbent assay (ELISA)
Immunoglobulin (Ig)G and free GM-CSF were measured using commercial ELISA kits (BioLegend, San Diego, CA, USA). GM-CSF autoantibodies were measured by ELISA, as reported previously 7.
Neutrophil function assays
Neutrophil CD64 index, phagocytosis and killing and oxidative burst were measured by flow cytometry in the Cincinnati Children's Hospital Medical Center (CCHMC) Clinical Immunology laboratory. Phagocytosis and killing of Staphylococcus aureus was measured in adherent neutrophils using the acridine orange method. Oxidative burst was measured in neutrophils in whole blood using dihydrorhodamine 123 dye following phorbol-12-myristate-13 acetate stimulation. These functions were measured under basal conditions and following GM-CSF priming (10 ng/ml for 30 min) of heparinized whole blood samples or washed neutrophils.
GM-CSF stimulation of PB granulocytes
Red blood cells from 0·5 ml of whole blood were lysed with ammonium chloride buffer (ACK) at RT, pelleted and washed with Dulbecco's modified Eagle's medium (DMEM). Pellets were resuspended in DMEM or day 14 ileal LPMC conditioned media and stimulated for 20 min at 37°C with 10 ng/ml of GM-CSF (R&D Systems, Minneapolis, MN, USA); 0·5 ml heparinized whole blood from the same sample was also stimulated for 20 min at 37°C with 10 ng/ml of GM-CSF and then lysed, pelleted and washed in DMEM. Cells were fixed with 1% paraformaldehyde overnight at 4°C. Next day the cells were washed with phosphate-buffered saline (PBS) and permeabilized with ice-cold 100% methanol and stored at −20°C. On the following day the cells were washed with PBS and stained for intracellular staining with phosphorylated STAT5 (pSTAT5) 14.
Flow cytometry analysis of PBMC and LPMC cell populations and granulocyte GM-CSF: pSTAT5 signalling
PBMC and LPMC were stained with CD3, CD4, CD19, and CD138 (BD Biosciences, San Jose, CA, USA) at the time of harvest and analysis was performed 14. For CD patients and healthy and disease controls, whole PB (WPB) and lysed PB (LPB) samples after GM-CSF stimulation were stained with CD3, CD4 and pSTAT5 (BD Biosciences, San Jose, CA, USA). GM-CSF (CD116) (BD Biosciences) receptor expression was stained on fresh cells. At least 104 cells were analysed on a fluorescence activated cell sorter (FACS)Calibur (Becton Dickinson) and data collected were evaluated on CellQuest software 14,15. The GM-CSF : pSTAT5 stimulation index (SI) was defined as: [(granulocyte pSTAT5 mean fluorescence intensity (MFI) following GM-CSF stimulation − basal granulocyte pSTAT5 MFI)/basal granulocyte pSTAT5 MFI] × 100. Peripheral blood GM-CSF neutralizing capacity was defined as: [(granulocyte pSTAT5 SI in whole blood − granulocyte pSTAT5 SI in washed cells)/granulocyte pSTAT5 SI in washed cells] × 100 × −1.
Statistical analysis
Statistical analyses were performed using GraphPad Prism© version 5. Continuous variables were analysed using the unpaired t-test, two-sample t-test, Mann–Whitney U-test, one-way analysis of variance (anova) with Bonferroni's multiple comparison test, Kruskal–Wallis with Dunn's multiple comparison test for multiple comparisons or linear test-for-trend. Discrete variables were analysed using Fisher's exact test or χ2 test. A P-value < 0·05 was considered significant.
Ethical considerations
The patient-based studies were approved by the CCHMC Institutional Review Board, and consent was obtained from parents and adult subjects and assent from paediatric subjects aged 11 years and above.
Results
Clinical and demographic characteristics
The clinical and demographic characteristics of the controls and CD patients are summarized in Table 1. For the CD patients enrolled at the time of ileocaecal resection, 31% were receiving mesalamine, 50% corticosteroids, 31% an immune modulator and 56% infliximab. Neutrophil function was measured in established patients on therapy, with 29% receiving mesalamine, 43% an immune modulator, 29% corticosteroids and 74% infliximab at the time of the blood draw. GM-CSF neutralizing capacity (NC) was determined prior to therapy in 25% of these patients. For the remainder, 30% were receiving mesalamine, 39% an immune modulator (thiopurine or methotrexate), 22% corticosteroids and 44% infliximab at the time of the blood draw. The median (IQR) serum GM-CSF autoantibody concentration increased from 0·4 (0·2, 0·7) μg/ml in healthy controls to 3 (1, 6) μg/ml in CD patients and 57 (39, 552) μg/ml in disease controls with PAP (P < 0·0001, Kruskal–Wallis test).
Table 1.
