Abstract
Hookworm infections and tuberculosis are co-endemic in many parts of the world. It has been suggested that infection with helminth parasites could suppress the predominant Th1 (IFN-γ-mediated) response needed to control Mycobacterium tuberculosis (Mtb) infection and enhance susceptibility to infection and/or disease. To determine the role of coincident hookworm infection on responses at steady state and on Mtb – specific immune responses in latent tuberculosis (TB), we examined the cellular responses in individuals with latent TB with or without concomitant hookworm infection. By analyzing the expression of Th1, Th2 and Th17 subsets of CD4+ T cells, we were able to demonstrate that the presence of coincident hookworm infection significantly diminished both spontaneously expressed and Mtb – specific mono – and dual – functional Th1 and Th17 cells. Hookworm infection, in contrast, was associated with expanded frequencies of mono – and dual – functional Th2 cells at both steady state and upon antigen – stimulation. This differential induction of CD4+ T cell subsets was abrogated upon mitogen stimulation. In addition, coincident hookworm infection was associated with increased adaptive T regulatory (aTreg) cells but not natural regulatory T cells (nTregs) in latent TB. Finally, the CD4+ T cell cytokine expression pattern was also associated with alterations in the systemic levels of Th1 and Th2 cytokines. Thus, coincident hookworm infection exerts a profound inhibitory effect on protective Th1 and Th17 responses in latent tuberculosis and may predispose toward the development of active tuberculosis in humans.
INTRODUCTION
Soil transmitted helminths (STHs) are complex eukaryotic organisms, characterized by their ability to maintain long-standing infections in humans, sometimes lasting decades. Hence, parasitic helminths are a major health care problem worldwide, infecting more than two billion people, mostly in resource-limited countries. In addition, helminth parasites are often clinically asymptomatic due, in large part, to the parasites' ability to manipulate the host immune system to enhance their survival and to restrict local inflammatory pathology (1). Modulation of the host immune response involves a variety of strategies including induction of regulatory networks and dysregulation of innate and adaptive immune responses (1). The immune down modulation associated with helminth infections is mostly parasite-antigen specific, but some bystander effects on routine vaccinations, allergic processes, and autoimmune diseases have been noted (2, 3). Helminth infections are known to impair immune responses to oral cholera (4, 5), tetanus toxoid (6, 7) and bacille Calmette-Guérin (BCG) vaccinations (8) and to thereby reduce their efficacy in helminth-endemic populations.
Hookworm infections are common intestinal helminth infections (affecting 740 million people worldwide) known to cause intestinal injury and blood loss (9). These infections occur throughout the tropics and subtropics and in many regions of the world have an overlapping geographic distribution with Mycobacterium tuberculosis (Mtb). The control of Mtb infection requires a clearly delineated Th1 response (IL-12, IFN-γ and TNF-α and, to a lesser extent, Th17 response (IL-17 and IL-23). Both Th1 and Th17 responses have been shown to be important in the induction and maintenance of protective immune responses in mouse models of Mtb infection or for control of human Mtb infection (as seen in latent TB) (10–12). During latency, Mtb is contained within granulomas, where the mycobacteria reside in macrophages and in which growth and replication appears to be constrained. Maintenance of the granulomatous lesion is mediated by CD4+ and CD8+ T cells (13).
Mycobacteria-specific T cells mediate delayed-type hypersensitivity reactions to purified protein derivative (PPD), and this reaction (in the absence of demonstrable active infection) is generally considered to indicate latent TB (14). More recently, interferon-γ release assays (IGRAs) that enable the detection of circulating T lymphocytes responsive to specific Mtb antigens have been used to detect latent TB (15). Mtb induces prototypical Th1 and Th17 responses in CD4+ and CD8+ T cells both in mouse models and in human infection, in addition to inducing activation of macrophages (with predominant Nos2 production) and both Toll-like receptor- and Nod-like receptor-mediated NF-κB activation (10–12). Finally, multi-functional CD4+ Th1 cells, co-expressing IFN-γ, TNF-α and IL-2 and dual – functional cells expressing IFN-γ/IL-2 and IFN-γ/TNF-α have been shown to be associated with protection against active pulmonary disease in TB (16–18).
Age-specific prevalence studies have indicated that infections with hookworms usually precede the acquisition of tuberculin skin test positivity (19). Thus, in co-infected individuals, hookworm infection often precedes the acquisition of latent TB. We hypothesized that immune responses in latent TB would be modulated by the regulatory immune networks often seen in chronic helminth infection. To this end, we examined the induction of Th1, Th2, and Th17 responses in latent TB individuals with or without active hookworm infections. We observed that the presence of hookworm infection altered profoundly the Mtb-specific responses in individuals with coincident latent TB, specifically by diminishing the frequencies of mono – and dual – functional Th1 and Th17 cells and by expanding Th2 subset differentiation. This alteration in T helper cell subset frequencies was reflected in systemic levels of T cell derived cytokines.
