Abstract
Increased cell migration and invasion lead to cancer metastasis and are crucial to cancer prognosis. In this study, we explore whether FBXW7 plays any role in metastatic process. We show that depletion of FBXW7 induces epithelial-mesenchymal transition (EMT) in human colon cancer cells along with the increase in cell migration and invasion. Moreover, FBXW7 deficiency promotes the generation of colon cancer stem-like cells in tumor-sphere culture. mTOR inhibition by rapamycin suppresses FBXW7 loss-driven EMT, invasion and stemness. Our results define the FBXW7/mTOR axis as a novel EMT pathway that mediates cancer invasion.
1. Introduction
The lethal consequences of solid cancers are related to their metastasis to other organ sites. Therefore tremendous research effort has been concentrated on understanding the metastatic processes. Increasing evidence shows that epithelial-mesenchymal transition (EMT) plays a crucial role in tumor progression and metastasis [1]. EMT is characterized by the loss of epithelial characteristics and acquisition of a mesenchymal phenotype, which confers the ability for cancer cells to invade adjacent tissue and migrate to distant sites [2]. Identification of genes that regulate the EMT phenotypic switching will lead to understandings of the longstanding puzzles of metastasis and new therapeutic targets for metastatic cancer.
The cancer stem cell (CSC) hypothesis provides an attractive model of tumor development and progression, holding that solid tumors are hierarchically organized and sustained by a minority of the tumor cell population with stem cell properties, such as self-renewal, tumorigenicity and multilineage differentiation capacity [3]. Therapeutic resistance, underlying tumor recurrence and the lack of curative treatments in metastatic disease, raise the question whether conventional anticancer therapies target the right cells. Indeed, these treatments might miss CSCs, which represent a more chemoresistant and radioresistant subpopulation within cancer [4]. Recently, a direct link between EMT process and the gain of stem cell competence was demonstrated in cultured cancer cells [5; 6]. In particular, it was shown that the induction of EMT program not only allowed cancer cells to disseminate from the primary tumor, but also promoted their self-renewal capability. Furthermore, the expression of stemness and EMT markers in cancer cells are associated with resistance to conventional anti-cancer therapies and treatment failure, highlighting the urgency of improving tools for detecting and eliminating minimal residual disease.
F-box and WD repeat domain containing 7 (FBXW7) encodes a substrate adaptor for an SCF E3 ubiquitin ligase complex and lies at the nexus of many pathways which control cell growth, cell differentiation, and tumorigenesis by negatively regulating the abundance of different oncoproteins, including c-Myc, c-Jun, cyclin E, Notch, Aurora-A, mTor, KLF5, and MCL-1 [7; 8]. FBXW7 as a human tumor suppressor gene is further supported by the discovery of FBXW7 gene mutations in cancers from a wide spectrum of human tissues with overall 6% point mutation frequency [7; 8]. Furthermore, deletion of the Fbxw7 gene in mice leads to embryonic lethality, but heterozygous mice develop normally [9; 10]. Although they do not develop spontaneous tumors, radiation exposure gives rise to different types of tumors, including a range of epithelial cancers and that one allele inactivation of Fbxw7 accelerates tumor development in p53 heterozygous mice [11]. This paper presents that depletion of FBXW7 in colon cancer cells induces EMT and cancer stem cell-like characteristics, which can be suppressed by mTOR inhibitor, rapamycin.
2. Materials and methods
2.1 Cell culture and rapamycin treatment
The human colon cancer cell lines HCT116 and DLD-1 FBXW7+/+ and FBXW7−/− were kind gifts from Dr. Bert Vogelstein [12]. All the cell lines were grown in McCoy’s 5A with 10% FBS at 37°C in a 5% CO2/95% air atmosphere. Cells were treated with rapamycin at final concentration of 100 nM.
