Abstract
Background
Chronic Allograft Dysfunction (CGD) is a common outcome in kidney transplants, but its pathogenesis is unclear. We investigated the CGD phenotype and single nucleotide polymorphisms (SNPs) associated with CGD.
Method
This prospective study enrolled 2,336 transplants from seven transplant centers in North America. CGD was defined as a greater than 25% rise in serum creatinine relative to a 3 month post-transplant baseline, requiring a kidney biopsy. We genotyped 2,724 SNPs in the initial 979 transplants which form the test cohort.
Results
CGD occurred 11.2 times per 100 person-years at a median of 509 ± 387 days from the three month baseline. CGD was independently associated with death- censored, allograft failure, in an adjusted analysis [HR=20.6 (11.8–35.8, p<0.001)]. Among 366 transplant recipients with CGD, 91% had inflammation on biopsy scores. 94 (26%) had inflammatory changes consistent with a diagnosis of concomitant acute rejection. SNPs in FM06 and FM03, potential drug metabolism genes, were associated with CGD, after accounting for multiple testing.
Conclusion
CGD phenotype with concomitant inflammation is associated with increased risk of allograft failure. SNPs associated with CGD in novel drug metabolism and transport genes, will be validated in subsequent transplants.
Keywords: chronic allograft dysfunction, single nucleotide polymorphisms
Introduction
The natural history of chronic allograft dysfunction (CGD) has been described as the early initiation of tubulointerstitial injury1 leading to eventual worsening interstitial fibrosis (ci) and tubular atrophy (ct) as graded by the Banff scoring of kidney biopsies and glomerular damage.2 The early phase of CGD can occur by three months post-transplant.1 This irreversible damage can lead to declining kidney function and allograft failure. However, the rate of decline in kidney function and development of allograft failure can vary in recipients with CGD.3 Therefore, there is a need for further characterizing the CGD phenotype to better understand which CGD phenotypes will rapidly develop allograft failure.
By using a multicenter cohort of kidney and simultaneous kidney-pancreas (SPK) transplant recipients, we prospectively studied the phenotype of CGD. Then, we studied the association of single nucleotide polymorphism (SNPs) with CGD. We also studied the association of SNPs with severity of interstitial fibrosis and tubular atrophy. SNPs can point to novel pathways that are important for development of CGD. SNPs have been instrumental in pointing to novel pathways in transplant4 and non-transplant disease states.5, 6 SNPs have also been important in understanding drug metabolism7, toxicity8,9 and drug dosing of tacrolimus in the transplant setting.7,10 In the future, SNPs may also identify high risk transplant recipients that will need close follow-up.
Materials and Methods
Clinical Assessment
Between October 1, 2005 and June 26, 2010, 2,336 kidney and SPK recipients at 7 transplant centers were enrolled in a prospective cohort. Patients were consented at the time of or soon after transplantation. All kidney transplant recipients at the 7 transplant centers were eligible. The Institutional Review Boards at each of the participating sites, approved the study.
The management of the immunosuppression was determined by the treating physician at the specific transplant center. The clinical data was collected in a central database, at the time of transplant and regularly until allograft failure. The local pathologist provided the biopsy scores using the standard Banff criteria. All biopsies studied were biopsies done for cause. CGD was a clinical diagnosis defined as a greater than 25% rise in serum creatinine, relative to a 3 month baseline creatinine, that required a biopsy. The baseline creatinine was established based on the average of three measurements of creatinine taken at least one week apart. This baseline was re-established for each participant six weeks after the initiation of AR treatment. This threshold of 25% rise in creatinine was based on a retrospective study of the participating transplant centers.11 All biopsies were processed by the local pathologist. After the 3 month baseline creatinine, there were 882 biopsies (mean number of biopsies ± S.D.= 0.4 ± 0.8, range 0–7) and 200 subjects (8.6 %) had more than one biopsy. AR was defined by the treating physician and 98% were biopsy confirmed.12 Death censored allograft failure was defined as return to dialysis or retransplantation. A log-rank test was done to assess death censored allograft failure after CGD.
SNP Genotyping
SNP genotyping was conducted using DNA from peripheral blood in 979 initial transplant recipients, as previously described.12 This cohort was referred to as the test cohort and the remaining recipients as the non-test cohort. The blood samples for DNA extraction were collected at the time of consent. The SNPs for genotyping were selected if they were known or believed to be functional within biologically relevant genes to transplantation and included genes in pathways related to immunity, cell signaling, cell growth and proliferation, drug absorption, disposition, metabolism and excretion. In the absence of functional variants, intragenic tagging variants were selected. The entire list of 2,724 SNPs has been published previously.12 Most of these SNPs were genotyped using a customized Affymetrix GeneChip having a total of 3,404 SNP assay and determined using a Affymetrix GeneChip Scanner 3000 Targeted Genotyping System (Affymetrix, Santa Clara, CA). 13, 14 Genotyping for additional variants that could not be accommodated on the SNP chip was conducted using the SNPlex (Applied Biosystems Inc, Foster City, CA) and Sequenom (Sequenom Inc, San Diego, CA) platforms, as per manufacturer’s recommendation. Details of this additional SNP genotyping and the genotyping quality control measures have been described previously. 12,15,16
Data Analysis
Association of CGD with allograft failure
The association of CGD with time to allograft failure was studied using a Cox proportional hazards model 17 with CGD being a time-varying covariate. (SAS v9.2, The SAS Institute, http://www.sas.com) The initial analysis was conducted among 2,336 kidney and SPK recipients. Backward selection with a retention p-value of 0.10 was performed to create a baseline model that adjusts for potential confounders. All baseline variables in Table 1 were eligible for backward selection, except for Hispanic ethnicity, history of diabetes, cold ischemia time, need for dialysis post-transplant, plasmapheresis prior to transplant, and use of mycophenolate mofetil.
Table 1.
Characteristics of kidney transplant recipients (n=2,336). P-value for comparisons between recipients with CGD and without CGD.
| Characteristic | All n= 2,336 | No CGD n = 1,970 | CGD n= 366 | p-value |
|---|---|---|---|---|
| 0.21 | ||||
| Ethnicity: White | 75 % | 76 % | 72 % | |
| Black | 19 % | 19 % | 20 % | |
| Asian | 3 % | 3 % | 3 % | |
| Other | 2 % | 2 % | 4 % | |
| Not Known | <1 % | < 1% | < 1% | |
| Hispanic | 2 % | 1 % | 2% | 0.25 |
| Male | 63 (%) | 63 % | 61 % | 0.32 |
| Mean age at enrollment in years | 49 ±14 | 50 ±14 | 46 ±16 | < 0.001 |
| Cause of End Stage Kidney Disease: | 0.03 | |||
| Diabetes | 28 % | 27% | 31% | |
| Glomerular disease | 21 % | 20% | 25% | |
| HTN | 14 % | 15% | 11% | |
| Polycystic kidney disease | 13 % | 13% | 10% | |
| Other | 21 % | 21% | 21% | |
| Unknown | 3 % | 4% | 2% | |
| History of diabetes | 36% | 36% | 38% | 0.37 |
| Living donor transplant | 58 % | 58% | 58% | 0.95 |
| Mean donor age in years | 40 ±14 | 40±14 | 43±14 | <0.01 |
| Male donor* | 48 % | 48% | 49% | 0.3 |
| Cold Ischemia time >24 h* | 8 % | 8% | 8% | 0.74 |
| Prior kidney Transplant | 13 % | 13% | 15% | 0.3 |
| Need for dialysis in the first 14 days post transplant* | 10% | 9% | 14% | 0.005 |
| Final PRA present * | 42% | 42% | 43% | 0.82 |
| T or B Crossmatch positive* | 6 % | 5% | 8% | 0.11 |
| Plasmapheresis prior to transplants | 3 % | 2% | 5% | 0.01 |
| Zero HLA mismatches | 12 % | 13% | 9% | 0.065 |
| Antibody Induction: | <0.0001 | |||
| Monoclonal | 44 % | 46% | 35% | |
| None | 4 % | 4% | 2% | |
| Other | 3 % | 2% | 5% | |
| Polyclonal | 50 % | 48% | 58% | |
| Smoking status:* | ||||
| Never | 58 % | 58% | 58% | 0.03 |
| Past | 32 % | 33% | 29% | |
| Current | 9 % | 9% | 13% | |
| Pre-emptive transplant | 29 % | 29% | 27% | |
| Steroid withdrawal by day 14 post-transplant | 39 % | 38% | 42% | 0.53 |
| Use of Mycophenolate Mofetil | 99% | 99% | 99% | |
| CNI type: | 0.17 | |||
| Cyclosporine | 28 % | 26% | 38% | 0.79 |
| Tacrolimus | 69 % | 71% | 59% | <0.0001 |
| None | 3 % | 3% | 3% | |
| SPK** | 6 % | 5% | 7% | |
| Prior Non-kidney Transplants* | 10 % | 9% | 13% | 0.22 |
| CMV Recipient/Donor Status* | 0.06 | |||
| Recipient (−)/Donor (−) | 20 % | 20% | 21% | <0.01 |
| Recipient (+) | 63 % | 64% | 56% | |
| Recipient (−)/Donor (+) | 17 % | 16% | 23% | |
| Acute Rejection in first 3 months post-transplant | 9 % | 8% | 13% | |
| <0.01 |
Missing data: Living donor gender missing in 7 subjects, need for dialysis in the first 14 days post-transplant missing in 4 subjects, Cold Ischemia time missing in 209 subjects, Final PRA missing in 7 subjects, Plasmapheresis prior to transplant missing in 57 subjects, B/T cell crossmatch missing in 51 subjects, Smoking status missing in 7 subject, Use of Mycophenolate Mofetil and CNI type was missing in 215 subjects, Prior non-kidney transplant missing in 44 subjects, CMV recipient/donor status missing in 44 subjects.
SPK= Simultaneous kidney pancreas transplants
SNPs Associated with CGD
Among recipients with SNP data, using an additive genetic model, separate Cox proportional hazards models were used to investigate the association of each SNP with time to CGD, adjusting for recipient race and stratifying by transplant center.18 Each SNP was added into the baseline model individually, while stratifying for transplant center and adjusting for recipient race (African-American vs. non-African American race). For the final models, the p-values for SNPs association, below the false discovery rate (FDR) set at 20%, were considered statistically significant after accounting for multiple testing.
SNPs Associated with Severity of Tubular Atrophy and Interstitial Fibrosis
To assess severity of tubular atrophy and interstitial nephritis, the test cohort with 979 genotyped recipients was divided into three groups: no biopsy, biopsy with ct-score less than or equal to 1, and ct-score greater than or equal to 2. Since these outcomes were not found to be ordinal, we then conducted a multinomial logistic regression analysis, adjusted for transplant center. Then, backward selection with a retention p-value of 0.10 was performed to create a baseline model that adjusts for potential confounders. All variables in Table 1 were eligible for backward selection. For the final models, the p-values for SNPs association, below the false discovery rate (FDR) set at 20%, were considered statistically significant after accounting for multiple testing.
Results
Baseline Characteristics and Outcomes
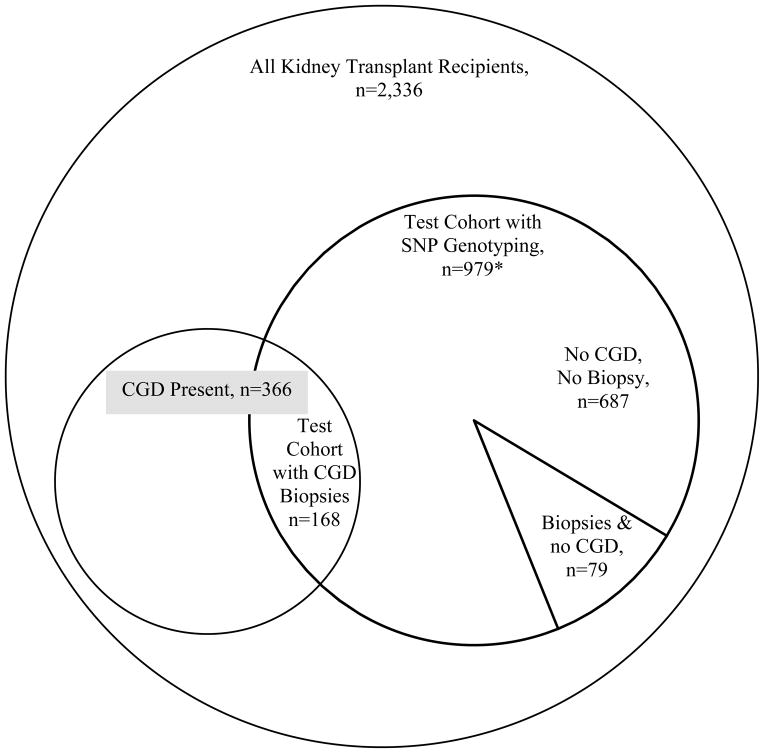
Seven transplant centers enrolled 2,336 kidney transplant recipients. (Table 1 and Figure 1) The creatinine at the three month baseline was 1.4 ± 0.48 mg/dl. After the first three months post-transplant, 366 (16 %) recipients experienced CGD with a rate of 11.2 per 100 person-years. The mean time to CGD diagnosis was 509 ± 387 days from the three month baseline. Creatinine at the time of CGD was 2.7 ± 1.5 mg/dl. The factors that were independently associated with CGD were donor age and recipient characteristics such as age, smoking status and recipient-donor CMV status, after adjusting for African-American recipient race,.
Figure 1.
A Venn-Diagram showing the sub-population of kidney transplant recipients in each analysis. All test cohort subjects with SNP genotyping were eligible for analysis of severity of tubular atrophy and interstitial fibrosis However, the analysis excluded the less than 1% (30) biopsies from 24 subjects with missing biopsy scores and excluded all 21 subjects with 3 biopsies from a single center that had no ct scores ≥2 (resulting in 687 with no biopsies, 79 with biopsies and no CGD and 168 CGD biopsies, n=934.)
CGD and Allograft Survival
During the mean follow-up of 21 months post-transplant, 66 (3%) died and 75 (3%) developed death-censored allograft failure. Death was not statistically different between the CGD group versus the non-CGD group [14 (4%) vs. 52 (3%), p=0.23]. Death-censored allograft failure was significantly higher in the CGD group versus the non-CGD group [56 (15%) vs 19 (1%), p<0.0001]. In a multivariate model, CGD was independently associated with increased risk of death-censored allograft failure [HR=20.6 (11.8 – 35.8, p<0.0001)]. In this model younger recipient age, longer duration of dialysis pre-transplant and higher creatinine at the three month baseline, were also independently associated with increased risk of death-censored allograft failure. The recipients without a biopsy who had a rise in serum creatinine were assessed to ensure that the cases were not misclassified. A chart review was conducted for recipients that had a 50% rise in creatinine from the 3 month baseline, but had no biopsy. The 17 recipients with such a rise in creatinine, had dehydration or intercurrent medical illness as the cause of the rise.
Biopsy findings in CGD
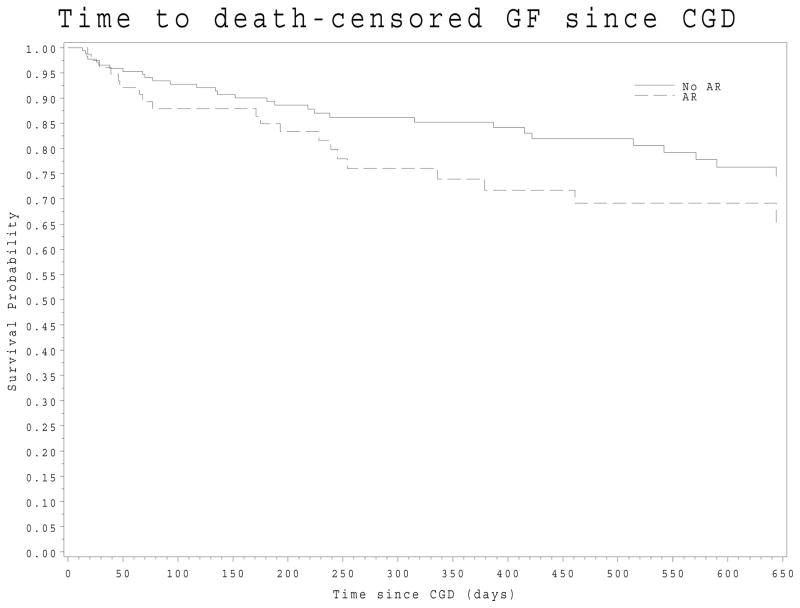
The CGD biopsy scores (Table 2) among the 366 recipients with i and t scores reported by the local pathologists, revealed significant amount of interstitial inflammation and tubulitis. Ninety-four (26% of 366) of the CGD biopsies had a Banff score consistent with AR with t-score more than 1 and i-score more than 1. (Table 2) The respective tables also show the presence of C4d positive status. These CGD biopsies with AR had chronic as well as acute changes. The rate of death censored allograft failure after the diagnoses of CGD did not differ between the CGD subgroup with concomitant AR and the CGD subgroup without AR (log-rank test, p-value=0.22). (Figure 2) The mean creatinine (IQR) after the diagnosis of CGD remained elevated from the reference creatinine in both sub-groups, 1.80 (1.50 – 3.10) mg/dl in subgroup with concomitant AR and 1.90 (1.50–2.45) mg/dl in the subgroup without concomitant AR. This creatinine was measured at a median (IQR) of 21 (12 – 30) days after the CGD event. Therefore, these groups were analyzed together for the SNP analysis.
Table 2.
The distribution of pathology scores and laboratory data in the patients with CGD. Panel A shows data for all CGD biopsies (n= 366) whereas Panel B shows data on 94 CGD biopsies with concomitant acute rejection.
| Panel A: All CGD biopsies (n=366)* | ||||
|---|---|---|---|---|
| Biopsy Score | 0 | 1 | 2 | 3 |
| i (interstitial inflammation) | 45% | 22% | 13% | 20% |
| t (tubulitis) | 44% | 21% | 19% | 15% |
| ci (interstitial fibrosis) | 33% | 43% | 19% | 4% |
| ct (tubular atrophy) | 25% | 51% | 19% | 5% |
| cv (vascular fibrosis) | 63% | 19% | 16% | 2% |
| cg (glomerulopathy) | 88% | 7% | 2% | 3% |
| ah (arteriolar hyaline thickening) | 71% | 20% | 6% | 3% |
| v (intimal artertitis) | 89% | 9% | 1% | 1% |
| g (glomerulitis) | 86% | 8% | 5% | 1% |
| Other Laboratory data | Focal Positive | Diffuse Positive | Negative | Not Done |
|---|---|---|---|---|
| C4D staining | 8% | 13% | 70% | 9% |
| Panel B: CGD biopsies with concomitant AR (n=94) | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Biopsy Score | ||||
| i (interstitial inflammation) | 0% | 0% | 36% | 64% |
| t (tubulitis) | 0% | 0% | 49% | 51% |
| ci (interstitial fibrosis) | 30% | 44% | 21% | 4% |
| ct (tubular atrophy) | 27% | 48% | 20% | 5% |
| cv (vascular fibrosis) | 75% | 10% | 12% | 3% |
| cg (glomerulopathy) | 94% | 4% | 1% | 1% |
| ah (arteriolar hyaline thickening) | 77% | 19% | 3% | 1% |
| v (intimal artertitis) | 71% | 24% | 2% | 3% |
| g (glomerulitis) | 85% | 9% | 5% | 1% |
| Other Laboratory data | Focal Positive | Diffuse Positive | Negative | Not Done |
|---|---|---|---|---|
| C4D staining positive | 17% | 24% | 59% | <1% |
Less than (30) 1% missing biopsy scores.
ci and ct scores correlated (Spearman correlation coefficient=0.88, p<0.0001); i and t scores correlated (Spearman correlation coefficient=0.81, p<0.0001)
ci and ct scores correlated (Spearman correlation coefficient=0.87, p<0.0001); i and t scores correlated (Spearman correlation coefficient=0.55, p<0.0001)
Figure 2.
Death-censored Allograft Failure after Developing CGD. In this Kaplan-Meier, the dashed line presents the group with concomitant acute rejection and the solid line represent the group with no concomitant acute rejection. (Log-rank test p-value 0.22)
SNPs Associated with CGD
The initial 979 patients which represent the test cohort were genotyped for SNPs and their characteristics are described in Table 3. There were some differences in the characteristics of the test cohort compared to the remaining non-test cohort. These differences were primarily due to center specific practices and referral patterns such as use of induction agents, use of dialysis post-transplant19, proportion of pre-sensitized candidates and steroid withdrawal. Transplant centers’ volume did vary from year to year, thus patients in the test and non-test cohort do not represent the same proportion of patients from all the seven centers. Therefore, the analysis in the test cohort was stratified by transplant center in order to minimize the effects of these center specific characteristics. In this test cohort, a Cox proportional hazards model was developed to adjust for recipient race and other confounders, and stratifying by transplant center. In this adjusted model, donor age and recipient characteristics such as smoking status, recipient-donor CMV status, and age were independently associated with CGD. In this test cohort composed of the 979 initial subjects, with 194 (20% of 979) CGD events, the top 15 SNPs associated with CGD, all p<0.01, are shown in Table 4. Four SNPs were significantly associated with CGD after accounting for a FDR of 20%. These 4 SNPs were in genes FM06 and FM03. These genes are flavin-containing mono-oxygenases (FMOs) consisting of five microsomal enzymes important for the oxidative metabolism of environmental toxins and therapeutic agents.20 Thus, these SNPs could play a role in metabolism of immunosuppressive agents. Our previously published study in this population, has shown that another SNP in FMO3 was associated with tacrolimus levels.15 This other SNP in FMO3 was not in linkage disequilibrium with the SNPs shown in Table 4. (r2<0.3) There is significant sequence homology between FMO6 and FMO3.20,21 Supplemental Table 1 shows the association of all SNPs genotyped with CGD.
Table 3.
Characteristics of kidney transplant recipients genotyped (Test cohort n=979) and the rest (non-test cohort). P-value for comparisons between recipients in test versus validation cohort.
| Characteristic | All n= 2,336 | Test Cohort n =979 | Non-Test Cohort n=1,357 | p-value |
|---|---|---|---|---|
| 0.05 | ||||
| Ethnicity: White | 75 % | 77 % | 74 % | |
| Black | 19 % | 17 % | 20 % | |
| Asian | 3 % | 3 % | 3 % | |
| Other | 2 % | 2 % | 3 % | |
| Not Known | <1 % | < 1% | 0 % | |
| Hispanic | 2 % | 2 % | 1 % | 0.99 |
| Male | 63 % | 63 % | 63 % | 0.89 |
| Mean age at enrollment in years | 49 ±14 | 49 ±14 | 49 ±14 | 0.20 |
| Cause of End Stage Kidney Disease: | 0.87 | |||
| Diabetes | 28 % | 31 % | 26 % | |
| Glomerular disease | 21 % | 20 % | 21 % | |
| HTN | 14 % | 12 % | 16 % | |
| Polycystic kidney disease | 13 % | 12 % | 13 % | |
| Other | 21 % | 21 % | 21 % | |
| Unknown | 3 % | 4 % | 3 % | |
| History of diabetes | 36% | 38 % | 35 % | 0.89 |
| Living donor transplant | 58 % | 59 % | 57 % | 0.84 |
| Mean donor age in years | 40 ± 14 | 40 ± 14 | 40 ± 14 | 0.07 |
| Male donor* | 48 % | 46 % | 49 % | 0.12 |
| Cold Ischemia time >24 h* | 8 % | 7 % | 9 % | 0.25 |
| Prior kidney Transplant | 13 % | 14 % | 13 % | 0.63 |
| Need for dialysis in the first 14 days post transplant* | 10% | 8 % | 10 % | 0.005 |
| Final PRA present * | 42% | 35 % | 47 % | <0.001 |
| T or B Crossmatch positive* | 6 % | 5 % | 6 % | 0.92 |
| Plasmapheresis prior to transplants | 3 % | 3% | 2% | 0.35 |
| Zero HLA mismatches | 12 % | 11 % | 12 % | 0.45 |
| Antibody Induction: | <0.0001 | |||
| Monoclonal | 44 % | 40 % | 47 % | |
| None | 4 % | 1 % | 5 % | |
| Other | 3 % | 4 % | 2 % | |
| Polyclonal | 50 % | 54 % | 46 % | |
| Smoking status:* | ||||
| Never | 58 % | 60 % | 57 % | 0.39 |
| Past | 32 % | 31 % | 33 % | |
| Current | 9 % | 9 % | 10 % | |
| Pre-emptive transplant | 29 % | 31 % | 26 % | |
| Steroid withdrawal by day 14 post-transplant | 39 % | 48 % | 30 % | 0.58 |
| Use of Mycophenolate Mofetil | 99% | 99 % | 99 % | |
| CNI type: | 0.03 | |||
| Cyclosporine | 28 % | 22 % | 35 % | 0.90 |
| Tacrolimus | 69 % | 75 % | 62 % | 0.58 |
| None | 3 % | 3 % | 3 % | |
| SPK** | 6 % | 6 % | 5 % | |
| Prior Non-kidney Transplants* | 10 % | 11 % | 9 % | 0.90 |
| CMV Recipient/Donor Status* | 0.88 | |||
| Recipient (−)/Donor (−) | 20 % | 22 % | 19 % | 0.43 |
| Recipient (+) | 63 % | 62 % | 64 % | |
| Recipient (−)/Donor (+) | 17 % | 16 % | 17 % |
Missing data: Living donor gender missing in 7 subjects, need for dialysis in the first 14 days post-transplant missing in 4 subjects, Cold Ischemia time missing in 209 subjects, Final PRA missing in 7 subjects, Plasmapheresis prior to transplant missing in 57 subjects, B/T cell crossmatch missing in 51 subjects, Smoking status missing in 7 subject, Use of Mycophenolate Mofetil and CNI type was missing in 215 subjects, Prior non-kidney transplant missing in 44 subjects, CMV recipient/donor status missing in 44 subjects.
SPK= Simultaneous kidney pancreas transplants
Table 4.
Multivariate model results with top 15 SNPs (ranked by p-value) associated with time to CGD, stratified by transplant center.(n=979) All variant data from dbSNP Build 132
| SNP | Genec | Variant | HRa | 95% CI | P Value | Minor allele in AA | MAF in AA | Minor allele in non-AA | MAF in non-AA |
|---|---|---|---|---|---|---|---|---|---|
| rs7886938 | FMO6d | Coding-synonb | 1.60 | 1.26–2.04 | 1.47E-04 | A | 0.19 | A | 0.16 |
| rs7889839 | FMO6d | Missenseb | 1.60 | 1.25–2.04 | 1.58E-04 | G | 0.19 | G | 0.16 |
| rs2272797 | FMO6d | Missenseb | 1.57 | 1.23–2.01 | 3.15E-04 | A | 0.17 | A | 0.16 |
| rs909530 | FMO3d | Coding-synon | 1.50 | 1.20–1.87 | 3.52E-04 | T | 0.48 | T | 0.25 |
| rs3769148 | ZAK | Missense | 0.68 | 0.54–0.85 | 5.61E-04 | A | 0.11 | A | 0.47 |
| rs2369679 | AK7 | Missense | 1.58 | 1.20–2.08 | 1.07E-03 | C | 0.03 | C | 0.17 |
| rs2239360 | FANCA | Intron | 1.42 | 1.15–1.76 | 1.22E-03 | C | 0.35 | T | 0.34 |
| rs10916 | CYP1B1 | Untranslated-3 | 1.45 | 1.16–1.83 | 1.38E-03 | G | 0.3 | G | 0.21 |
| rs2242480 | CYP3A4 | Intron | 1.59 | 1.19–2.13 | 1.59E-03 | C | 0.29 | T | 0.11 |
| rs916864 | PON3 | Intronb | 0.62 | 0.46–0.84 | 1.78E-03 | T | 0.09 | T | 0.22 |
| rs609636 | CYP4F12 | Missense | 0.37 | 0.20–0.69 | 1.85E-03 | T | 0.07 | T | 0.06 |
| rs609290 | CYP4F12 | Missense | 0.37 | 0.20–0.69 | 1.89E-03 | T | 0.07 | T | 0.06 |
| rs1639 | PON2 | Intron | 0.63 | 0.47–0.85 | 2.13E-03 | G | 0.1 | G | 0.23 |
| rs7190823 | FANCA | Missense | 1.39 | 1.12–1.72 | 2.59E-03 | T | 0.32 | C | 0.42 |
| rs12448860 | FANCA | Intron | 1.37 | 1.11–1.69 | 3.17E-03 | T | 0.37 | A | 0.42 |
(Abbreviations: AA= African Americans, MAF= Minor Allele Frequency, synon=synonymous, untranslated-3= variant in the untranslated 3′ end of gene, HR= Hazard Ratio)
Analysis conducted using a Cox proportional hazards model stratified by transplant center and adjusted by recipient race. Hazard ratio and 95% CI of CGD for each risk allele. Model also adjusted for confounders such as donor age and recipient characteristics such as African-American race, smoking status, recipient-donor CMV status, and age.
UCSC predicted variant relative to gene track
There was linkage disequilibrium (LD) between the three SNPs in FMO6 namely rs7886938 and rs7886938 and rs7889839 with r2=1.0 in both non-AAs and greater than 0.88 in AA’s. The FMO3 SNP, namely rs909530 is not in LD with FMO6 SNPs in non-AAs and AAs, with r2 less than 0.8.
SNPs in FANCA, rs7190823 and rs12448860 are in LD in non-AA only with r2=0.98.
SNPs in PON2 and PON3 rs1639 and rs916864, respectively are in LD with r2= 0.84 in non-AAs and 0.90 in AAs.
SNPs in CYP4F12, rs609636 and rs609290 are in LD in r2= 1.0 in AA and non-AA.
SNPs statistically significant after accounting for an false-discovery rate of 20%.
SNPs Associated with Severity of Tubular Atrophy and Interstitial Fibrosis
For the analysis of severity of these chronic scores among the 979 recipients in the test cohort, we excluded the less than 1% (30) biopsies from 24 subjects with missing scores and excluded all 21 subjects with 3 biopsies from a single center that had no ct scores ≥2. Then the test cohort comprised of a total of 934 recipients: 687 with no biopsies for cause, 168 with CGD biopsies and 79 with biopsies and no CGD. All 79 recipients with biopsies and no CGD did not have a 25% or greater rise in creatinine from their baseline. In this observational study, the need for biopsy was determined by the treating physician and physicians did order biopsies for other causes such as proteinuria. In order to assess the severity of ct-scores, we divided the test cohort into 3 groups: ct score ≥2 (n=52), ct-score ≤1 (n=195) and no biopsy group (n=687). During the median follow-up of 21 months post-transplant, the rate of return to dialysis or re-transplantation was 23% in the ct ≥ 2 group, 8% in the ct ≤ 1 and less than 1% in the no biopsy group. The top 15 SNPs potentially associated with ct ≥2 versus ct ≤ 1 (p<0.01), in a multivariate analysis adjusted for important confounders, are shown in Table 5. Confounders included SPK versus kidney alone, donor age, recipient age, recipient African American race, and baseline creatinine at 3 months post-transplant, smoking status and steroid withdrawal status at 14 days post-transplant. In this adjusted model, the top two SNPs (p<0.001) that were potentially associated with severity of ct-score were rs8179183 and rs3828034 in LEPR, a gene belonging to the family of cytokine receptors. The third SNPs were rs593421 in CYP4F12 gene, which is a member of the cytochrome P450 drug metabolizing enzyme. None of the top 15 SNPs were significant after accounting for an FDR of 20%. Supplemental Table 2 shows the association of all SNPs genotyped with severity of ct-scores. There was significant correlation between the ct (tubular atrophy) and ci (interstitial fibrosis scores) (Spearman correlation=0.88, p<0.0001), therefore the analysis was not repeated for ci scores.
Table 5.
SNPs associated with severity of ct-scores (chronic tubular atrophy) using an adjusted multinomial logistic regression with 3 outcome groups, ct score ≥2 (n=52), ct-score ≤ 1 (n=195) and no biopsy group (n=687). The top 15 SNPs are ranked by p-values for the t ≥ 2 versus t ≤ 1 groups. All variant data from dbSNP Build 132
|
|
||||||
|---|---|---|---|---|---|---|
| ct ≥ 2 vs ct ≤ 1 | ||||||
|
| ||||||
| SNP | Geneb | Variant | Allele | ORa | 95% CI | P Value |
| rs8179183 | LEPR | Missense | C | 2.67 | [1.538,4.627] | 4.77E-04 |
| rs3828034 | LEPR | Intron | C | 2.74 | [1.537,4.873] | 6.27E-04 |
| rs593421 | CYP4F12 | Coding Synon | C | 0.38 | [0.211,0.68] | 1.15E-03 |
| rs491347 | LRP5 | Intron | C | 2.20 | [1.35,3.57] | 1.53E-03 |
| rs2853559 | VDR | Intron | T | 0.41 | [0.232,0.717] | 1.84E-03 |
| rs2238136 | VDR | Intron | A | 2.20 | [1.337,3.63] | 1.95E-03 |
| rs667126 | LRP5 | Intron | C | 2.17 | [1.328,3.539] | 1.96E-03 |
| rs875444 | RXRA | Intron | G | 0.44 | [0.26,0.746] | 2.29E-03 |
| rs2306862 | LRP5 | Coding Synon | T | 2.44 | [1.376,4.344] | 2.31E-03 |
| rs7533315 | MTHFR | Intron | T | 2.03 | [1.286,3.197] | 2.33E-03 |
| rs688755 | CYP4F12 | Coding Synon | C | 0.41 | [0.235,0.73] | 2.33E-03 |
| rs2237580 | PON1 | Intron | G | 3.12 | [1.486,6.561] | 2.66E-03 |
| rs2955617 | SHBG | Unknown | G | 0.45 | [0.267,0.759] | 2.76E-03 |
| rs915057 | SYNE2 | Intron | T | 2.08 | [1.284,3.357] | 2.87E-03 |
| rs2066471 | MTHFR | Intron | A | 2.20 | [1.309,3.699] | 2.90E-03 |
(Abbreviations: synon=synonymous, OR= Odds Ratio)
= Analysis conducted using a multinomial logistic regression model, Odds ratio and 95% CI for each risk allele. The model was adjusted for transplant center, SPK versus kidney alone, donor age, recipient age, recipient African American race, and baseline creatinine at 3 months post-transplant, smoking status and steroid withdrawal status at 14 days post-transplant.
There was linkage disequilibrium (LD) between the two SNPs in LEPR rs8179183 and rs3828034 with r2=0.94 in AA but not in non-AA (r2=0.12).
The two SNPs in CYP4F12, rs593421 and rs688755 are in LD with r2=0.99 in both in AA and non-AA. None of these SNPs are in the LD with the the CYP4F12 SNPs shown in Table 4.
The two SNPs in VDR, rs2853559 and rs2238136 are not in LD in AA and non-AA with r2<0.3
Discussion
The goal of this study was to describe the CGD phenotype, its impact on allograft survival and genetic variants associated with CGD. We found that CGD was associated with a significant risk of death-censored allograft failure. CGD biopsies in 28% of the transplant recipients had findings consistent with AR concomitantly with chronic changes. This suggests a role of ongoing inflammation in development of many cases of CGD. Four SNPs in the microsomal enzyme genes, FMO3 and FMO6, were associated with CGD, after accounting for multiple testing. These SNPs may play a role in metabolism of immunosuppressive agents. Higher severity of tubular atrophy (ct) scores on biopsies, were associated with a higher risk of death-censored allograft failure. Different SNPs were potentially associated with severity of tubular atrophy namely SNPs in genes of LEPR, a cytokine receptor and CYP4F12, a member of cytochrome P450 family.
Our study is consistent with others22 describing CGD with biopsies showing concomitant interstitial inflammation and tubulitis. Based on the previously published natural history of “chronic allograft nephropathy” using protocol biopsies, the early onset of tubular atrophy and interstitial nephritis occurs by three months post-transplant and is the predominant feature at 1 year post-transplant.1 In contrast, the present study shows that CGD phenotype has not only these chronic scores but also acute inflammatory changes with significant i-scores and t-scores. (Table 2) These acute inflammatory changes were present despite defining CGD as occurring after three months post transplant, excluding the most common window for AR namely during the first three months post-transplant and reestablishing the baseline creatinine six weeks after initiating treatment for AR. It is not surprising to see this difference since the present study only had biopsies for cause. Also the recipients in the present study were higher risk compared to this previous study 1 because the present study included African Americans, individuals with positive crossmatch and some recipients requiring plasmapheresis prior to transplantation. In a study of failed allografts, tubular atrophy/interstitial fibrosis represented 31% of the causes of allograft failure.23 These chronic changes were due to immunological causes in 9% of the allograft failure cases. In another cohort enrolled at time of a biopsy for late allograft dysfunction. In this cross-sectional cohort, inflammation along with tubular atrophy changes was a phenotype that was described among four of the six main types of biopsy findings.24 In the present study, the rates of death censored allograft failures in recipients with CGD events, with (n=94) and without concomitant AR (n=272) was similar. (Figure 2) It is important to note that the CGD subgroup without AR also had concomitant interstitial inflammation and tubulitis (Table 2), but not enough to meet the Banff criteria of AR. Thus our findings of poor kidney function are consistent with literature. Even, sub-clinical inflammation, along with interstitial fibrosis, on protocol biopsies in recipients, has been previously associated with reduced allograft survival.25, 22
Prior genomics studies have focused on the phenotype of AR and CGD (defined by ct and ci scores only) separately. As seen in this study, both entities can occur concomitantly with increased risk of allograft failure. Therefore, this study is the first to focus on a phenotype of CGD with concomitant interstitial inflammation and tubulitis. The most significant SNPs associated with CGD, after accounting for multiple testing, were in the FM03 and FMO6 genes. With the exception of FMO5, the human isoforms of FMO are encoded within a single gene cluster on human chromosome 1q23-25. These genes are flavin-containing mono-oxygenases which produce proteins that catalyze the oxidation of many substrates, often in conjunction with cytochrome P450s. There is significant sequence homology between FMO6 and FMO3.26,27 FMO3 is the major adult isoform of the enzyme and is found in the liver. It is involved in the metabolism of drugs such as voriconazole, cimetidine, rantidine, tamoxifen, sulindac, nicotine and busulfan.28,29 Little is known about the effect of FMO3 on common drugs used in transplantation. Although we previously identified the FMO3 SNP, rs1800822, to be associated with higher tacrolimus troughs in kidney transplant patients.16 Rs1800822 is not in linkage disequilibrium (r2<0.3) with the SNPs shown in Table 4, suggesting that these SNPs may be involved through intracellular metabolism of tacrolimus or other mechanisms. FMO6 is poorly studied and effect of these variants has yet to be defined. Rs7886938 in FM06 and rs909530 in FM03 are synonymous coding SNPs. Recent discoveries have shown that SNPs in nucleotides that code for synonymous codons, can influence the rate of translation of mRNA transcripts and thereby influence the amount of protein produced and the post-translational modification of the protein.30
Our study is the first to study SNPs potentially associated with severity of chronic tubular atrophy as determined by ct-scores. It is well known that there is variation in severity of decline in kidney function3; hence it is not surprising that different SNPs were associated with risk of CGD and severity of ct-scores. This study describes the potential genetic factors associated with this variation, after accounting for relevant clinical factors such as donor age. The most significant SNPs potentially associated with CGD severity were in the LEPR and CYP4F12 genes. LEPR is a gene belonging to the family of cytokine receptors. These cytokine receptors stimulate gene transcription by activating cytosolic STAT proteins. The LEPR or leptin receptor, possesses strong homology to the signal-transducing subunits of IL-6 receptor. IL-6 is a B-cell stimulatory factor2 or IFN-beta-2.31 LEPR codes for a pro-inflammatory cytokine receptor gene and leptin plays a role in regulatory T cell proliferation.32–36 CYP4F12 is a member of the cytochrome P450 superfamily of enzymes, but the exact physiological function of this member as it relates to transplantation is not known. The cytochrome P450 enzymes catalyze many reactions involved in drug metabolism and synthesis of steroids.37
Previous studies have found SNPs, that are associated with chronic allograft dysfunction. These SNPs include rs699 in AGT 38, rs2069762 in IL2 in a Japanese population39, rs1801131 in MTHFR40, rs1800629 in TNFA41. These previously published studies are much smaller than the present study. The present study could not replicate these findings. Differences in population being studied could explain the lack of duplication. There could also be differences in the CGD phenotype among studies. None of the previously published studies provided detailed description and analysis using biopsy results.
The current study has several limitations. It is possible that the association of SNPs in other candidate genes was not seen in our study due to the SNP’s small effect size and our limited sample size. Another potential limitation is that the biopsy slides were not read by a centralized pathologist who was blinded to the clinical information. The local pathologist did not describe inflammation in areas of tubular atrophy42 since this type of inflammation is not in the standard Banff definition of AR.2 Nonetheless, the clinic phenotype of CGD was strongly associated with reduced death-censored allograft survival. Lastly, different clinical entities that could give rise to CGD and reduce our power to detect associations with SNPs. However, the study found SNPs associated with CGD despite this limitation of the CGD definition. We currently lack the power to study SNP-SNP interactions from multiple genes, due to the limited sample size.
In summary, this study has described a phenotype of CGD that has concomitant inflammation. This phenotype is strongly associated with allograft failure. We have analyzed SNPs associated with CGD, after accounting for important clinical factors. SNPs potentially involved in drug metabolism genes such as FM03 and FM06, are novel findings in this study. This study will validate the top SNPs (Table 4–5) in an ongoing, larger non-test cohort of 2,000 kidney recipients after accounting for multiple testing. In the future, with larger cohorts of kidney recipients and careful phenotyping, there may be enough power to conduct a genome–wide association study.
Supplementary Material
Supplement Table 1. Multivariate Analysis Multivariate model showing all SNPs (ranked by p-value) associated with time to CGD, stratified by transplant center and adjusted for recipient race. Model also adjusted for confounders such as donor age and recipient characteristics such as African-American race, smoking status, recipient-donor CMV status, and age. Analysis conducted using a Cox proportional hazards model.
Supplement Table 2. Association of all SNPs with severity of ct-scores (chronic tubular atrophy) using an adjusted multinomial logistic regression with 3 outcome groups, ct score ≥ 2 (n=52), ct-score ≤ 1 (n=195) and no biopsy group (n=687). SNPs are ranked by p-values for the ct ≥ 2 versus ct ≤ 1 groups. The model was adjusted for transplant center, SPK versus kidney alone, donor age, recipient age, recipient African American race, and baseline creatinine at 3 months post-transplant, smoking status and steroid withdrawal status at 14 days post-transplant.
Acknowledgments
Funding Source
This work was supported by the National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119) and DeKAF (5U01-AI058013)
We acknowledge the dedication and hard work of our coordinators at each of the six clinical sites: University of Alberta, Nicoleta Bobocea, Tina Wong, Adrian Geambasu and Alyssa Sader; University of Manitoba, Myrna Ross and Kathy Peters; University of Minnesota, Mandi DeGrote and Jill Nagorski; Hennepin County Medical Center, Lisa Berndt; Mayo Clinic, Tom DeLeeuw; University of Iowa, Wendy Wallace and Tammy Lowe; University of Alabama, Jacquelin Vaughn and Tena Hilario. We also acknowledge the dedicated work of our research scientists: Marcia Brott, Becky Willaert, Jennifer Vigliaturo, and Brian Kasel.
Abbreviations
- CGD
chronic allograft dysfunction
- AR
acute rejection
- SNP
single nucleotide polymorphism
- eGFR
estimated glomerular filtration rate
- SPK
simultaneous kidney pancreas transplant
- FDR
false discovery rate
- i
interstitial inflammation
- t
tubulitis
- ci
interstitial fibrosis
- ct
tubular atrophy
- cv
vascular fibrosis
- cg
glomerulopathy
- ah
arteriolar hyaline thickening
- v
intimal artertitis
- g
glomerulitis
Footnotes
Requests for reprints and published: isran001@umn.edu
Presented in part as two oral presentations at the 2011 ATC in Philadephia
Conflict of Interest
None to report
DeKAF Genomics Investigators
J. Michael Cecka, M.D., UCLA Immunogenetics Center, Los Angeles, CA 90095, mcecka@ucla.edu
John Connett, Ph.D., Division of Biostatistics. University of Minnesota, Minneapolis, MN 55455, john-c@biostat.umn.edu
Fernando G. Cosio, M.D., Division of Nephrology, Mayo Clinic, Rochester, MN 55905, Cosio.Fernando@mayo.edu
Robert Gaston, M.D., University of Alabama, Division of Nephrology, Birmingham, AL 35294-0006, rgaston@uab.edu
Sita Gourishankar, M.D., Division of Nephrology and Immunology, University of Alberta, Edmonton, Alberta, Canada, sitag@ualberta.ca
Joseph P. Grande, M.D., Ph.D., Mayo Clinic College of Medicine, Rochester MN 55905, Grande.Joseph@mayo.edu
Lawrence Hunsicker, M.D., Nephrology Division, Iowa City, IA 52242-1082, lawrence-hunsicker@uiowa.edu
Bertram Kasiske, M.D., Department of Medicine, Hennepin County Medical Center and the University of Minnesota, Minneapolis, MN 55415, kasis001@umn.edu
Rosalyn Mannon, M.D., University of Alabama, Division of Nephrology, Birmingham, AL 35294-0006, rmannon@uab.edu
David Rush, M.D., Health Sciences Center, Winnipeg MB, Canada, drush@exchange.hsc.mb.ca
Gretchen Crary, M.D, M.B.A., Hennepin County Medical Center, University of Minnesota, Minneapolis, MN 55415-1829, Gretchen.Crary@hcmed.org
Author Contributions:
Ajay Israni, MD, MS, - Research design, writing of paper, performance of research, contributed to analytical tools.
Robert Leduc, PhD - Research design, writing of paper, performance of research, contributed to analytical tools, data analysis.
Pamala A. Jacobson, PharmD - Research design, writing of paper, performance of research.
Winston Wildebush - Writing of paper, performance of research, data analysis
William S. Oetting, PhD, - Research design, writing of paper, performance of research.
Weihua Guan, PhD - Research design, writing of paper, contributed to analytical tools, data analysis.
David Schladt, MS, - Research design, writing of paper, performance of research, contributed to analytical tools, data analysis.
Arthur J. Matas, MD, - Research design, writing of paper, performance of research.
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. New England Journal of Medicine. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 2.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American Journal of Transplantation. 2008;8:753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Heim-Duthoy KL, Tortorice K, Rao KV. The variable nature of chronic declines in renal allograft function. Transplantation. 1991;51:330–4. doi: 10.1097/00007890-199102000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Brown KM, Kondeatis E, Vaughan RW, et al. Influence of donor C3 allotype on late renal-transplantation outcome. [see comment] New England Journal of Medicine. 2006;354:2014–23. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 5.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Zeiss C, EYC Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson P, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, Matas AJ, Lamba V, Julian BA, Mannon RB, Israni A for DeKAF investigators. Novel Polymorphisms Associated with Tacrolimus Trough Concentrations: Results from a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91:300–8. doi: 10.1097/TP.0b013e318200e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson P, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, Lamba V, Mannon RB, Julian BA, Israni A for the DEKAF investigators. Genetic Determinants of Mycophenolate Related Anemia and Leukopenia Following Transplantation. Transplantation. 2011;91:309–16. doi: 10.1097/TP.0b013e318200e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson PA, Schladt D, Israni A, et al. Genetic and clinical determinants of early, acute calcineurin inhibitor-related nephrotoxicity: Results from a kidney transplant consortium. Transplantation. 2012;93:624–31. doi: 10.1097/TP.0b013e3182461288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing equation for tacrolimus using genetic variants and clinical factors. British Journal of Clinical Pharmacology. 2011;72:948–57. doi: 10.1111/j.1365-2125.2011.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasiske B, Gaston R, Gourishankar S, et al. Long-term deterioration of kidney allograft function. American Journal of Transplantation. 2005;5:1405–14. doi: 10.1111/j.1600-6143.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 12.Israni A, Leduc R, Holmes J, et al. Single Nucleotide Polymorphisms, Acute Rejection and Severity of Tubulitis in Kidney Transplantation, Accounting for Center-to-Center Variation. Transplantation. 2010;90:1401–8. doi: 10.1097/TP.0b013e3182000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Ness B, Ramos C, Haznadar M, et al. Genomic variation in myeloma: design, content, and initial application of the Bank On A Cure SNP Panel to detect associations with progression-free survival. BMC Med. 2008:6. doi: 10.1186/1741-7015-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardenbol PBJ, Jain M, Nilsson M, Namsaraev EA, Karlin-Neumann GA, et al. Multiplexed genotyping with sequence-tagged molecular inversion probes. Nat Biotechnol. 2003;21:673–8. doi: 10.1038/nbt821. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson P, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, Matas AJ, Lamba V, Julian BA, Mannon RB, Israni A for DeKAF investigators. Novel Polymorphisms Associated with Tacrolimus Trough Concentrations: Results from a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91(3):300–8. doi: 10.1097/TP.0b013e318200e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson P, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, Lamba V, Mannon RB, Julian BA, Israni A for the DEKAF investigators. Genetic Determinants of Mycophenolate Related Anemia and Leukopenia Following Transplantation. Transplantation. 2011;91(3):309–16. doi: 10.1097/TP.0b013e318200e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life-tables. Journal of the Royal Statistics Society. 1972;34:187–220. [Google Scholar]
- 18.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 19.Akkina SK, Connaire JJ, Israni AK, Snyder JJ, Matas AJ, Kasiske BL. Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. American Journal of Transplantation. 2009;20:1351–8. doi: 10.1111/j.1600-6143.2009.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hines RN, et al. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol Pharmacol. 2002 doi: 10.1124/mol.62.2.320. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez D, et al. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004 doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Grimm PC, Nickerson P, Gough J, et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. Journal of the American Society of Nephrology. 2003;14:1662–8. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- 23.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. American Journal of Transplantation. 2009;9:527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 24.Matas AJ, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: preliminary data from the DeKAF study. American Journal of Transplantation. 10:315–23. doi: 10.1111/j.1600-6143.2009.02943.x. [DOI] [PubMed] [Google Scholar]
- 25.Park WD, Griffin MD, Cornell LD, et al. Fibrosis with inflammation at one year predicts transplant functional decline. Journal of the American Society of Nephrology. 21:1987–97. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisamuddin IM, Yang VW. Genetic polymorphisms of human flavin-containing monooxygenase 3: implications for drug metabolism and clinical perspectives. Pharmacogenomics. 2007;8:635–43. doi: 10.2217/14622416.8.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hines RN, Hopp KA, Franco J, Saeian K, Begun FP. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol Pharmacol. 2002;62:320–5. doi: 10.1124/mol.62.2.320. [DOI] [PubMed] [Google Scholar]
- 28.Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Jr, DRT In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab Dispos. 2010;38:25–31. doi: 10.1124/dmd.109.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parte P, Kupfer D. Cyclic oxidation by human FMO1 and FMO3 of tamoxifen to tam-N-oxide and its reduction back to tamoxifen by human P450s: Model cyclic reactions for novel triphenylethyleneamine SERMs. Drug Metabolism and Disposition. 2005;33:1446–52. doi: 10.1124/dmd.104.000802. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Saha S, Shabalina SA, et al. Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science. 329:1534–7. doi: 10.1126/science.1191701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang S, He F. Phylogeny of a growth hormone-like cytokine superfamily based upon 3D structure. J Mol Evol. 2003;56:131–6. doi: 10.1007/s00239-002-2385-2. [DOI] [PubMed] [Google Scholar]
- 32.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. Journal of Clinical Endocrinology & Metabolism. 2007;92:2272–9. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 33.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Lago F, Dieguez C, Gomez-Reino J, et al. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine & Growth Factor Reviews. 2007;18:313–25. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Lord GM, Lord GM. Leptin as a proinflammatory cytokine. Contributions to Nephrology. 2006;151:151–64. doi: 10.1159/000095326. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Honma M, Ishida-Yamamoto A, et al. Adiponectin and leptin modulate cell proliferation and cytokine secretion of normal human keratinocytes and T lymphocytes. Journal of Dermatological Science. 59:143–5. doi: 10.1016/j.jdermsci.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Dhar M, Sepkovic DW, Hirani V, et al. Omega oxidation of 3-hydroxy fatty acids by the human CYP4F gene subfamily enzyme CYP4F11. Journal of Lipid Research. 2008;49:612–24. doi: 10.1194/jlr.M700450-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Azarpira N, Bagheri M, Raisjalali GA, Aghdaie MH, Behzadi S, Salahi H, Rahsaz M, Darai M, Ashraf MJ, