Abstract
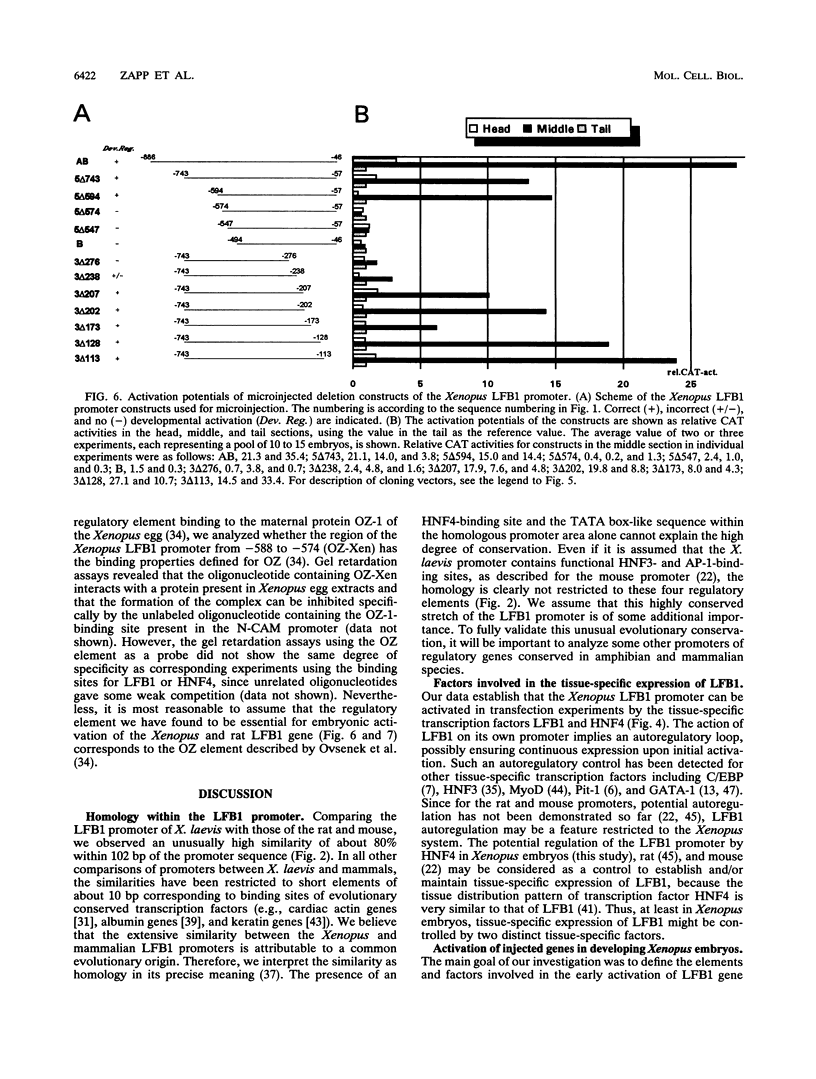
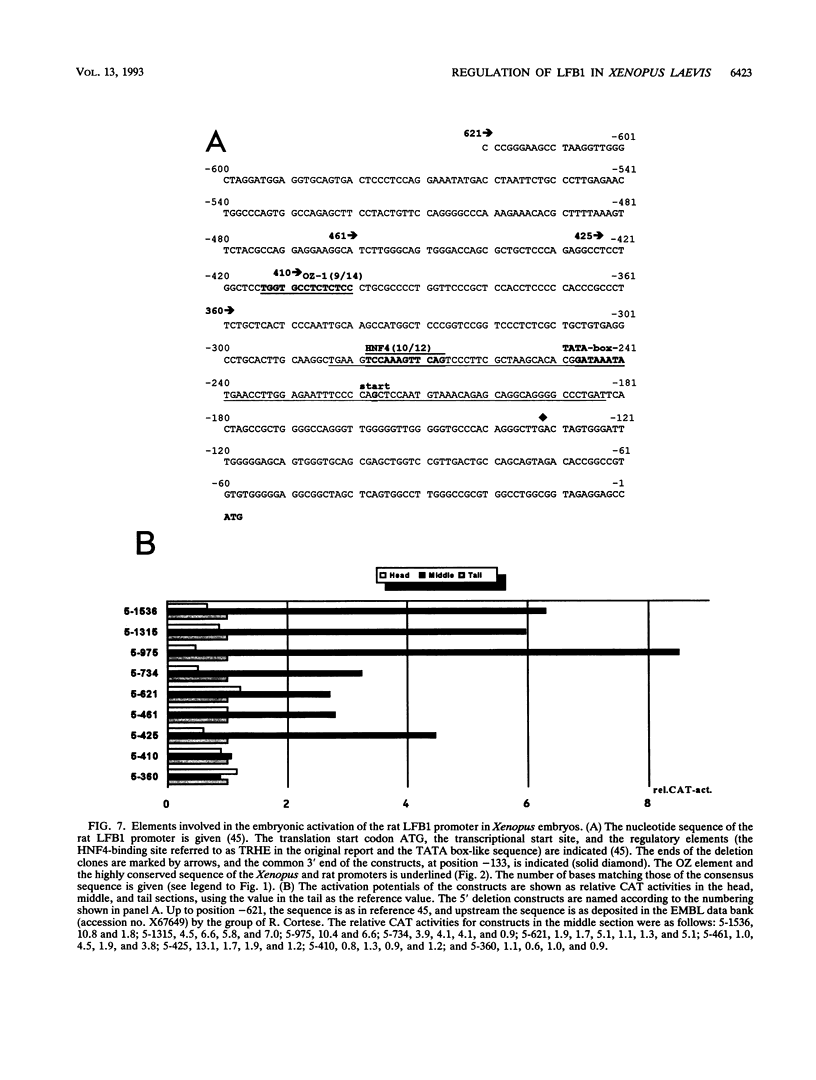
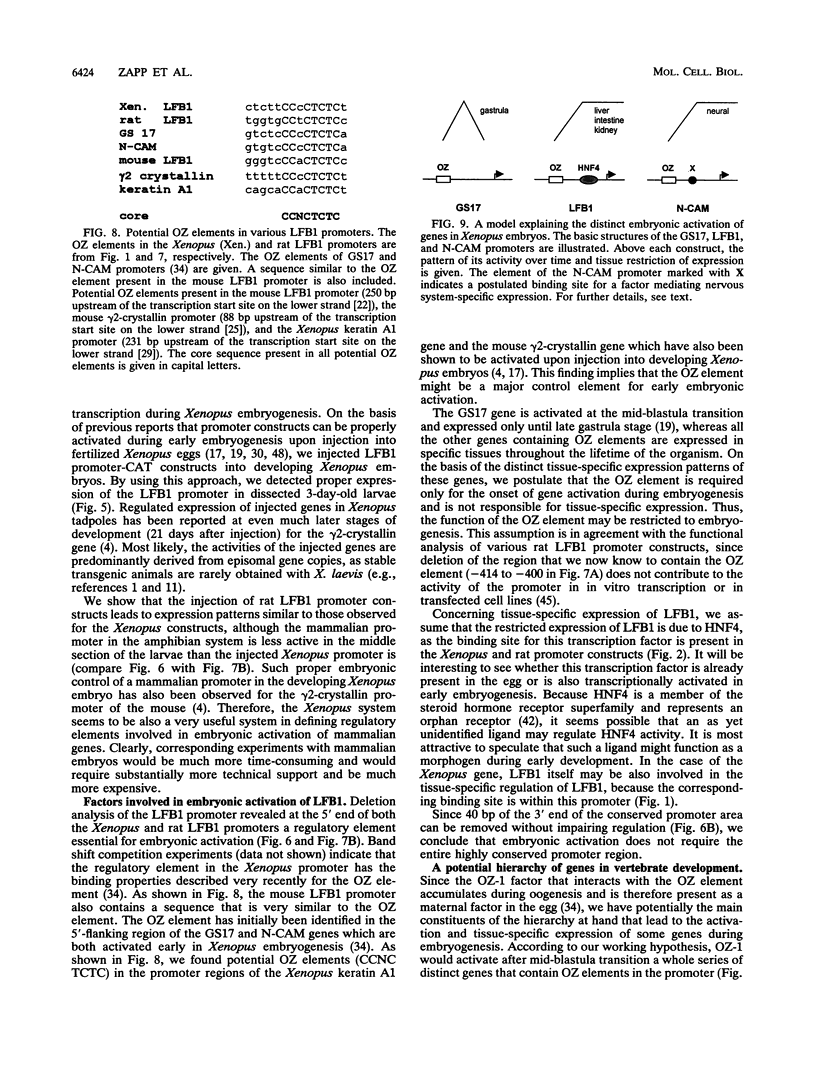
LFB1 (HNF1) is a tissue-specific transcription factor found in the livers, stomachs, intestines, and kidneys of vertebrates. By analyzing the promoter of the Xenopus LFB1 gene, we identified potential autoregulation by LFB1 and regulation by HNF4, a transcription factor with a tissue distribution similar to that of LFB1. Injection of LFB1 promoter-chloramphenicol acetyltransferase constructs into Xenopus eggs revealed embryonic activation that is restricted to the region of the developing larvae expressing endogeneous LFB1. Proper embryonic activation was also observed with a rat LFB1 promoter. Deletion analysis of the Xenopus and rat promoters revealed that in both promoters embryonic activation is absolutely dependnet on the presence of an element that contains CCNCTCTC as the core consensus sequence. Since this element is recognized by the maternal factor OZ-1 previously described by N. Ovsenek, A. M. Zorn, and P. A. Krieg (Development 115:649-655, 1992), we might have identified the main constituents of a hierarchy that leads via LFB1 to the activation of tissue-specific genes during embryogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Muellener D. B., Ryffel G. U. Persistence, methylation and expression of vitellogenin gene derivatives after injection into fertilized eggs of Xenopus laevis. Nucleic Acids Res. 1984 Mar 12;12(5):2283–2302. doi: 10.1093/nar/12.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowski S., Zapp D., Weber H., Eberle G., Zoidl C., Senkel S., Klein-Hitpass L., Ryffel G. U. Developmental regulation and tissue distribution of the liver transcription factor LFB1 (HNF1) in Xenopus laevis. Mol Cell Biol. 1993 Jan;13(1):421–431. doi: 10.1128/mcb.13.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld M., Maury M., Chouard T., Yaniv M., Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. 1991 Oct;113(2):589–599. doi: 10.1242/dev.113.2.589. [DOI] [PubMed] [Google Scholar]
- Brakenhoff R. H., Ruuls R. C., Jacobs E. H., Schoenmakers J. G., Lubsen N. H. Transgenic Xenopus laevis tadpoles: a transient in vivo model system for the manipulation of lens function and lens development. Nucleic Acids Res. 1991 Mar 25;19(6):1279–1284. doi: 10.1093/nar/19.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Yaniv M., Cortese R. Hepatocyte dedifferentiation and extinction is accompanied by a block in the synthesis of mRNA coding for the transcription factor HNF1/LFB1. EMBO J. 1990 Jul;9(7):2257–2263. doi: 10.1002/j.1460-2075.1990.tb07396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. P., Ingraham H. A., Treacy M. N., Albert V. R., Wilson L., Rosenfeld M. G. Autoregulation of pit-1 gene expression mediated by two cis-active promoter elements. Nature. 1990 Aug 9;346(6284):583–586. doi: 10.1038/346583a0. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- De Simone V., De Magistris L., Lazzaro D., Gerstner J., Monaci P., Nicosia A., Cortese R. LFB3, a heterodimer-forming homeoprotein of the LFB1 family, is expressed in specialized epithelia. EMBO J. 1991 Jun;10(6):1435–1443. doi: 10.1002/j.1460-2075.1991.tb07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin L. D., Pearman B. Distribution, expression and germ line transmission of exogenous DNA sequences following microinjection into Xenopus laevis eggs. Development. 1987 Jan;99(1):15–23. doi: 10.1242/dev.99.1.15. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. The generation of diversity and pattern in animal development. Cell. 1992 Jan 24;68(2):185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Hannon R., Evans T., Felsenfeld G., Gould H. Structure and promoter activity of the gene for the erythroid transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Melton D. A. Diffusible factors in vertebrate embryonic induction. Cell. 1992 Jan 24;68(2):257–270. doi: 10.1016/0092-8674(92)90469-s. [DOI] [PubMed] [Google Scholar]
- Jonas E. A., Snape A. M., Sargent T. D. Transcriptional regulation of a Xenopus embryonic epidermal keratin gene. Development. 1989 Jun;106(2):399–405. doi: 10.1242/dev.106.2.399. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Developmental regulation of a gastrula-specific gene injected into fertilized Xenopus eggs. EMBO J. 1985 Dec 16;4(13A):3463–3471. doi: 10.1002/j.1460-2075.1985.tb04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler W., Kaling M., Ross K., Wagner U., Ryffel G. U. BAP, a rat liver protein that activates transcription through a promoter element with similarity to the USF/MLTF binding site. Nucleic Acids Res. 1990 Dec 11;18(23):6943–6951. doi: 10.1093/nar/18.23.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler W., Wagner U., Ryffel G. U. Tissue-specificity of liver gene expression: a common liver-specific promoter element. Nucleic Acids Res. 1988 Apr 25;16(8):3165–3174. doi: 10.1093/nar/16.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. J., Conley P. B., Chen L., Sladek F. M., Darnell J. E., Jr, Crabtree G. R. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992 Jan 30;355(6359):457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Smith E., Litvin O., Costa R. H., Darnell J. E., Jr HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 1990 Aug;4(8):1427–1436. doi: 10.1101/gad.4.8.1427. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Schibler U. A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell. 1989 Jun 30;57(7):1179–1187. doi: 10.1016/0092-8674(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Lok S., Breitman M. L., Chepelinsky A. B., Piatigorsky J., Gold R. J., Tsui L. C. Lens-specific promoter activity of a mouse gamma-crystallin gene. Mol Cell Biol. 1985 Sep;5(9):2221–2230. doi: 10.1128/mcb.5.9.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Crabtree G. R. HNF-1, a member of a novel class of dimerizing homeodomain proteins. J Biol Chem. 1991 Jan 15;266(2):677–680. [PubMed] [Google Scholar]
- Mendel D. B., Hansen L. P., Graves M. K., Conley P. B., Crabtree G. R. HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991 Jun;5(6):1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- Miyatani S., Winkles J. A., Sargent T. D., Dawid I. B. Stage-specific keratins in Xenopus laevis embryos and tadpoles: the XK81 gene family. J Cell Biol. 1986 Nov;103(5):1957–1965. doi: 10.1083/jcb.103.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Garrett N., Gurdon J. B. Upstream sequences required for tissue-specific activation of the cardiac actin gene in Xenopus laevis embryos. EMBO J. 1986 Dec 1;5(12):3185–3193. doi: 10.1002/j.1460-2075.1986.tb04628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Taylor M. V., Garrett N., Gurdon J. B. The CArG promoter sequence is necessary for muscle-specific transcription of the cardiac actin gene in Xenopus embryos. EMBO J. 1989 Apr;8(4):1153–1161. doi: 10.1002/j.1460-2075.1989.tb03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaci P., Nicosia A., Cortese R. Two different liver-specific factors stimulate in vitro transcription from the human alpha 1-antitrypsin promoter. EMBO J. 1988 Jul;7(7):2075–2087. doi: 10.1002/j.1460-2075.1988.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsenek N., Zorn A. M., Krieg P. A. A maternal factor, OZ-1, activates embryonic transcription of the Xenopus laevis GS17 gene. Development. 1992 Jun;115(2):649–655. doi: 10.1242/dev.115.2.649. [DOI] [PubMed] [Google Scholar]
- Pani L., Quian X. B., Clevidence D., Costa R. H. The restricted promoter activity of the liver transcription factor hepatocyte nuclear factor 3 beta involves a cell-specific factor and positive autoactivation. Mol Cell Biol. 1992 Feb;12(2):552–562. doi: 10.1128/mcb.12.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck G. R., de Haën C., Teller D. C., Doolittle R. F., Fitch W. M., Dickerson R. E., Chambon P., McLachlan A. D., Margoliash E., Jukes T. H. "Homology" in proteins and nucleic acids: a terminology muddle and a way out of it. Cell. 1987 Aug 28;50(5):667–667. doi: 10.1016/0092-8674(87)90322-9. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Kugler W., Wagner U., Kaling M. Liver cell specific gene transcription in vitro: the promoter elements HP1 and TATA box are necessary and sufficient to generate a liver-specific promoter. Nucleic Acids Res. 1989 Feb 11;17(3):939–953. doi: 10.1093/nar/17.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorpp M., Kugler W., Wagner U., Ryffel G. U. Hepatocyte-specific promoter element HP1 of the Xenopus albumin gene interacts with transcriptional factors of mammalian hepatocytes. J Mol Biol. 1988 Jul 20;202(2):307–320. doi: 10.1016/0022-2836(88)90460-3. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Regional biosynthetic markers in the early amphibian embryo. J Embryol Exp Morphol. 1984 Apr;80:289–319. [PubMed] [Google Scholar]
- Sladek F. M., Darnell J. E. Mechanisms of liver-specific gene expression. Curr Opin Genet Dev. 1992 Apr;2(2):256–259. doi: 10.1016/s0959-437x(05)80282-5. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Snape A. M., Jonas E. A., Sargent T. D. KTF-1, a transcriptional activator of Xenopus embryonic keratin expression. Development. 1990 May;109(1):157–165. doi: 10.1242/dev.109.1.157. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Tian J. M., Schibler U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 1991 Dec;5(12A):2225–2234. doi: 10.1101/gad.5.12a.2225. [DOI] [PubMed] [Google Scholar]
- Toniatti C., Demartis A., Monaci P., Nicosia A., Ciliberto G. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990 Dec;9(13):4467–4475. doi: 10.1002/j.1460-2075.1990.tb07897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Wilson C., Cross G. S., Woodland H. R. Tissue-specific expression of actin genes injected into Xenopus embryos. Cell. 1986 Nov 21;47(4):589–599. doi: 10.1016/0092-8674(86)90623-9. [DOI] [PubMed] [Google Scholar]