2.2 Western blotting and antibodies
Standard methods were used for western blotting. Cells were lysed in lysis buffer and total protein contents were determined by the Bradford method. 30μg of proteins were separated by SDS-PAGE under reducing conditions and blotted onto a polyvinylidene difluoride membrane (Millipore). Membranes were probed with specific antibodies. Blots were washed and probed with respective secondary peroxidase-conjugated antibodies, and the bands visualized by chemoluminescence (Amersham Biosciences). Antibody against fibronectin was purchased from Abcam (Cambridge, MA, USA). E-cadherin, N-cadherin, vimentin, and β-actin antibodies were from Cell Signaling technology (Danvers, MA, USA).
2.3 Confocal immunofluorescence microscopy
Cells were plated on culture slides (Costar, Manassas, VA, USA). After 24 hrs, the cells were rinsed with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS, and cell membrane was permeabilized using 0.5% Triton X-100. These cells were then blocked for 30 min in 10% BSA (Sigma, Aldrich St. Louis, MO, USA) in PBS and incubated with primary antibodies in 10% BSA overnight at 4°C. After three washes in PBS, the slides were incubated for 1 hour in the dark with FITC-conjugated secondary goat anti-mouse, or goat anti-rabbit antibodies (Invitrogen, Grand Island, NY, USA). After three further washes, the slides were stained with 4-,6-diamidino-2-phenylindole (DAPI; Sigma, Aldrich St. Louis, MO, U SA) for 5 min to visualize the nuclei, and examined using an Carl Zeiss confocal imaging system (LSM 780) (Carl Zeiss, Jena, Germany).
2.4 Wound healing assay
Cells were seeded in 6cm culture plates, and the monolayer cells were wounded by scratching with sterile plastic 200μl micropipette tips and photographed using phase-contrast microscopy immediately and 48hrs after wounding. The assays were independently performed in triplicate. The migration distance of each cell was measured after the photographs were converted to Photoshop files.
2.5 Cell invasion and motility assay
Invasion of cells was measured in Matrigel (BD, Franklin Lakes, NJ, USA) -coated Transwell inserts (6.5 mm, Costar, Manassas, VA, USA) containing polycarbonate filters with 8μm pores as detailed previously [13]. The inserts were coated with 50μl of 1mg/ml Matrigel matrix according to the manufacturer’s recommendations. 2×105 cells in 200μl of serum-free medium were plated in the upper chamber, whereas 600μl of medium with 10% fetal bovine serum were added to lower well. After 24 hr incubation, cells that migrated to the lower surface of the membrane were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. For each membrane, five random fields were counted at ×10 magnification. The mean cell number was calculated and data were presented as mean ± s.d. from three independent experiments done in triplicate. Motility assays were similar to Matrigel invasion assay except that the Transwell insert was not coated with Matrigel.
2.6 Spheroid Formation Assay
The capability of self-renewal was assessed using Corning Ultra-Low Attachment Surface (Corning). Different number of HCT116 and DLD-1 FBXW7+/+ and FBXW7−/− cells were seeded and incubated in a cell culture incubator for 1 week in McCoy’s 5A supplemented with 10% FBS or serum free medium and phase-contrast images were obtained.
2.7 Statistical analysis
Data was described as the mean ± s.d., and analyzed by Student’s two-tailed t-test. The limit of statistical significance was P<0.05. Statistical analysis was done with SPSS/Win11.0 software (SPSS, Inc., Chicago, Illinois, USA).
3. Results
3.1 Depletion of FBXW7 induces EMT in colon cancer cells
Both HCT116 and DLD-1 FBXW7−/− cells exhibited fibroblastic morphology, compared to their respective control cells (Fig. 1A and Supplemental Fig. 1A). In consistent with this observation, western blot analyses of molecular markers associated with epithelial and mesenchymal phenotypes showed that depletion of FBXW7 decreased the levels of epithelial markers (E-cadherin and α-catenin) and increased the levels of mesenchymal markers (fibronectin and vimentin) (Fig. 1B and Supplemental Fig. 1B), which was further confirmed by immunochemical staining (Fig. 1C). Finally, qRT-PCR studies showed that expression levels of Twist-1 and Snail-1 increase in FBXW7−/− cells in comparison to its wild-type control cells (Fig. 1D). These results clearly indicate that depletion of FBXW7 in colon cancer cells induces EMT.
Figure 1.
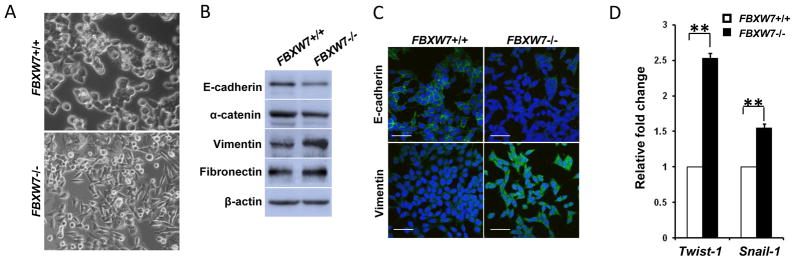
Depletion of FBXW7 in HCT116 cells leads to Epithelial-mesenchymal transition. (A) Morphology comparison of HCT116 FBXW7−/− and its control cells under bright field. (B) Western blot analysis of indicated EMT markers in HCT116 FBXW7−/− and its control cells. (C) Immunofluorescence staining for indicated EMT markers (Green) in HCT116 FBXW7−/− and its control cells. DAPI staining is blue. Scale bar is 20μm. (D) qRT-PCR analysis of Twist-1 and Snail-1 expression in HCT116 FBXW7−/− and its control cells. Data were presented as means ± Standard deviation from three independent experiments in triplicates. **P <0.01 was obtained from Student’s t-test.
2.2 Depletion of FBXW7 promotes migratory and invasive capacities of colon cancer cells in vitro
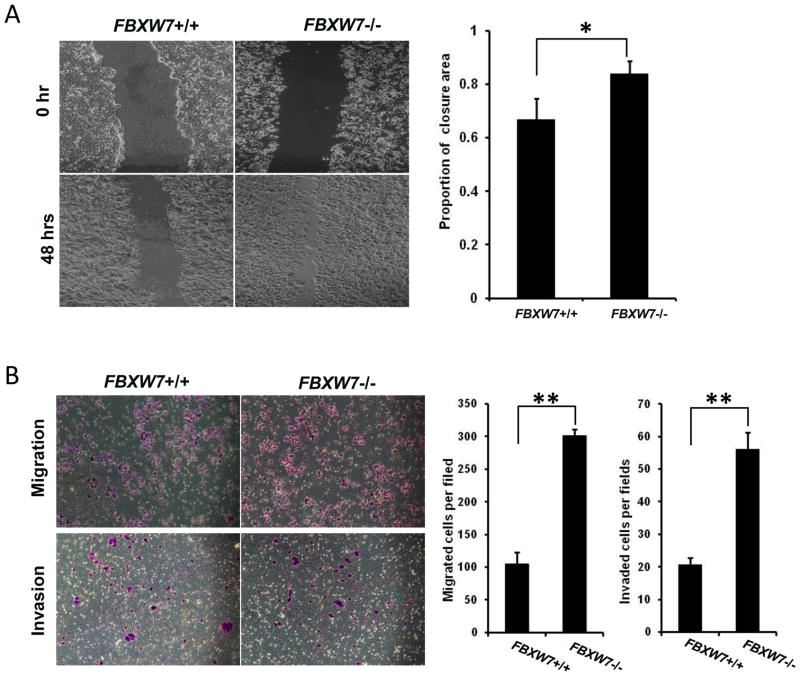
We next examined whether FBXW7 could modulate the migratory and invasive capacities of colon cancer cells. The effect of FBXW7 on cell migration was first assessed by a wound healing assay. HCT116 FBXW7−/− cells had significantly faster closure of the wound area compared to their respective control cells (Fig. 2A). This result was confirmed by Boyden’s chamber assay (Fig. 2B). Moreover, HCT116 FBXW7−/− cells showed a greater degree of invasion through Matrigel (Fig. 2B). Taken together, these results indicate that depletion of FBXW7 promotes migratory and invasive behaviors in colon cancer cells.
Figure 2.
Depletion of FBXW7 in HCT116 cells enhances cell migration and invasion. (A) Migration of HCT116 FBXW7−/− and its control cells was measured by wound healing assay. (B) Migration and invasion ability of HCT116 FBXW7−/− and its control cells were determined by uncoated or Matrigel-coated transwell assay. Data were presented as means ± Standard deviation from three independent experiments in triplicates. *P < 0.05 and **P < 0.01 were obtained from Student’s t-test.
3.3 Depletion of FBXW7 promotes emergence of stem cell-like behavior in colon cancer cell lines
Increasing evidence has linked EMT with acquisition of molecular and functional traits of stem cells in normal and neoplastic cell populations [5; 6]. Consistent with this concept, we found that depletion of FBXW7 in HCT116 cells significantly increased expression of the stem cell transcription factors or stem cell markers OCT4, SOX2 and NANOG (Fig. 3A). In terms of sphere formation assay, the FBXW7−/− cells were able to form large and more tumor-spheres in low adherent plates (Fig. 3B and Supplemental Fig. 2). Increased stem cell markers and tumor-sphere formation indicates that depletion of FBXW7 increases the cancer stem-like phenotype of colon cancer cells.
Figure 3.
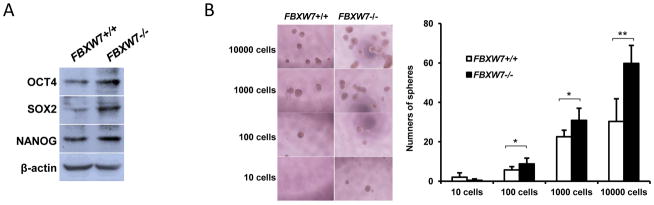
Depletion of FBXW7 in HCT116 cells induces CSC characterises. (A) Expression of stem cell markers in HCT116 FBXW7−/− and its control cells was analyzed by Western blotting. β-actin was used as a loading control. (B) Number of spheres was quantified using sphere formation assay for HCT116 FBXW7−/− and its control cells; left panels showed representative spheres. Data were presented as means ± Standard deviation from three independent experiments in triplicates. *P < 0.05 and ** P < 0.01 were based on Student’s t-test.
3.4 Rapamycin inhibits depletion of FBXW7-induced EMT and stem cell-like behavior in colon cancer cell lines
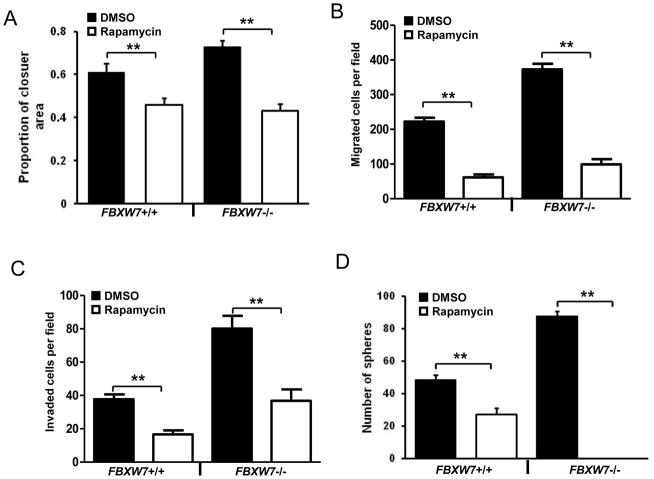
mTOR, which is one of FBXW7 downstream substrates, has been reported to involve in regulation of motility, EMT and metastasis of colorectal cancer [14]. To define the mechanism underlies FBXW7-loss mediated motility and stem-like characteristics, we investigated whether mTOR inhibition could suppress these processes. As shown in Fig. 4A, treatment of rapamycin, a mTOR inhibitor, significantly decreased the closure rate of FBXW7−/− cells by wound healing assay. Consistent with this result, rapamycin significantly decreased migration (Fig. 4B) and invasion (Fig. 4C) of FBXW7−/− cells using a Boyden chamber assay. Finally, rapamycin significantly suppressed the colon tumor-sphere formation of FBXW7−/− cells (Fig. 4D and Supplemental Fig. 3). Interestingly, rapamycin treatment also has significant effect on these biological phenotypes in HCT116 FBXW7+/+ cells (Fig. 4). However, with rapamycin treatment, FBXW7−/− cells have similar migratory and invasive behavior as FBXW7+/+ cells (Fig. 4A to C), and FBXW7−/− cells failed to form tumor-sphere while FBXW7+/+ cells were still able (Fig. 4D). These results clearly indicate that FBXW7 regulates cancer cell motility and stem-like characteristics possibly via mTOR signaling.
Figure 4.
Rapamycin alleviates the enhanced migration, invasion and stemness induced by depletion of FBXW7. Wound healing assay (A), uncoated (B) or Matrigel-coated (C) transwell assay of HCT116 FBXW7−/− cells treated with DMSO control or Rapamycin. (D) Number of spheres was quantified using sphere formation assay for 104 HCT116 FBXW7−/− cells treated with DMSO control or Rapamycin. Data were presented as means ± Standard deviation from three independent experiments in triplicates. **p < 0.01 was based on Student’s t-test.
4. Discussion
The tumor suppressor FBXW7 is mutated in a widely range of human cancers. Our data highlight a pivotal role for FBXW7 in colon cancer progression, possibly in other types of human cancer as well, through repressing cancer epithelial cells to acquire mesenchymal characteristics and invasive behavior. Depletion of FBXW7 leads to the increase in expression of EMT master regulatory genes TWIST-1 and SNAIL-1. To our knowledge, this is the first report connecting FBXW7 with EMT.
The key mechanistic finding in our study is that mTOR inhibition by rapamycin suppresses FBXW7 loss-induced motility and invasiveness. mTOR is a central component of several complex signaling networks that regulate cell growth, metabolism and proliferation. A recent study reports a role for mTOR in regulating EMT, motility, and metastasis of colorectal cancer [14]. Our previous study has shown that mTOR is one of FBXW7 targets [15]. Depletion of FBXW7 leads to elevated mTOR signaling activity [15; 16; 17], subsequently inducing EMT and increasing cell motility and invasiveness, which is suppressed in our experiments by mTOR inhibitor. However we are unable to fully exclude the possibility that other FBXW7 pathways are involved in these processes. For example, a few recent studies have shown that Aurora-A plays a role in EMT [18; 19; 20]. Aurora-A has also been demonstrated as one of FBXW7 targets [11; 21]. Thus, it is also possible that FBXW7 regulates EMT through Aurora-A. Further study will be granted to investigate this possibility.
Another important finding is that depletion of FBXW7 in colon cancer cell promotes stem-like characteristics. The connection between stemness and FBXW7 has been reported and has been attributed to multiple pathways, such as Notch, c-Myc, c-Jun [7, 8]. Moreover, two recent studies showed that FBXW7 plays a role in the generation of induced pluripotent stem cells [22; 23]. Our data demonstrated that rapamycin, a mTOR inhibitor, suppresses FBXW7 loss-induced stemness, suggesting accumulation of mTOR in FBXW7-depleted cells play a role in induction of stem-like properties.
In conclusion, EMT and stem cell-like properties are essential for tumor cells to disseminate from adjacent tissues and seed new tumors in distant sites. Our results demonstrated that FBXW7 regulated these two essential characteristics of metastatic disease through mTOR signaling pathway.
Supplementary Material
Highlights.
Depletion of FBXW7 induces epithelial-mesenchymal transition.
Depletion of FBXW7 promotes the cell migration and invasiveness.
Depletion of FBXW7 promotes the generation of colon cancer stem-like cells.
rapamycin suppresses FBXW7 loss-driven EMT, stemness and invasion.
Acknowledgments
We thank Dr. Bert Vogelstein for providing HCT116 and DLD-1 and their derivative FBXW7−/− cell lines. This work was supported by National Natural Science Foundation of China (grant numbers 81172528, 31271461 to G. W.); Doctoral Fund of Ministry of Education of China (grant number 20110131110035 to G. W.); Shandong Provincial Natural Science Foundation, China (grant number ZR2011HM034 to G. W.); by the National Institutes of Health, National Cancer Institute grant (grant number R01 CA116481 to J. H.M.); the Low Dose Scientific Focus Area, Office of Biological & Environmental Research, US Department of Energy (grant number DE-AC02-05CH11231 to J. H.M.); and Laboratory Directed Research & Development Program (LDRD) (to J. H.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 7.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Li G. Role of the ubiquitin ligase Fbw7 in cancer progression. Cancer Metastasis Rev. 2012;31:75–87. doi: 10.1007/s10555-011-9330-z. [DOI] [PubMed] [Google Scholar]
- 9.Tetzlaff MT, Yu W, Li M, Zhang P, Finegold M, Mahon K, Harper JW, Schwartz RJ, Elledge SJ. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci U S A. 2004;101:3338–45. doi: 10.1073/pnas.0307875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsunematsu R, Nakayama K, Oike Y, Nishiyama M, Ishida N, Hatakeyama S, Bessho Y, Kageyama R, Suda T, Nakayama KI. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004;279:9417–23. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 11.Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, Brown K, Bryson S, Balmain A. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–9. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 13.Rice J. Metastasis: The rude awakening. Nature. 2012;485:S55–7. doi: 10.1038/485S55a. [DOI] [PubMed] [Google Scholar]
- 14.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Huang Y, Wang Z, Li X, Louie A, Wei G, Mao JH. Temporal mTOR inhibition protects Fbxw7-deficient mice from radiation-induced tumor development. Aging (Albany NY) 2013;5:111–119. doi: 10.18632/aging.100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arabi A, Ullah K, Branca RM, Johansson J, Bandarra D, Haneklaus M, Fu J, Aries I, Nilsson P, Den Boer ML, Pokrovskaja K, Grander D, Xiao G, Rocha S, Lehtio J, Sangfelt O. Proteomic screen reveals Fbw7 as a modulator of the NF-kappaB pathway. Nat Commun. 2012;3:976. doi: 10.1038/ncomms1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Assoro AB, Liu T, Quatraro C, Amato A, Opyrchal M, Leontovich A, Ikeda Y, Ohmine S, Lingle W, Suman V, Ecsedy J, Iankov I, Di Leonardo A, Ayers-Inglers J, Degnim A, Billadeau D, McCubrey J, Ingle J, Salisbury JL, Galanis E. The mitotic kinase Aurora-A promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERalpha(+) breast cancer cells. Oncogene. 2013 doi: 10.1038/onc.2012.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou CH, Yang NK, Liu TY, Tai SK, Hsu DS, Chen YW, Chen YJ, Chang CC, Tzeng CH, Yang MH. Chromosome Instability Modulated by BMI1-AURKA Signaling Drives Progression in Head and Neck Cancer. Cancer Res. 2013;73:953–66. doi: 10.1158/0008-5472.CAN-12-2397. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lu N, Niu B, Chen X, Xie J, Cheng N. Overexpression of Aurora-A enhances invasion and matrix metalloproteinase-2 expression in esophageal squamous cell carcinoma cells. Mol Cancer Res. 2012;10:588–96. doi: 10.1158/1541-7786.MCR-11-0416. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YW, Kim IJ, Wu D, Lu J, Stock WA, Jr, Liu Y, Huang Y, Kang HC, DelRosario R, Jen KY, Perez-Losada J, Wei G, Balmain A, Mao JH. Pten regulates Aurora-A and cooperates with Fbxw7 in modulating radiation-induced tumor development. Mol Cancer Res. 2012;10:834–44. doi: 10.1158/1541-7786.MCR-12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu D, Davis MP, Abreu-Goodger C, Wang W, Campos LS, Siede J, Vigorito E, Skarnes WC, Dunham I, Enright AJ, Liu P. MiR-25 regulates Wwp2 and Fbxw7 and promotes reprogramming of mouse fibroblast cells to iPSCs. PLoS One. 2012;7:e40938. doi: 10.1371/journal.pone.0040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okita Y, Matsumoto A, Yumimoto K, Isoshita R, Nakayama KI. Increased efficiency in the generation of induced pluripotent stem cells by Fbxw7 ablation. Genes Cells. 2012;17:768–77. doi: 10.1111/j.1365-2443.2012.01626.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.