Clinical and demographic characteristics
| Disease classification | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Behaviour | ||||||||
| n | Age (years) | Male gender | Disease duration (years) | L1 | L2 | L3 | B1 | B2B3 | |
| CTR NC | 11 | 13 (7, 18) | 73% | – | – | – | – | – | – |
| CTR PMN | 10 | 10·8 (7, 15) | 40% | – | – | – | – | – | – |
| CD NC | 55 | 11 (1, 18) | 64% | 2·6 (0, 16) | 13% | 27% | 60% | 76% | 24% |
| CD PMN | 45 | 16·2 (1, 23) | 64% | 4·3 (0, 14) | 14% | 24% | 62% | 64% | 36% |
| CD Sur. | 16 | 13 (7, 18) | 56% | 4·1 (1, 10) | 25% | 0% | 75% | 0% | 100% |
CTR: healthy control; CD: Crohn disease; NC: subjects in which peripheral blood granulocyte–macrophage colony-stimulating factor (GM-CSF) neutralizing capacity was determined; PMN: subjects in which neutrophil function was measured; CD Sur: CD subjects in which ileal surgical specimens were analysed. Date are shown as the mean (range) or frequency. L1, ileal location, L2, colon-only location, L3, ileocolonic location, B1, inflammatory behaviour, B2B3, stricturing/penetrating behaviour.
GM-CSF autoantibodies are enriched within total IgG produced by ileal stricture LPMC
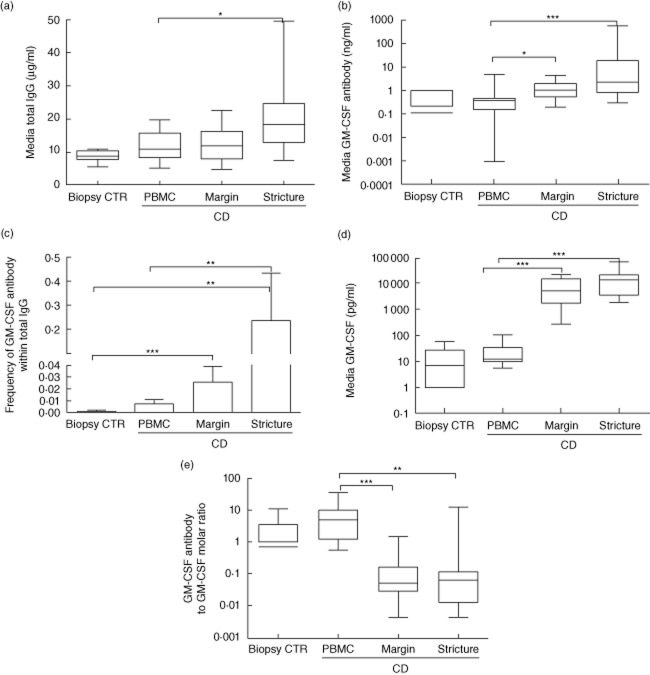
We first asked whether LPMC isolated from ileocaecal resection specimens would produce a higher proportion of GM-CSF autoantibodies within total IgG than control ileum. LPMC were isolated from the mid-point of the strictured segment of ileocaecal resection specimens and the visually unaffected proximal margin and cultured for 14 days (Supplementary Fig. S1a). Histological scoring confirmed a high degree of inflammation and chronic architectural changes within the ileal stricture; this was reduced substantially at the proximal margin (Supplementary Fig. S1b). IgG production by control ileum in 14-day culture was comparable to CD PBMC or CD ileal margin LPMC (Fig. 1a). IgG production by ileal LPMC was increased in cells isolated from the ileal stricture compared to PBMC (Fig. 1a, P = 0·02, Kruskal–Wallis test). Consistent with this, the frequency of CD19+ B lymphocytes, but not CD19+/CD138+ plasma cells or CD3+ T lymphocytes, was also increased in the ileal stricture LPMC cultures at the time of cell isolation and plating (9 ± 2%), compared to ileal margin LPMC (4 ± 1%) and PBMC (3 ± 0·4%) (P = 0·007, Kruskal–Wallis test). Seven of 10 control ileal samples produced detectable levels of GM-CSF autoantibodies, with a median [interquartile range (IQR)] concentration of 0·1 (0, 0·2) μg/ml. The median (IQR) GM-CSF autoantibody concentration increased from 0·4 (0·2, 0·5) ng/ml for CD PBMC cultures to 1·1 (0·6, 2) ng/ml for CD ileal margin LPMC and 2·3 (0·8, 19) ng/ml for the ileal stricture LPMC (Fig. 1b, P = 0·002, Kruskal–Wallis test). The frequency of GM-CSF autoantibodies within total IgG was increased significantly from a mean [standard deviation (s.d.)] of 0·002 (0·002) in control ileal samples to 0·01 (0·01) in CD PBMC cultures and 0·24 (0·67) in CD ileal stricture LPMC (Fig. 1c, P < 0·0001, Kruskal–Wallis test). Free GM-CSF (not bound to GM-CSF autoantibodies) was detected in six of 10 control ileal sample cultures at a median (IQR) concentration of 7 (0, 27) pg/ml. Free GM-CSF was detected at a median (IQR) concentration of 13 (10, 34) pg/ml in CD PBMC cultures, compared to 5338 (1755, 14 866) pg/ml for CD ileal margin LPMC and 13 895 (3590, 22 672) pg/ml for the ileal stricture LPMC (Fig. 1d, P < 0·0001, Kruskal–Wallis test). This resulted in a lower molar ratio of GM-CSF autoantibodies to free GM-CSF in the CD ileal LPMC cultures compared to the CD PBMC cultures (Fig. 1e, P < 0·0001, Kruskal–Wallis test). These data confirmed that free GM-CSF and GM-CSF autoantibodies were produced by LPMC isolated from both the ileal stricture and the proximal margin in CD, and that GM-CSF autoantibody production was enriched within total IgG for CD ileum compared to control ileum.
Fig. 1.
Granulocyte–macrophage colony-stimulating factor (GM-CSF) and GM-CSF autoantibody production by control and Crohn disease (CD) ileum. Control ileal biopsies, CD peripheral blood mononuclear cells (PBMC) and CD ileal stricture and proximal margin lamina propria mononuclear cell (LPMC) were isolated and cultured for 14 days and (a) total immunoglobulin (Ig)G and (b) GM-CSF autoantibody concentration in the media were determined by enzyme-linked immunosorbent assay (ELISA); (c) the frequency of GM-CSF autoantibodies within total IgG was determined. (d) GM-CSF concentration in the media was determined by ELISA and (e) the molar ratio of GM-CSF autoantibody : GM-CSF was calculated. Data are shown as the median [interquartile range (IQR)] or mean (standard deviation), differences between groups were tested by Kruskal–Wallis with Dunn's multiple comparison test (MCT) or analysis of variance (anova) with Bonferroni's MCT, *P < 0·05; **P < 0·01; ***P < 0·001; CTR (control) n = 10, CD PBMC n = 17, CD ileal margin LPMC n = 12, CD ileal stricture LPMC n = 12.
Neutrophil bacterial killing is reduced in CD and GM-CSF enhances bacterial killing on washed CD neutrophils
We then asked whether CD patients would exhibit a reduction in neutrophil bacterial killing compared to controls. As shown in Fig. 2a, mean (s.d.) killing was reduced from 93(1)% in controls to 85(1)% in CD patients, P = 0·0003. GM-CSF primes neutrophil bacterial killing in part via induction of cell surface CD11B and CD18, which then promotes adhesion and phagocytosis. This response is reduced substantially in PAP neutrophils when GM-CSF stimulation is performed in whole blood samples which contain GM-CSF autoantibodies, compared to washed neutrophils in which serum has been removed 4. We therefore asked whether GM-CSF stimulation would increase bacterial killing by control or CD neutrophils, and whether this would vary for whole blood versus washed samples. GM-CSF stimulation increased bacterial killing by both washed and non-washed control neutrophils (Fig. 2b). By comparison, GM-CSF stimulation induced an increase in neutrophil bacterial killing only on washed CD neutrophils, with an increase in the mean (s.d.) killing from 87(6)% under basal conditions to 94(3)% following stimulation (Fig. 2b, P < 0·0001, Kruskal–Wallis test). Neither phagocytic capacity nor oxidative burst changed under these conditions (data not shown). These studies confirmed that GM-CSF stimulation would enhance bacterial killing by washed CD neutrophils; this was abrogated in whole blood samples.
Fig. 2.
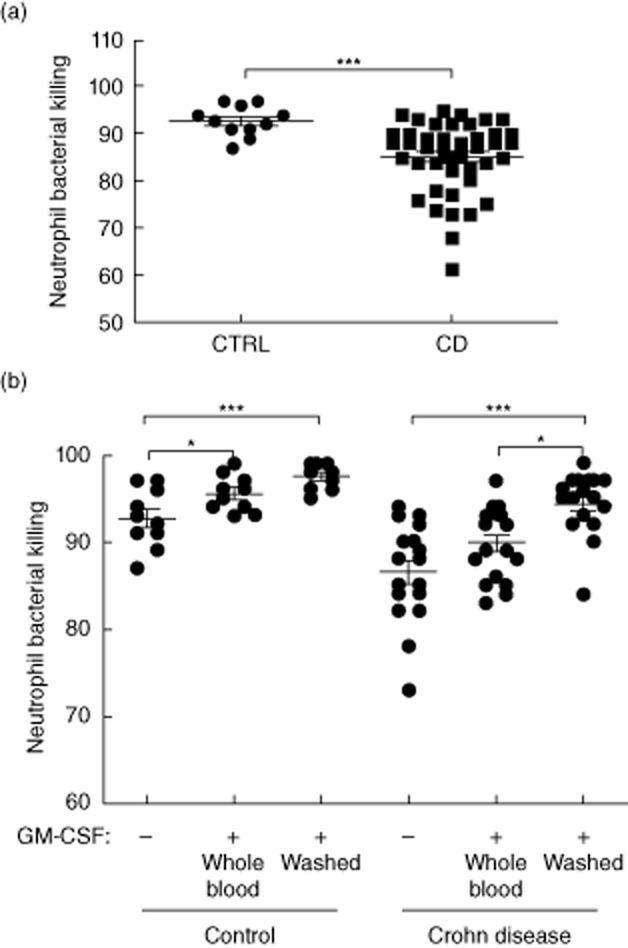
Neutrophil bacterial killing in controls and Crohn disease (CD) patients. The frequency of Staphyloccus aureus killing by isolated adherent peripheral blood neutrophils was determined (a) under basal conditions and (b) following granulocyte–macrophage colony-stimulating factor (GM-CSF) stimulation in whole blood or washed cells in controls (CTRL) and CD patients. Data are shown as scatter-plots with means, differences between groups were tested by Mann–Whitney U- or Kruskal–Wallis tests with Dunn's multiple comparison test, *P < 0·05; ***P < 0·001; control (CTR) n = 10, CD n = 44 for basal killing and n = 19 for GM-CSF primed killing.
Neutrophil bacterial killing is reduced in CD patients with elevated GM-CSF autoantibodies
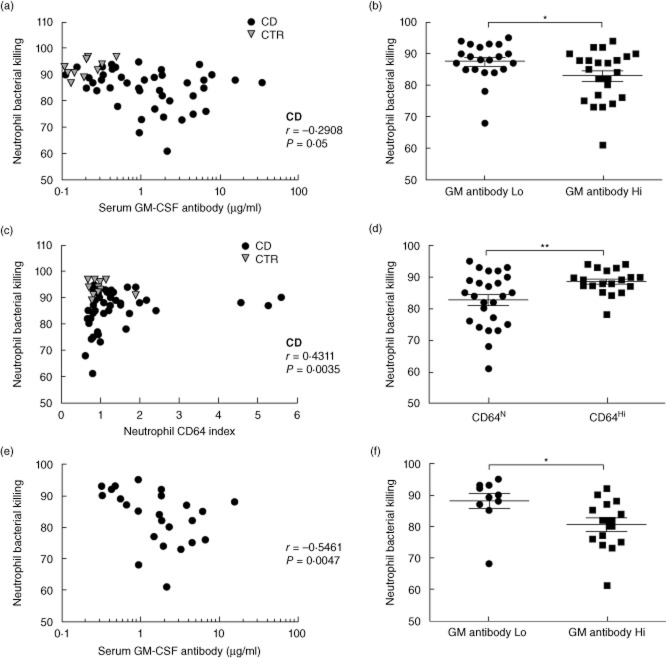
We then asked whether GM-CSF autoantibodies would be associated with differences in CD neutrophil bacterial killing. We measured the neutrophil CD64 index to control for neutrophil activation, which has been associated with disease activity in CD. Neutrophil bacterial killing decreased as serum GM-CSF autoantibodies increased within the CD patients (Fig. 3a, r = −0·2908, P = 0·05, Spearman's correlation). Previous studies found that neutrophil anti-microbial functions decreased between serum GM-CSF autoantibody concentrations of 1 μg/ml to 10 μg/ml. When we stratified by a serum GM-CSF autoantibody concentration of 1 μg/ml, we observed a decrease in mean (s.d.) bacterial killing from 88(6)% to 83(8)% (Fig. 3b, P = 0·05). Neither medication exposures, the CD64 index, phagocytic capacity nor oxidative burst varied between CD patients with higher versus lower serum GM-CSF autoantibodies (data not shown). Neutrophil bacterial killing increased with neutrophil activation, as measured by the CD64 index (Fig. 3c, r = 0·4311, P = 0·004). When we stratified by the CD64 index, we observed that all the patients with reduced bacterial killing were in the subgroup with a normal CD64 index (Fig. 3d). We therefore asked whether serum GM-CSF autoantibodies would be associated with neutrophil bacterial killing in the subset of CD patients with a normal CD64 index. As shown in Fig. 3e, bacterial killing decreased as serum GM-CSF autoantibodies increased (r = −0·5461, P = 0·005, Spearman's correlation). When we stratified by a serum GM-CSF autoantibody concentration of 1 μg/ml, we observed a decrease in mean (s.d.) bacterial killing from 88(8) to 80(8)% (Fig. 3f, P = 0·01). These data confirmed that elevated GM-CSF autoantibodies are associated with reduced neutrophil bacterial killing in paediatric CD patients.
Fig. 3.
Serum granulocyte–macrophage colony-stimulating factor (GM-CSF) autoantibody concentration and neutrophil bacterial killing. The frequency of Staphyloccus aureus killing by isolated adherent peripheral blood neutrophils and the neutrophil CD64 activation index were determined by flow cytometry and serum GM-CSF autoantibody concentration was determined by enzyme-linked immunosorbent assay (ELISA). The association between serum GM-CSF autoantibody concentration and neutrophil bacterial killing was tested by Spearman's correlation in (a) the entire Crohn disease (CD) cohort, n = 44 or (e) the CD patients with neutrophil CD64 activation index in the normal range, <1·3, n = 25. Neutrophil bacterial killing is shown in (b) for the entire CD cohort stratified by serum GM-CSF autoantibody concentration of 1 μg/ml and (f) the CD patients with neutrophil CD64 activation index in the normal range, <1·3, stratified by serum GM-CSF autoantibody concentration of 1 μg/ml. Differences between groups were tested by Mann–Whitney U-test. (c) The association between neutrophil CD64 activation index and bacterial killing was tested by Spearman's correlation, n = 44. (d) Neutrophil bacterial killing is shown for the entire CD cohort stratified by CD64 activation index of 1·3. Differences between groups were tested by unpaired t-test. *P < 0·05; **P < 0·01; ***P < 0·001.
GM-CSF signalling is reduced in CD patients with elevated GM-CSF autoantibodies and complicated behaviour
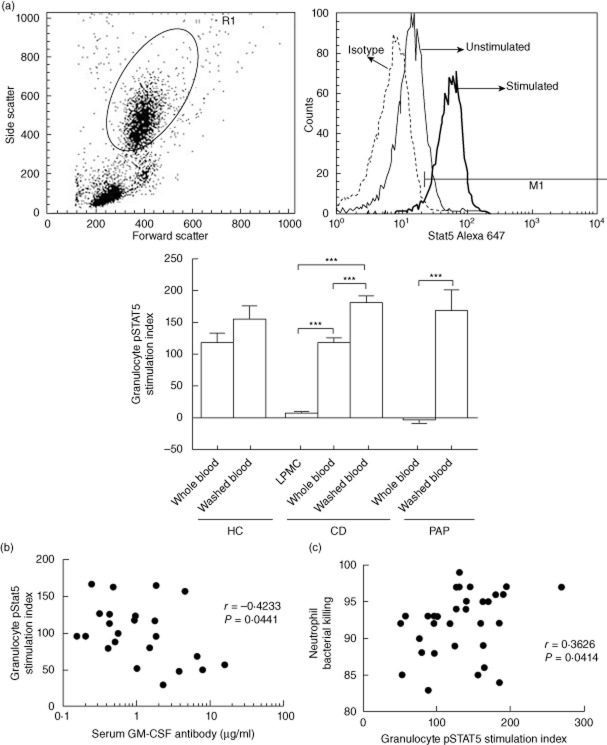
Finally, we asked whether CD peripheral blood samples would exhibit GM-CSF neutralizing capacity (NC), and whether this would vary with GM-CSF autoantibody concentration or patient outcomes. We developed a flow cytometry assay to measure the change in intracellular tyrosine pSTAT5 MFI on peripheral granulocytes following GM-CSF stimulation 14. Representative results for the granulocyte gate and the change in the pSTAT5 MFI with GM-CSF stimulation are shown in Fig. 4a. We found that the granulocyte pSTAT5 stimulation index (SI) was reduced in paediatric CD when cells were stimulated in day 14 ileal LPMC conditioned media and in whole peripheral blood (PB) samples, compared to washed cells (Fig. 4a). By comparison, we observed a trend towards a reduction in the PB granulocyte pSTAT5 SI in healthy controls which did not reach significance, and a complete reduction in the PB granulocyte pSTAT5 SI in disease controls with PAP and high levels of neutralizing GM-CSF Ab. The granulocyte pSTAT5 SI did not vary between the three groups for washed cells, demonstrating that there was no intrinsic defect in granulocyte GM-CSF signalling. Consistent with this, cell surface abundance of the GM-CSF receptor alpha subunit (CD116) did not differ between the CD patients and healthy controls (Supplementary Fig. S2). As serum GM-CSF autoantibodies increased, the granulocyte pSTAT5 SI in whole blood decreased (Fig. 4b, r = −0·4233, P = 0·04, Spearman's correlation). By comparison, there was no association between serum GM-CSF autoantibodies and the granulocyte pSTAT5 SI in washed cells (r = −0·1068, P = 0·6278). Consistent with this, as the granulocyte pSTAT5 SI increased, neutrophil bacterial killing increased (Fig. 4c, r = 0·3626, P = 0·04).
Fig. 4.
Granulocyte–macrophage colony-stimulating factor (GM-CSF) : signal transducter and activator of transcription 5 (STAT5) stimulation index in granulocytes from Crohn disease (CD) patients and healthy and disease controls. The change in intracellular tyrosine phosphorylated STAT5 (pSTAT5) mean fluorescence intensity (MFI) in peripheral granulocytes within whole blood samples, washed blood cells or lamina propria mononuclear cell (LPMC) conditioned media (LPMC) following GM-CSF stimulation was measured by flow cytometry and a pSTAT5 stimulation index was calculated for healthy controls (HC), CD patients, and disease controls with primary alveolar proteinosis (PAP). (a) Representative results for the granulocyte gate and the change in the pSTAT5 MFI with GM-CSF stimulation are shown. Data are shown as the mean (standard error of the mean), differences between groups were compared by t-test or analysis of variance (anova) with Bonferroni's multiple comparison test, ***P < 0·001; n = 11 for HC (healthy controls); n = 9 for CD LPMC conditioned media, n = 55 for CD whole and washed blood, n = 6 for PAP. The association between (b) serum GM-CSF autoantibody concentration and granulocyte pSTAT5 stimulation index (SI) in whole blood (n = 23) and (c) granulocyte pSTAT5 SI and neutrophil bacterial killing (n = 32) was tested by Spearman's correlation.
We utilized the difference in the pSTAT5 SI between washed and peripheral blood samples to calculate the GM-CSF neutralizing capacity (NC) of the peripheral blood samples. The mean [standard error of the mean (s.e.m.)] peripheral blood GM-CSF NC increased from 25(5) in CD with inflammatory behaviour to 46(9) in CD with stricturing/internal penetrating behaviour, compared with 92(2) for CD day 14 ileal stricture LPMC conditioned media (Fig. 5a, anova with Bonferroni's multiple comparison test for B1 versus B2B3 serum, *P < 0·05). The median value for GM-CSF NC within the CD group was equal to 25%. The rates of stricturing/penetrating behaviour and surgery were increased significantly for CD patients with GM-CSF NC > 25% (Fig. 5b). Finally, we asked whether rates of stricturing/internal penetrating behaviour would vary with combined results for GM-CSF autoantibody concentration and GM-CSF NC. The frequency of complicated disease behaviour was equal to 60% in CD patients with both serum GM-CSF autoantibody ≥ 1·6 μg/ml and GM-CSF NC > 25% (n = 15), compared to 11% in those with one or neither factor (n = 36, P = 0·0011). The same result was observed for the frequency of surgery (53% versus 11%, P = 0·008). Consistent with this, the LR [95% confidence interval (CI)] for developing complicated behaviour or requiring surgery increased from 2 (1, 3) and 1·7 (1, 3) in CD patients with elevated GM-CSF autoantibodies alone to 5 (2, 11) and 5 (2, 10), respectively, in CD patients with both elevated GM-CSF autoantibodies and GM-CSF NC.
Fig. 5.
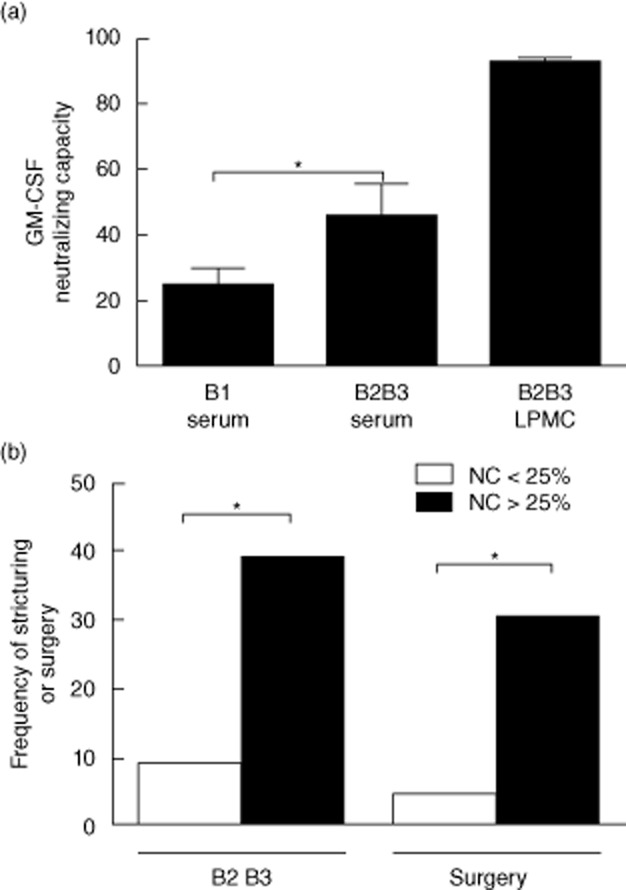
Granulocyte–macrophage colony-stimulating factor (GM-CSF) neutralizing capacity and disease behaviour. Results for the GM-CSF : phosphorylated signal transducer (pSTAT5) and activator of transcription 5 (STAT5) granulocyte stimulation assay were used to calculate the (a) GM-CSF neutralizing capacity (NC) of the peripheral blood sample (serum) or ileal lamina propria mononuclear cell (LPMC) conditioned media and are shown as the mean (standard error of the mean) for Crohn disease (CD) with inflammatory behaviour (B1, n = 42) and CD with stricturing behaviour (B2B3, n = 13). Differences between groups were tested with analysis of variance (anova) with Bonferroni's multiple comparison test, *P < 0·05. (b) The frequency of stricturing/internal penetrating behaviour or surgery is shown for CD patients stratified by peripheral blood GM-CSF NC of 25% (n = 55). Differences between groups were tested by Fisher's exact test, *P < 0·05.
Discussion
Endogenous cytokine autoantibodies play a role in a number of autoimmune and chronic inflammatory disorders 3. Given the advent of therapeutic anti-cytokine antibody use in IBD, a better understanding of the role of endogenous cytokine autoantibodies in pathogenesis and treatment response is potentially of great importance 1. To our knowledge, our study is the first to show that both free GM-CSF and GM-CSF autoantibodies are produced by LPMC harvested from CD ileal surgical samples. Normalization of the GM-CSF autoantibody concentration within the culture media to total IgG demonstrated that production of GM-CSF autoantibodies were increased markedly in LPMC isolated from the stricture, suggesting a specific local tissue response. A functional effect was suggested by our studies demonstrating an association between higher serum GM-CSF autoantibody concentration and lower neutrophil bacterial killing, and between higher GM-CSF neutralizing capacity of peripheral blood samples and increased rates of complicated behaviour. It will be important in future studies to determine whether in-vivo ileal production of GM-CSF autoantibodies occurs prior to the development of stricturing, in association with reduced ileal and peripheral blood neutrophil bacterial killing and elevated GM-CSF NC. Collectively, these data are consistent with the hypothesis that prolonged exposure to the cognate cytokine, in this case GM-CSF, leads to loss of T cell tolerance, and expansion of an existing pool of B cells producing GM-CSF autoantibodies 3.
We found that expression of the GM-CSF receptor alpha subunit, CD116, was intact on circulating granulocytes isolated from CD patients, compared to healthy controls. GM-CSF-induced STAT5 tyrosine phosphorylation did not vary between healthy controls, disease controls with PAP and CD patients when washed granulocytes were stimulated following removal of all serum components. This confirmed that there was no intrinsic defect in granulocyte GM-CSF responsiveness in CD. Conversely, when GM-CSF was used to stimulate granulocytes in peripheral blood samples which contained serum, or day 14 ileal LPMC conditioned media, we observed a progressive increase in GM-CSF neutralizing capacity between healthy controls, CD patients with inflammatory behaviour, CD patients with stricturing/penetrating behaviour and disease controls with PAP. This was consistent with a circulating factor such as GM-CSF autoantibodies regulating GM-CSF bioactivity. A recent study in adult CD patients demonstrated a reduction in CD116 expression, and GM-CSF-induced STAT3 tyrosine phosphorylation, in whole peripheral blood samples obtained from adult CD and ulcerative colitis patients 16. The reason for the different result for CD116 expression is not clear, although in the previous study only whole peripheral blood samples were tested. It would have been important to also test washed cells, to determine whether removal of serum restored GM-CSF signalling on granulocytes, as was observed in the current study.
The GM-CSF receptor is expressed on both IEC and immune cells, and GM-CSF exerts pleotropic effects in the gut which would be anticipated to regulate mucosal inflammation and injury 8. A critical function is likely to be priming of neutrophil anti-microbial function n 4. Mice genetically deficient in GM-CSF or the GM-CSF receptor are more susceptible to ileal injury following NSAID exposure and colonic injury following DSS administration 7,9. Conversely, GM-CSF administration ameliorates DSS-induced colonic injury in mice, and reduces symptoms in some children and adults with CD 7,9,11,12. Whether variation in clinical responses to GM-CSF is due to endogenous GM-CSF autoantibodies is not known, but would be worthy of future study.
Our previous studies have shown that patients with elevated GM-CSF autoantibodies exhibit an increase in intestinal permeability, independent of the degree of mucosal inflammation, and increased titres of several anti-microbial serology 7,17. In the current study we have extended this work by determining that neutrophil bacterial killing is reduced in CD patients with higher serum concentrations of serum GM-CSF autoantibodies. In-vitro stimulation with GM-CSF on washed CD neutrophils activated STAT5 and enhanced bacterial killing. This effect was abrogated in whole peripheral blood samples, consistent with the presence of blocking GM-CSF autoantibodies. These results have implications for strategies to boost neutrophil anti-microbial function via GM-CSF administration in CD patients.
Our study adds to the growing literature regarding the role of cytokine autoantibodies in IBD 5–7. We have found for the first time that both GM-CSF and GM-CSF autoantibodies are expressed in the affected tissue in ileal surgical specimens, and that CD patients with higher levels of GM-CSF autoantibodies exhibit a reduction in neutrophil bacterial killing, while those with elevated peripheral blood GM-CSF NC are more likely to require surgery. We have reported previously that elevated levels of GM-CSF autoantibodies are associated with increased rates of stricturing behaviour and surgery in adult and paediatric CD. In the current study, we found that GM-CSF NC was also increased in the peripheral blood in CD patients who experience complicated behaviour. CD patients with both elevated GM-CSF autoantibodies and high GM-CSF NC experienced the highest rates of stricturing/penetrating behaviour and surgery. This would be consistent with a mechanism in which higher absolute local production of GM-CSF autoantibodies with greater intrinsic GM-CSF blocking ability would be required to reduce ileal GM-CSF bioactivity and affect disease behaviour. In terms of utility as a diagnostic test, our data suggest that an initial screening test using GM-CSF autoantibodies alone will detect most patients who will experience complicated behaviour [negative LR (95% CI) of 0·26 (0·1, 1)], while a second test to determine which patients with elevated GM-CSF autoantibodies also have higher GM-CSF NC would then have sufficient positive predictive value [positive LR of 5 (2, 11)] to guide the earlier introduction of disease-modifying therapy in this subset of high-risk patients.
Acknowledgments
This work was supported by the Integrative Morphology and Flow Cytometry cores of the National Institutes of Health (NIH)-supported Cincinnati Children's Hospital Research Foundation Digestive Health Center (1P30DK078392-01), a CCFA Research Fellowship award (A.S.) and NIH grant R01 DK078683 (L.A.D.). Neutrophil functions including bacterial killing, phagocytic capacity and oxidative burst were measured in the CCHMC CLIA/CAP certified Clinical Immunology Laboratory.
Disclosures
The authors have no financial arrangement(s) with the company whose product figures prominently in the submitted manuscript or with the company making a competing product.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Isolation of LPMC from ileocaecal resection specimens. (a) Lamina propria mononuclear cells (LPMCs) were isolated from the mid-point of the strictured segment, and the proximal ileal margin, of ileocaecal resection specimens. A representative specimen shows a stricture (arrows) at the ileocaecal valve region. The mucosa in the stricture appears cobblestoned and the wall is extremely thick. Asterisk indicates proximal margin and arrowhead points to the vermiform appendix. (b) The Crohn's Disease Histologic Index of Severity (CDHIS) was determined for haematoxylin and eosin (H&E)-stained slides prepared from the stricture and proximal margin and is shown as the mean (standard error of the mean, ***P = 0·0001 by t-test, n = 16.
Fig. S2. Granulocyte CD116 abundance in healthy controls and Crohn disease (CD) patients. Cell surface abundance of the granulocyte–macrophage colony-stimulating factor (GM-CSF) receptor CD116 was measured by flow cytometry in healthy controls (HC) and CD patients. (a) Representative granulocyte gate and results for CD116 for CD and HC are shown. Data are shown as the mean (standard error of the mean) for the frequency of granulocytes expressing CD116 and the CD116 mean fluorescence intensity (MFI) on granulocytes, n = 11 for healthy controls (HC) and n = 39 for CD.
References
- 1.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932. doi: 10.1136/gutjnl-2011-300904. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Uchida K, Nakagaki K, et al. High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 2010;21:263–273. doi: 10.1016/j.cytogfr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 5.Ebert EC, Das KM, Mehta V, et al. Non-response to infliximab may be due to innate neutralizing anti-tumour necrosis factor-alpha antibodies. Clin Exp Immunol. 2008;154:325–331. doi: 10.1111/j.1365-2249.2008.03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert EC, Panja A, Das KM, et al. Patients with inflammatory bowel disease may have a transforming growth factor-beta-, interleukin (IL)-2- or IL-10-deficient state induced by intrinsic neutralizing antibodies. Clin Exp Immunol. 2009;155:65–71. doi: 10.1111/j.1365-2249.2008.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Uchida K, Jurickova I, et al. Granulocyte–macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease. Gastroenterology. 2009;136:1261–1271. doi: 10.1053/j.gastro.2008.12.046. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egea L, Hirata Y, Kagnoff MF. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4:723–731. doi: 10.1586/egh.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernasconi E, Favre L, Maillard MH, et al. Granulocyte–macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–441. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Gilbert S, Groschwitz K, et al. Loss of GM-CSF signalling in non-haematopoietic cells increases NSAID ileal injury. Gut. 2010;59:1066–1078. doi: 10.1136/gut.2009.203893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsen JR, Rosh J, Heyman M, et al. Phase I trial of sargramostim in pediatric Crohn's disease. Inflamm Bowel Dis. 2010;16:1203–1208. doi: 10.1002/ibd.21204. [DOI] [PubMed] [Google Scholar]
- 12.Korzenik JR, Dieckgraefe BK, Valentine JF, et al. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 13.Sainathan SK, Hanna EM, Gong Q, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2007;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey R, Jurickova I, Ballard E, et al. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willson TA, Kuhn BR, Jurickova I, et al. STAT3 genotypic variation and cellular STAT3 activation and colon leukocyte recruitment in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:32–43. doi: 10.1097/MPG.0b013e318246be78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JI, Kominsky DJ, Jacobson N, et al. Defective leukocyte GM-CSF receptor (CD116) expression and function in inflammatory bowel disease. Gastroenterology. 2011;141:208–216. doi: 10.1053/j.gastro.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nylund CM, D'Mello S, Kim MO, et al. Granulocyte–macrophage colony-stimulating factor autoantibodies and increased intestinal permeability in Crohn disease. J Pediatr Gastroenterol Nutr. 2011;52:542–548. doi: 10.1097/MPG.0b013e3181fe2d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.