MATERIALS AND METHODS
Study population
We studied a group of 42 individuals with latent TB, 21 of who were positive by stool microscopic examination for hookworm infection in Tamil Nadu, South India (Table 1). Latent TB was diagnosed by a positive IGRA, using the QuantiFERON-TB Gold In-Tube (Cellestis, Valencia, CA). All subjects had normal chest radiographs. None of the subjects had pulmonary symptoms (cough, fever, chest pain, hemoptysis) nor a positive sputum for Mtb by smear microscopy and culture. All individuals were examined as part of a clinical protocol approved by Institutional Review Boards of both the National Institute of Allergy and Infectious Diseases and the National Institute for Research in Tuberculosis (NCT00375583), and informed written consent was obtained from all participants.
Table 1.
| Study population | |||
|---|---|---|---|
| LTB | HW/LTB | P value | |
| Sample size (n) | 21 | 21 | - |
| Age Median (Range) | 37 (19–76) | 32 (20–58) | - |
| Sex (M/F) | 12/9 | 18/3 | - |
| Eggs in stool | Negative | Positive | - |
| Immunological profile | |||
| CD4+T cells [absolute numbers] GM (Range) |
970.62 (659–1503) | 956 (538–2084) | NS |
| Effector memory T cells [% of CD4+T cells] GM (Range) |
27.04 (15.31–52.16) | 34.33 (19.70–79.88) | NS |
| Central memory T cells [% of CD4+T cells] GM (Range) |
47.5 (21.97–65.92) | 34.58 (16.95–46.46) | p=0.0049 |
| Naive T cells [% of CD4+T cells] GM (Range) |
15.78 (8.79–25.97) | 23.13 (7.37–49.46) | p=0.0216 |
Parasitologic examination
Stool samples were collected, transported to the laboratory at ambient temperatures, and examined by direct microscopy and by formal-gasoline concentration techniques, as described previously (19). Stool microscopy was used to exclude the presence of other intestinal helminths including Ascaris, Strongyloides, Trichuris, Enterobius, Taenia and Hymenolepsis. Filarial infection was excluded by the TropBio Og4C3 enzyme-linked immunosorbent assay (ELISA) (Trop Bio Pty. Ltd, Townsville, Queensland, Australia).
Flow cytometry analysis
Flow cytometry acquisition was done on BD FACS Canto II (BD Biosciences, San José, CA, USA). Analysis was done using FlowJo software v9.4.10 (TreeStar Inc., Ashland, OR, USA).
Total T cells and naïve, memory, and regulatory T cell subsets
Absolute CD4+T cell counts were enumerated in whole blood using BD Multiset™ 6-Color TBNK cocktail (BD Biosciences). Naïve and memory T cell phenotyping was performed using FITC-CD45RA (BD Pharmingen™; BD Biosciences) and APC-CCR7 (eBioscience, San Diego, CA, USA) staining in CD4+ and CD8+ T cells. Naïve cells were classified as CD45RA+CCR7+, effector memory cells as CD45RA−CCR7−, and central memory cells as CD45RA−CCR7+. Natural Tregs (nTregs) were classified as CD4+CD25+Foxp3+CD127dim (BD Pharmingen™ and eBioscience) (see Fig S3 for gating strategy).
Antigens
Mycobacterial antigens— recombinant early secreted antigen-6 and culture filtrate protein-10 (ESAT-6/CFP-10) (Fitzgerald Industries Intl. Inc, Acton, MA)— as well as total CFP from Mtb H37 Rv (Mtb CFP) were used as the antigenic stimuli. Final concentrations were 10 µg/ml for ESAT-6/ CFP-10 and 10 µg/ml for Mtb CFP. Phorbol ester (PMA) and ionomycin at concentrations of 12.5 ng/ml and 125 ng ml, respectively, were used as the positive control stimuli.
In Vitro Culture
Whole blood cell cultures were performed to determine the intracellular levels of cytokines. Briefly, whole blood was diluted 1:1 with RPMI-1640 medium supplemented with penicillin/streptomycin (100 U/100 mg/ml), L-glutamine (2 mM), and HEPES (10 mM) (all from Invitrogen, San Diego, CA) and distributed in 12-well tissue culture plates (Costar, Corning Inc., Corning, NY). The cultures were then stimulated with Mtb CFP or ESAT-6/ CFP-10 or PMA/ - onomycin or media alone in the presence of the costimulatory molecules CD49d/CD28 at 37°C for 6 h. FastImmune™ Brefeldin A solution (10 µg/ml) was added after 2 h. After 6 h, centrifugation, washing, and red blood cell lysis were performed. Cells were fixed using cytofix/ cytoperm buffer (BD Biosciences) and cryopreserved at –80°C.
Intracellular Cytokine Staining
The cells were thawed, washed, and then stained with surface antibodies for 30–60 min. Surface antibodies used were CD3 (Amcyan), CD4 (APC-H7), and CD8 (PE-Cy7). The cells were washed and permeabilized with BD Perm/Wash™ buffer (BD Biosciences) and stained with intracellular cytokines for an additional 30 min before washing and acquisition. Cytokine antibodies used were IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-10, IL-13, IL-17A, IL-17F, and IL-22. Eight-color flow cytometry was performed on a FACSCanto II flow cytometer with FACSDiva software v.6 (Becton Dickinson and Company, Cockeysville, MD). Lymphocyte gating was set by forward and side scatter, and 100,000 lymphocyte events were acquired. Gating for CD4+ T cells expressing cytokines was determined by FMO (fluorescence minus one). Data were collected and analyzed using Flow Jo software (TreeStar Inc., Ashland, OR). All data are depicted as frequency of CD4+ T cells expressing cytokine(s). Baseline values following media stimulation are depicted as baseline frequency, while frequencies following stimulation with antigens or PMA/ Ionomycin are depicted as net frequencies (with baseline values subtracted).
ELISA
The levels of cytokines in plasma were measured using Bioplex multiplex cytokine assay system (Biorad). The cytokines analyzed were IL-2, IFN-γ, TNF-α, IL-4, IL-5 and IL-13.
Statistical analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendency. Statistically significant differences between two groups were analyzed using the nonparametric Mann-Whitney U test and multiple comparisons were corrected using the Holm’s correction.
RESULTS
Hookworm infection is associated with constitutively decreased frequencies of mono – and dual – functional CD4+ Th1 and Th17 cells and increased frequencies of Th2 cells
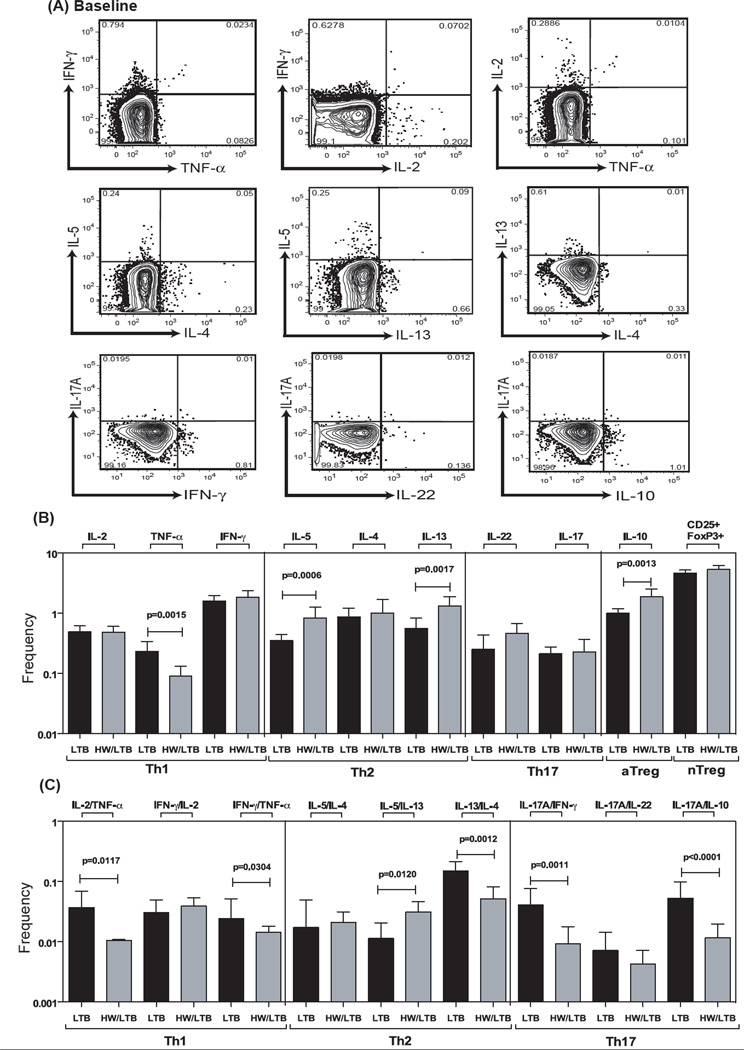
To determine the impact of hookworm infection on the steady state (or constitutive) Th1, Th2 and Th17 profile of latent TB individuals, we cultured whole blood from co-infected (HW/LTB) and latent TB only (LTB) individuals with media alone and measured the frequency of CD4+ T cells expressing each of the Th1-, Th2- and Th17-associated cytokines (Fig. 1A). As shown in Fig 1, the frequencies of CD4+ T cells expressing TNF-α alone or co – expressing TNF-α/IFN-γ, TNF-α/IL-2, IL-17A/IFN-γ or IL-17A/IL-10 were all significantly reduced in co-infected individuals compared to latent TB infected individuals. On the other hand, the frequencies of CD4+ T cells expressing IL-5 or IL-13 alone or co-expressing IL-5/IL-13 were significantly increased in co-infected individuals. Also, hookworm infection was associated with constitutively higher frequencies of CD4+ T cells expressing IL-10 (adaptive Tregs) but not natural regulatory T (nTregs) cells. Therefore, hookworm infection is associated with profound alterations in the repertoire of Th1, Th2 and Th17 subsets at steady state.
Figure 1. Hookworm infection is associated with decreased constitutively expressed frequencies of CD4+ Th1 and Th17 cells and increased frequencies of Th2 cells in latent TB.
Whole blood was cultured with media alone for 6 h and the baseline frequencies of Th1, Th2 and Th17 cells determined. (A) Representative whole-blood intracellular cytokine assay flow data from a latent TB individual showing expression of Th1, Th2 and Th17 cytokines at baseline. The plots shown are gated on CD3+CD4+ T cells. The baseline frequencies of (B) mono – and (C) dual – functional CD4+ Th1, Th2 and Th17 cells are shown. All individuals were latent TB infected with (HW/LTB; n=21) or without (LTB; n=21) concomitant hookworm infection. The bars represent geometric mean and 95% confidence intervals. P values were calculated using the Mann-Whitney test.
Hookworm infection is not associated with alterations in phorbol ester / ionomycin induced Th1, Th2 and Th17 responses
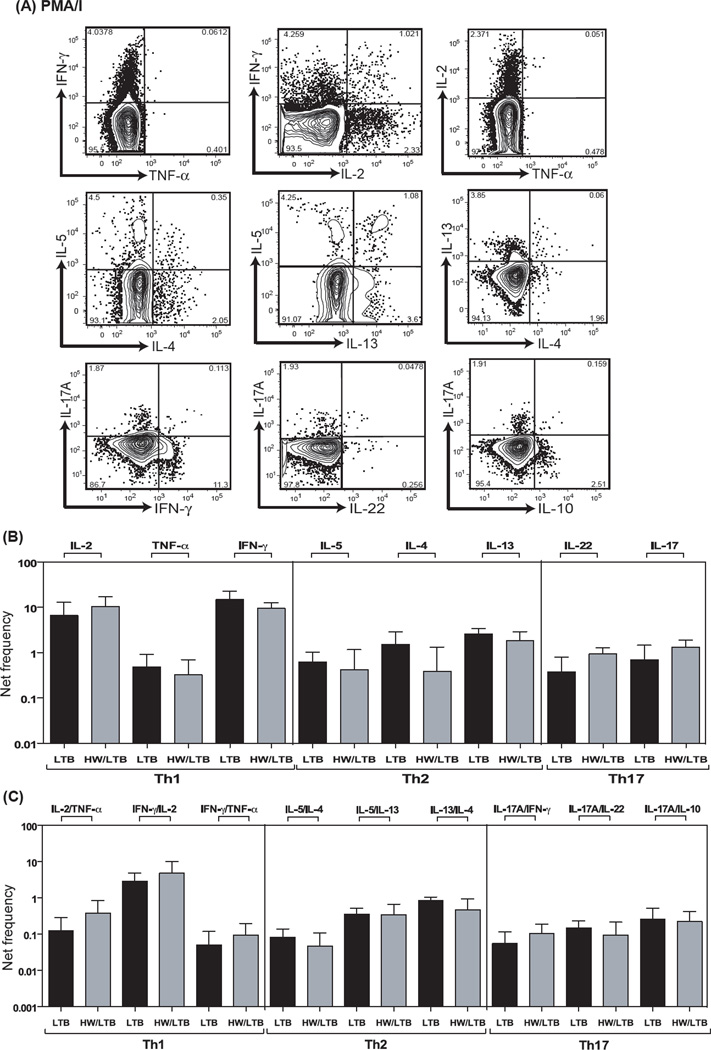
To determine whether the altered baseline repertoire of Th1, Th2 and Th17 cells influences the ability of CD4+ T cells to respond to a mitogenic stimulus, we stimulated whole blood from HW/LTB or LTB individuals with PMA/ ionomycin for 6 h and measured the frequencies of mono – and multi – functional CD4+ T cells expressing Th1, Th2 and Th17 cytokines (Fig. 2A). As shown in Fig 2B, the net frequency of mono – functional CD4+ Th1, Th2 and Th17 cells was not significantly increased in co-infected (HW/LTB) individuals compared to LTB infected individuals. Also as shown in Fig 2C, the frequencies of dual – functional CD4+ T cells expressing different combinations of dual cytokines of the Th1, Th2 and Th17 family were not significantly altered in co-infected individuals. Therefore, hookworm infection modulated the repertoire of CD4+ T cells in latent TB but did not impair the ability of these cells to respond appropriately to mitogenic stimulation.
Figure 2. Hookworm infection is not associated with alterations in the frequencies of mono – and dual – functional CD4+ Th1, Th2 and Th17 cells upon phorbol ester and ionomycin stimulation.
Whole blood was cultured with PMA/ Ionomycin for 6 h and the net frequencies of CD4+ Th1, Th2 and Th17 cells determined. (A) Representative whole-blood intracellular cytokine assay flow data from a latent TB individual showing expression of Th1, Th2 and Th17 cytokines following PMA/ ionomycin stimulation. The plots shown are gated on CD3+CD4+ T cells. (B) Net frequency of CD4+ T cells expressing only mono –functional Th1, Th2 and Th17 cytokines is shown as bar graphs. (C) Net frequency of CD4+ T cells expressing dual –functional Th1, Th2 and Th17 cytokines is shown as bar graphs. The bar represents the geometric mean of the frequency of CD4+ T cells expressing the respective cytokine(s), and the error bar represents the 95% confidence interval in hookworm uninfected (LTB, n = 21) and hookworm infected (HW/LTB, n = 21) latent TB individuals. Net frequencies were calculated by subtracting baseline frequency from the antigen – induced frequency for each individual. P values were calculated using the Mann-Whitney test.
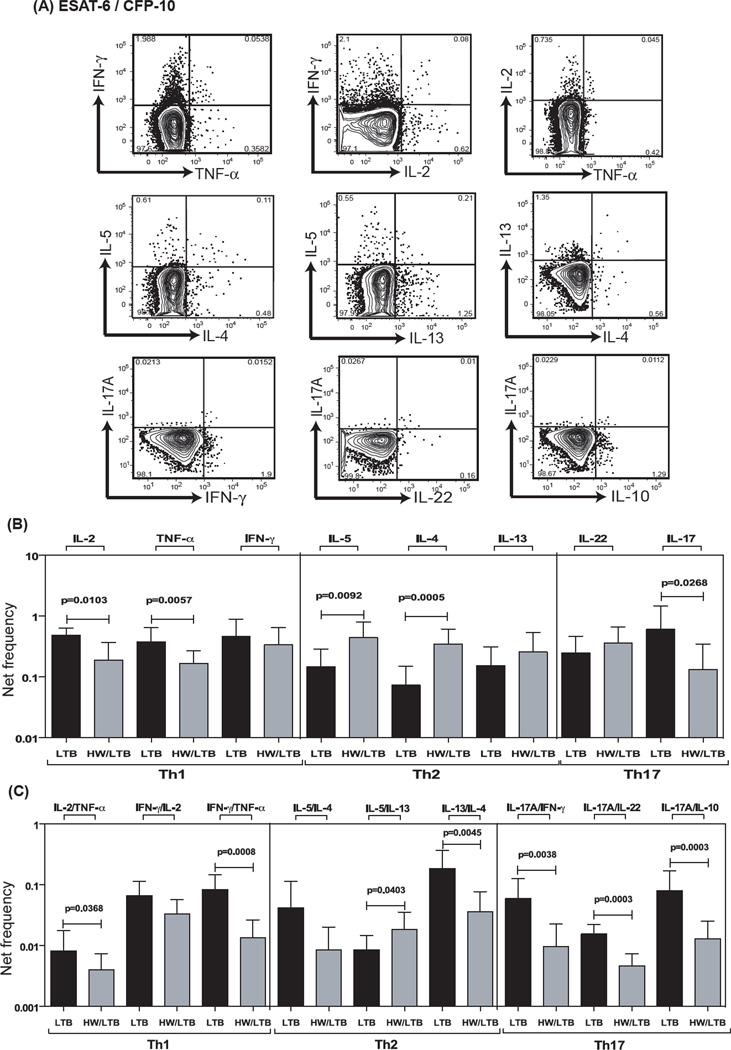
Hookworm infection is associated with decreased antigen – specific frequencies of mono – and dual – functional Th1 and Th17 cells and increased Th2 cells
To determine the impact of coexisting hookworm infection on mycobacterial antigen-specific Th1, Th2 and Th17 responses in latent TB, we stimulated whole blood from co-infected or singly infected individuals with mycobacterial antigens - ESAT-6/CFP-10 or total CFP for 6 h and measured the frequencies of mono – and dual – functional Th1, Th2 and Th17 cells (Figure 3A). As shown in Fig 3B, the frequency of CD4+ T cells expressing IL-2, TNF-α or IL-17A alone was significantly reduced while the frequency of CD4+ T cells expressing IL-5 or IL-13 alone was significantly increased following antigen - stimulation in HW/LTB individuals compared to those with LTB. Similarly, as shown in Fig 3C, the frequencies of CD4+ T cells co-expressing IL-2/TNF-α, IFN-γ/TNF-α, IL-17A/IFN-γ, IL-17A/IL-22 and IL-17A/IL-10 were all significantly reduced following ESAT-6/CFP-10– stimulation. In contrast, the frequency of CD4+ T cells co-expressing IL-5/IL-13 (but not IL-4/IL-13) was significantly increased following Mtb antigen – stimulation. In addition, as shown in Fig S1 and Fig S2, the frequencies of CD4+ T cells expressing IL-2 or TNF-α alone or co-expressing IL-2/TNF-α, IFN-γ/TNF-α, IL-17A/IFN-γ or IL-17A/IL-10 were significantly reduced following total CFP stimulation. Therefore, hookworm infection profoundly alters the antigen – stimulated Th1 and Th17 responses in latent TB.
Figure 3. Hookworm infection is associated with decreased antigen – specific frequencies of mono – and dual – functional CD4+ Th1 and Th17 cells and increased frequencies of Th2 cells in latent TB.
Whole blood was cultured with ESAT-6/CFP-10 for 6 h and the net frequencies of CD4+ Th1, Th2 and Th17 cells determined. (A) Representative whole-blood intracellular cytokine assay flow data from a latent TB individual showing expression of Th1, Th2 and Th17 cytokines following antigen stimulation. The plots shown are gated on CD3+CD4+ T cells. (B) Net frequency of CD4+ T cells expressing only mono –functional Th1, Th2 and Th17 cytokines is shown as bar graphs. (C) Net frequency of CD4+ T cells expressing dual –functional Th1, Th2 and Th17 cytokines is shown as bar graphs. The bar represents the geometric mean of the frequency of CD4+ T cells expressing the respective cytokine(s), and the error bar represents the 95% confidence interval in hookworm uninfected (LTB, n = 21) and hookworm infected (HW/LTB, n = 21) latent TB individuals. Net frequencies were calculated by subtracting baseline frequency from the antigen – induced frequency for each individual. P values were calculated using the Mann-Whitney test.
Hookworm infection is associated with altered systemic levels of Th1 and Th2 cytokines in latent TB
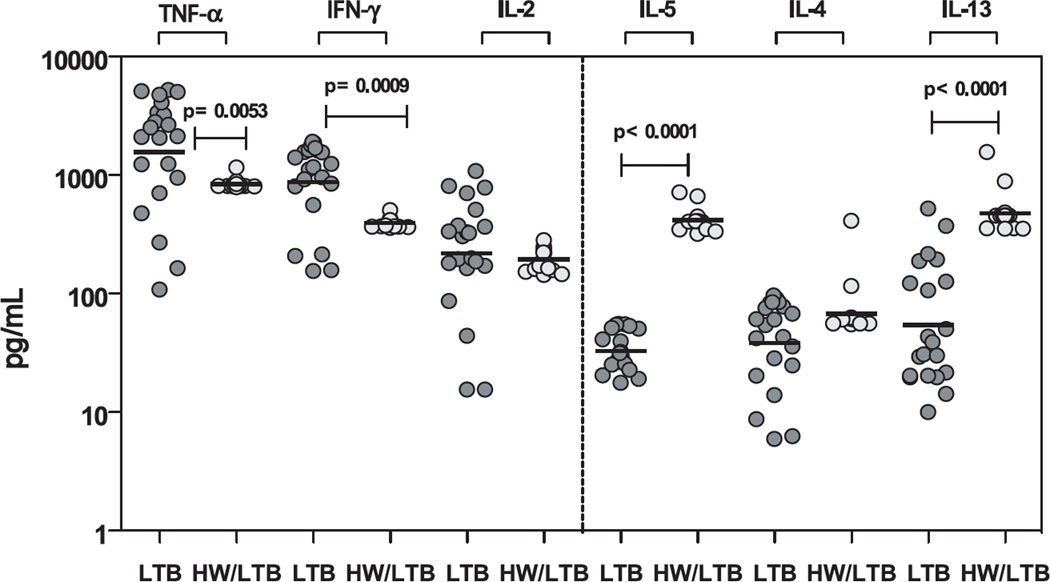
To examine whether alterations in the frequency of CD4+ Th1 and Th2 cells results in alterations in the circulating levels of the prototypical Th1 and Th2 cytokines, we measured the levels of IFN-γ, TNF-α, IL-2, IL-4, IL-5 and IL-13 in the plasma of HW/LTB co-infected and LTB-infected individuals. As shown in Figure 4, we observed significantly lower plasma levels of IFN-γ (GM of 873.6 pg/ml in LTB vs. 394.8 pg/ml in HW/LTB-infected, p = 0.0009) and TNF-α (GM of 1561 pg/ml in LTB vs. 842.1 pg/ml in HW/LTB, p = 0.0053) but not IL-2 in co-infected individuals compared to latent TB infected individuals. We also observed significantly higher levels of IL-5 (GM of 33.0 pg/ml in LTB vs. 417.6 pg/ml in HW/LTB, p < 0.0001) and IL-13 (GM of 54.5 pg/ml in LTB vs. 477.3 pg/ml in HW/LTB, p < 0.0001) in co-infected individuals compared to latent TB infected individuals. Thus, alterations in CD4+ T cell cytokine expression is reflected in the corresponding changes in systemic levels of Th1 and Th2 cytokines in coinfected individuals.
Figure 4. Hookworm infection is associated with alterations in the plasma levels of Th1 and Th2 cytokines in latent TB.
The plasma levels of Th1 - IFN-γ, IL-2, TNF-α and Th2 – IL-4, IL- 5, IL-13 cytokines were measured by ELISA in latent TB infected individuals with (HW/LTB; n=21) or without (LTB; n=21) concomitant hookworm infection. The results are shown as scatterplots with each circle representing a single individual. P values were calculated using the Mann-Whitney test.
DISCUSSION
A wide variety of studies have been performed to examine the possible effect of helminth infection on the induction of a protective immune response against mycobacteria (20, 21). Both intestinal and systemic helminths have been shown to modulate proliferation and IFN-γ production in response to PPD in helminth – TB coinfected individuals (20, 21). Some of these defects have been shown to be reversible following anthelmintic chemotherapy (20). We have previously demonstrated that concurrent filarial infection could inhibit the generation of potentially protective Th1 immune responses in latent TB infected individuals (22). In addition to a significant reduction of Th1 responses by PPD-specific T cells in helminth infected patients, it was shown that IL-23 and IL-17 production in response to both PPD and Mtb culture filtrate was also significantly lower in filarial-infected latent TB individuals when compared with latent TB controls without lymphatic filariasis (22). Furthermore, patients co-infected with filaria and Mtb exhibited a significant reduction of both Toll-like receptor (TLR)-2 and TLR-9 expression and in pro-inflammatory cytokine production and that treatment with antihelmintic drugs restored these responses suggesting that helminth-induced immunomodulatory effects are transient (23).
Intestinal helminth co-infection has also been shown to adversely impact antimycobacterial immune responses (24, 25). Other studies have also demonstrated that intestinal helminth infections may be one of the risk factors for the development of active pulmonary TB (26). Similarly, a recent study found that there was an association between Schistosoma mansoni infection and progression to TB disease in HIV-infected Ugandan individuals (27). Finally, the immunogenicity of BCG vaccination has been shown to be impaired in helminth-infected individuals, and this is associated with enhanced TGF-β production but not enhanced Th2 responses (24). Apart from human studies, a variety of animal model of co-infection have confirmed the influence of helminth infection on the immune response to TB (21, 28). Thus, although a variety of epidemiological studies have demonstrated potential association between the presence of helminth infections and the susceptibility to TB disease, very few studies have examined the mechanism behind this potential connection.
The interplay among different CD4+ T cell effector subsets plays a key role in defining immune responses to pathogens as well as to a variety of inflammatory stimuli and the prevention/development of autoimmunity. Not only do Th1 and Th17 cells play an important role in the establishment/maintenance of a variety of chronic inflammatory and autoimmune disorders, they appear to be critical in mediating resistance to a variety of intracellular infections (29, 30). In Mtb, for example, Th1 responses are absolutely necessary for inducing resistance, with Th17 responses important in inducing and maintaining memory and recall responses (10). Because immune-mediated protection against Mtb is characterized by strong mycobacterium-specific Th1 responses, it has been postulated that coincident infections with helminth parasites could modulate these immune responses by driving Th2 and/or Tregs that induce anti-inflammatory responses (3). However, the effect of co-infection on antigen – specific induction of protective or pathogenic Th1, Th2 and Th17 subsets have not been carefully examined.
The examination of constitutive or mitogen – induced immune responses revealed certain interesting differences between hookworm co-infected or LTB alone infected individuals. First, the constitutive frequencies of CD4+ cells producing Th1 and Th17 cytokines were significantly down-regulated in co-infected individuals. Since TNF-α and IL-17 have been reported to exhibit anti-mycobacterial activity either in primary or memory responses to infection (31–33), the fact that hookworm co-infection influences the induction of these T cells suggests a potential compromise in anti-bacterial immunity in the presence of this helminth infection. Second, hookworm infections were also associated with constitutively increased frequency of CD4+ T cells expressing antigen-driven Th2 cells, cells that have been implicated in susceptibility to TB infection because of their potential ability to down-modulate protective Th1 responses (34), to induce alternative activation of macrophages leading to diminished bactericidal responses (35) and to inhibit autophagy, also involved in bacterial killing (36). Thus, the increased frequency of Th2 cells observed in HW/LTB co-infections could potentially serve another possible mechanism by which there is an increased risk of promoting development of active TB. Third, while hookworm infection was associated with a profound modulation of Th1, Th2 and Th17 baseline repertoires, it did not impair the ability of these CD4+ T cells to respond appropriately to a positive stimulus. While hookworm infection mediates alterations in the baseline subsets of CD4+ T cells, we were interested in examining the effect of these alterations on mycobacterial – antigen specific responses in these latent TB infected individuals. We primarily focused on the expression of mono – and dual – functional expressing CD4+ Th1, Th2 and Th17 cells. While the role of CD4+ T cells, IFN-γ and TNF-α in resistance to TB is well established, the role multi – functional CD4+ T cells is still not clear (37). CD4+ T cells expressing IL-2 alone or those co-expressing IL-2 and IFN-γ or TNF-α and IFN-γ have been show to be potential correlates of protective immunity to Mtb (18, 38). Similarly, multi – functional CD4+ T cells co-expressing IFN-γ, TNF-α and IL-2 have also been shown to correlate with immunity to Mtb in a study comparing smear-positive TB to those with smear-negative TB or latent TB (16). Thus, mono – and dual – functional Th1 cells clearly play an important role in susceptibility or resistance to infection and/or disease. Our data in HW/LTB co-infected individuals suggests that both mono – and dual – functional antigen – specific Th1 and Th17 cells are functionally impaired following antigen – stimulation. Therefore, one of the potential mechanisms by which helminth infections impair the ability to respond to Mtb is by altering the antigen – specific immune responses of Th1, Th2 and Th17 cells, alterations that could have a profoundly detrimental effect by leading to reactivation of TB.
Helminth infections establish persistent infections, chronicity that is facilitated by mechanisms that dampen immune responses. The two major mechanisms by which helminth infections can modulate immune responses are by the induction of nTregs and CD4+ T cells expressing IL-10 (aTregs) (39). Foxp3-expressing T cells are nTregs that play an important role in peripheral tolerance and attenuation of immune responses (40). Foxp3 expression is known to be upregulated in human hookworm infections (41), and CD4+CD25+ T cells are known to mediate suppression as evidenced by decreased allergic inflammation in an animal model of helminth infection (42, 43). Similarly, IL-10 is known to mediate immune suppression in a variety of helminth infections (44). Hence, we wanted to examine the nature of the cells that might be responsible for this regulation. Interestingly, while we observed no significant differences in the frequency of nTregs, hookworm co-infection was associated with a significantly enhanced frequency of IL-10 expressing CD4+ Tcells, suggesting that adaptive Tregs might play the most important role in modulation of the T cell subsets observed in the present study. In addition, hookworm infections did not modulate the absolute numbers or the frequencies of effector memory T cells in latent TB – infecting individuals, suggesting that the altered CD4+ T cell repertoire is not due to alterations in T cell numbers or memory subsets.
There is a great deal of geographic overlap in the regions of high hookworm endemicity and susceptibility to TB (19). We therefore examined whether established immune responses against a given infection could affect the immune response to a subsequent infection. Our study clearly demonstrates the modulation of immune responses to Mtb by coincident hookworm infection. Hookworm-induced regulatory networks appear to be associated with a significant bystander/spillover effect on the immune responses against mycobacterial antigens in latent TB patients. Our findings may have significant implications for vaccine efficacy in helminth-endemic countries and potentially for understanding how latency in Mtb is broken. Understanding the pathways that helminth infections utilize to mediate bystander suppression/modulation to exogenous antigens and infections should enable new strategies to antagonize suppression for controlling deleterious infections and optimal boosting of vaccine efficacy.
Supplementary Material
Acknowledgments
We would like to thank the field staff at the Epidemiology Unit of NIRT in Tiruvallur for their assistance in the conduct of this study.
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Because T.B.N., and S.B. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMed Central for display and use by the public, and PubMed Central may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Footnotes
Author disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
REFERENCES
- 1.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper PJ, Chico ME, Losonsky G, Sandoval C, Espinel I, Sridhara R, Aguilar M, Guevara A, Guderian RH, Levine MM, Griffin GE, Nutman TB. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 6.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 7.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 12.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 11:343–354. doi: 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- 13.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann SH. Tuberculosis: back on the immunologists' agenda. Immunity. 2006;24:351–357. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Herrera V, Perry S, Parsonnet J, Banaei N. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin Infect Dis. 52:1031–1037. doi: 10.1093/cid/cir068. [DOI] [PubMed] [Google Scholar]
- 16.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman P, Lalvani A. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipner EM, Gopi PG, Subramani R, Kolappan C, Sadacharam K, Kumaran P, Prevots DR, Narayanan PR, Nutman TB, Kumaraswami V. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am J Trop Med Hyg. 2006;74:841–847. [PubMed] [Google Scholar]
- 20.Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 7:231–238. doi: 10.1097/COH.0b013e3283522c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafi W, Ribeiro-Rodrigues R, Ellner JJ, Salgame P. Coinfection-helminthes and tuberculosis. Curr Opin HIV AIDS. 2012;7:239–244. doi: 10.1097/COH.0b013e3283524dc5. [DOI] [PubMed] [Google Scholar]
- 22.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. Human Type 1 and 17 Responses in Latent Tuberculosis Are Modulated by Coincident Filarial Infection through Cytotoxic T Lymphocyte Antigen-4 and Programmed Death-1. J Infect Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl Trop Dis. 2009;3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 25.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11:551–558. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 27.Brown M, Miiro G, Nkurunziza P, Watera C, Quigley MA, Dunne DW, Whitworth JA, Elliott AM. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. Am J Trop Med Hyg. 2006;74:819–825. [PubMed] [Google Scholar]
- 28.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 42:2215–2220. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 31.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 32.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 33.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17-and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 35.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, Kaufmann SH. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 36.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 40:2139–2142. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 38.Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 40.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricci ND, Fiuza JA, Bueno LL, Cancado GG, Gazzinelli-Guimaraes PH, Martins VG, Matoso LF, de Miranda RR, Geiger SM, Correa-Oliveira R, Gazzinelli A, Bartholomeu DC, Fujiwara RT. Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl Trop Dis. 5:e1383. doi: 10.1371/journal.pntd.0001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MS, Maizels RM. Regulatory T cells induced by parasites and the modulation of allergic responses. Chem Immunol Allergy. 2006;90:176–195. doi: 10.1159/000088892. [DOI] [PubMed] [Google Scholar]
- 44.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.