In the past several years, there has been increasing interest in the roles of inflammation and immunity in hypertension. Inflammatory cells, including macrophages and T cells are commonly observed in the kidneys of hypertensive animals, and select immunosuppressive agents lower blood pressure and prevent end-organ damage in experimental hypertension.1, 2 Depending on their phenotype, macrophages can release reactive oxygen species, matrix metalloproteinases and cytokines that promote tissue damage, change gene expression, induce vascular remodeling and lead to vasoconstriction and renal damage. Indeed, mice with reduced monocytes and macrophages develop less vascular remodeling, endothelial dysfunction and vascular oxidative stress as compared to wild type mice in response to either angiotensin II or DOCA-salt challenge.3 Recently, genetic deletion of macrophages has been shown to blunt virtually all consequences of angiotensin II infusion, including blood pressure elevation, induction of vascular adhesion molecules, vascular dysfunction and superoxide production.4
It is fairly easy to imagine that innate immune cells like macrophages are involved in hypertension, but rather surprisingly, it seems that T cells of the adaptive immune system also contribute. Almost 50 years ago, White and Grollman showed that injection of lymph node cells from hypertensive rats could raise blood pressure in normotensive recipient rats.5 Svendsen and colleagues showed the thymus played a role in the hypertension caused by partial renal infarction and in the sustained phase of DOCA-salt hypertension.6 When lymphocytes from Lyon rats with genetic hypertension are transferred into Lyon Low pressure rats sustained hypertension develops in the recipients.7 Our group found that mice lacking lymphocytes are protected against several forms of hypertension, including angiotensin II, norepinephrine and DOCA-salt and that adoptive transfer of T cells can restore hypertension in these models. We have proposed that neo-antigens formed in target tissues such as the kidney and vasculature promote T cell activation, which produce cytokines that lead to vascular and renal dysfunction, promoting hypertension.8
In parallel with inflammation, there is unequivocal evidence that the central nervous system plays a critical role in hypertension. Lesions of the forebrain, specifically in the AV3V region, the subfornical organ (SFO) and organum vasculosum of the lateral terminalis (OVLT) prevent various forms of experimental hypertension.9–11 The SFO and OVLT are circumventricular organs that have a poorly formed blood brain barrier, and can be activated by circulating factors such as angiotensin II and salt. Fibers from these extend to the hypothalamus, in particular the paraventricular nucleus (PVN),12 which in turn relays signals to the rostral ventral lateral medulla (RVLM) and other brainstem centers. These brainstem sites integrate input from the baroreceptors and ultimately modulate sympathetic outflow to the kidney and splanchnic circulation.13, 14 As tangible evidence of the importance of these pathways, percutaneous renal denervation has recently proven effective in lowering blood pressure in humans with hypertension resistant to conventional therapy.15
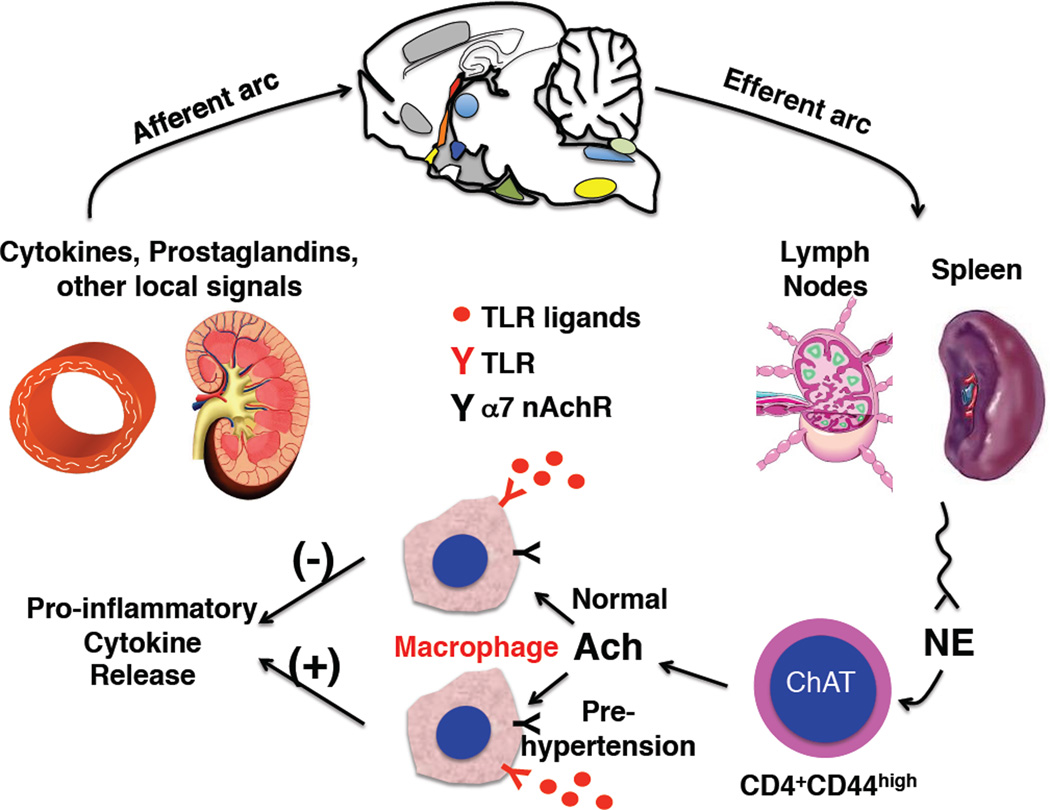
A major question is how and if these two seemingly very separate systems – the immune and nervous systems –interact to modulate blood pressure. Is there evidence that one can affect the other in promoting blood pressure elevation or alterations of vascular or renal function? It turns out that there is enormous interplay between the autonomic and immune systems. Cells of the immune system possess adrenergic and cholinergic receptors that significantly affect their function. Alpha2-adrenergic receptor stimulation enhances antigen uptake by dendritic cells,16 while beta adrenergic stimulation inhibits dendritic cell function.17 T cells possess both alpha and beta adrenergic receptors that have been variously reported to modify polarization, alter proliferation and change surface markers. Monocytes and macrophages also possess α1 and β1 adrenergic receptors that modulate pro-inflammatory cytokine production in response to toll-like receptor (TLR) agonists.18, 19 Likewise monocyte/macrophages possess α7 nicotinic receptors which suppress cytokine production.20, 21 Interestingly, it has recently been shown that a subset of CD4+ cells with a memory phenotype contain choline acetyltransferase and produce acetylcholine upon sympathetic nerve stimulation.22 Tracey and colleagues have described an inflammatory reflex, in which “danger’ signals, such as locally released cytokines and prostaglandins activate vagal afferent nerves that transmit information to the brainstem, the hypothalamus and higher centers. This orchestrates behavioral changes, reduces heart rate variability, increases vagal efferent activity and increases sympathetic outflow.23 Increased vagal and sympathetic stimulation of secondary lymphoid organs promotes acetylcholine release from the aforementioned T cells, which in turn decreases cytokine production by nearby macrophages and therefore dampens the inflammatory response (Figure). Thus, this represents a reflex circuit that is active in myriad illnesses including sepsis, myocardial infarction and multisystem organ failure.
Figure.
Abnormal cytokine release in pre-hypertension. Shown is the “inflammatory reflex” in which local signals in tissues such as vessels and the kidney activate vagal afferent signals to the brain. These ultimately increase vagal and sympathetic outflow. In secondary lymphoid organs, increased norepinephrine (NE) release acts on a subset of memory (CD44high) CD4+ T cells that contain choline acetyltransferase and produce acetylcholine (Ach). The released ach acts on a7 nicotinic receptors of adjacent splenocytes, including macrophages. In normal circumstances, this has an inhibitory effect on pro-inflammatory cytokine release in response to various stimuli such as toll-like receptor (TLR) activation. In the paper by Harwani et al, this effect of nicotinic stimulation is markedly altered in SHR prior to the development of hypertension, such that cytokine release is paradoxically increased. (Illustration: Ben Smith)
In this issue of Circulation Research, Harwani et al24 provide evidence that the effector limb of this reflex is profoundly disturbed in the setting of pre-hypertension. These investigators examined how angiotensin II and nicotine modulate TLR induced cytokine release from isolated splenocytes of young normotensive Wistar Kyoto (WKY) and SHR prior to the development of hypertension. As expected from the circuit shown in the Figure, nicotine pre-exposure suppressed TLR9-mediated interleukin-6 (IL-6) secretion in splenocytes of normal WKY rats, while angiotensin II had no effect. In contrast, pre-exposure to either nicotine or angiotensin II paradoxically increased IL-6 release in response to TLR7/8 and TLR9 stimulation in SHR splenocytes. Nicotine’s anti-inflammatory effect in WKY and pro-inflammatory response in SHR were also observed in vivo. Using flow cytometry, the investigators identified a population of activated macrophages (CD161a+) that seems responsible for production of IL-6 in SHR.
The differential effect of nicotine on IL-6 production could have significant implications for other aspects of inflammation in hypertension. Mice lacking IL-6 are protected against the development of hypertension.25 IL-6 synergizes with TGFβ to promote polarization of T cells to produce IL-17, while TGFβ promotes skewing of T cells to a regulatory phenotype in the absence of IL-6.26 Prior studies from our group have shown that T cells producing IL-17 are critical in the development of hypertension.27 These cells likely infiltrate the perivascular space and the kidney, and the secreted IL-17 promotes superoxide production and accumulation of other inflammatory cells. Thus, the increase in IL-6 in SHR macrophages might have a critical role in skewing responses of T cells that are pro-hypertensive.
It is of interest that SHR demonstrate abnormal macrophage responses prior to the onset of hypertension. This temporal relationship indicates that altered nicotinic responses might be a cause, rather than a consequence of hypertension. There is ample evidence that early life events in SHR affect blood pressure. Classic studies by McCarty and colleagues more than 20 years ago showed that cross fostering SHR pups to normal WKY dams led to a permanent decline in blood pressure and sympathetic outflow as the SHR aged.28, 29 This might be related to epigenetic events that occur early in life.30 Alterations in DNA methylation and histone modifications have recently been identified in several relevant genes in the SHR, including the sodium potassium chloride cotransporter 1, the angiotensin converting enzyme and the β-1 adrenergic receptor in SHR. 31 32, 33 In their current work, Harwani et al found that the α7 nAchR signaling is altered in the SHR, as the splenocyte responses were not blocked by its known inhibitor α-bungarotoxin. Future studies examining epigenetic modification of genes encoding signals downstream of the α7 nAchR might be quite revealing in this regard.
The studies by Harwani et al largely focused on splenocytes, and one can question how important this organ is in the genesis of hypertension. Studies often focus on the spleen, because it is a ready source of leukocytes in small animals. Antigen presentation by macrophages and dendritic cells occurs in secondary lymphoid organs, including the spleen and lymph nodes, which receive both vagal and sympathetic innervation. Thus, the interplay between T cells and macrophages shown in the Figure could also occur in lymph nodes. Of interest, removal of the celiac ganglion, which provides splenic innervation, prevents both angiotensin II and DOCA-salt hypertension.34, 35 This has been attributed to hemodynamic factors, however it is conceivable that an anti-inflammatory effect of denervation might also play a role.
Given these interactions, it is interesting to note that similarities exist between the immune and autonomic nervous systems.36 Both have synapses, develop memory and share a variety of signaling molecules. In both systems, signals from upstream cells (pre-ganglionic neurons and antigen presenting cells) markedly alter function of downstream cells (post-ganglionic neurons and T cells). Plasticity is a feature common to both, and DNA rearrangement, needed for T cell diversification, has been reported in the central nervous system. Chemokines, known to play critical roles in inflammatory cell homing, also contribute to development of the central nervous system.37
In summary, the study by Harwani et al further emphasizes that perturbations of the immune system and the resultant inflammation contribute to hypertension. It should be noted that despite intensive study for the past century, the etiology of most cases of human hypertension remains unknown. This study offers a new understanding of how the interplay between neural signaling and innate immune cells contributes to hypertension, and perhaps provides novel therapeutic directions for this common and devastating disease.
Acknowledgments
Sources of Funding:
Supported by NIH R01HL039006, P01HL058000, P01HL095070, P01GM015431 and R01HL105294-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None
LITERATURE CITED
- 1.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 2.Luft FC, Dechend R, Muller DN. Immune mechanisms in angiotensin II-induced target-organ damage. Ann Med. 2012;44(Suppl 1):S49–S54. doi: 10.3109/07853890.2011.653396. [DOI] [PubMed] [Google Scholar]
- 3.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 5.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–264. [PubMed] [Google Scholar]
- 6.Svendsen UG. Influence of neonatal thymectomy on blood pressure and hypertensive vascular disease in rats with renal hypertension. Acta Pathol Microbiol Scand A. 1975;83:199–205. doi: 10.1111/j.1699-0463.1975.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 7.Renaudin C, Bataillard A, Sassard J. Partial transfer of genetic hypertension by lymphoid cells in Lyon rats. J Hypertens. 1995;13:1589–1592. [PubMed] [Google Scholar]
- 8.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggy J, Fink GD, Johnson AK, Brody MJ. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res. 1977;40:I110–I117. [PubMed] [Google Scholar]
- 10.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H680–H685. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 11.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1564–R1571. doi: 10.1152/ajpregu.00034.2010. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res. 2001;921:78–85. doi: 10.1016/s0006-8993(01)03093-1. [DOI] [PubMed] [Google Scholar]
- 13.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep. 2011;13:221–228. doi: 10.1007/s11906-011-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 16.Yanagawa Y, Matsumoto M, Togashi H. Enhanced dendritic cell antigen uptake via alpha2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. J Immunol. 2010;185:5762–5768. doi: 10.4049/jimmunol.1001899. [DOI] [PubMed] [Google Scholar]
- 17.Maestroni GJ. Adrenergic modulation of dendritic cells function: relevance for the immune homeostasis. Curr Neurovasc Res. 2005;2:169–173. doi: 10.2174/1567202053586776. [DOI] [PubMed] [Google Scholar]
- 18.Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. alpha1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther. 2011;338:648–657. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol. 2010;47:1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi HK, Iwagaki H, Hamano R, Yoshino T, Tanaka N, Nishibori M. alpha7 Nicotinic acetylcholine receptor stimulation inhibits lipopolysaccharide-induced interleukin-18 and -12 production in monocytes. J Pharmacol Sci. 2006;102:143–146. doi: 10.1254/jphs.sc0060074. [DOI] [PubMed] [Google Scholar]
- 22.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal Modulation of the Innate Immune System is Pro-inflammatory in the Pre-hypertensive Spontaneously Hypertensive Rat, a Genetic Model of Essential Hypertension. Circ Res. 2012;111 doi: 10.1161/CIRCRESAHA.112.277475. xxx-xxx [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 Knockout Prevents Angiotensin II Hypertension. Role of Renal Vasoconstriction and Janus Kinase 2/Signal Transducer and Activator of Transcription 3 Activation. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cierpial MA, McCarty R. Hypertension in SHR rats: contribution of maternal environment. Am J Physiol. 1987;253:H980–H984. doi: 10.1152/ajpheart.1987.253.4.H980. [DOI] [PubMed] [Google Scholar]
- 29.Cierpial MA, Konarska M, McCarty R. Maternal influences on sympathetic-adrenal medullary system in spontaneously hypertensive rats. Am J Physiol. 1990;258:H1312–H1316. doi: 10.1152/ajpheart.1990.258.5.H1312. [DOI] [PubMed] [Google Scholar]
- 30.Cowley AW, Jr, Nadeau JH, Baccarelli A, Berecek K, Fornage M, Gibbons GH, Harrison DG, Liang M, Nathanielsz PW, O'Connor DT, Ordovas J, Peng W, Soares MB, Szyf M, Tolunay HE, Wood KC, Zhao K, Galis ZS. Report of the National Heart, Lung, and Blood Institute Working Group on epigenetics and hypertension. Hypertension. 2012;59:899–905. doi: 10.1161/HYPERTENSIONAHA.111.190116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HA, Cho HM, Lee DY, Kim KC, Han HS, Kim IK. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension. 2012;59:621–626. doi: 10.1161/HYPERTENSIONAHA.111.182428. [DOI] [PubMed] [Google Scholar]
- 32.Cho HM, Lee HA, Kim HY, Han HS, Kim IK. Expression of Na+-K+-2Cl- cotransporter 1 is epigenetically regulated during postnatal development of hypertension. Am J Hypertens. 2011;24:1286–1293. doi: 10.1038/ajh.2011.136. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Q, Yuan H, Xing X, Liu J, Huang Z, Du X. Methylation of adrenergic beta1 receptor is a potential epigenetic mechanism controlling antihypertensive response to metoprolol. Indian J Biochem Biophys. 2011;48:301–307. [PubMed] [Google Scholar]
- 34.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1965–H1973. doi: 10.1152/ajpheart.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 36.Habibi L, Ebtekar M, Jameie SB. Immune and nervous systems share molecular and functional similarities: memory storage mechanism. Scand J Immunol. 2009;69:291–301. doi: 10.1111/j.1365-3083.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- 37.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]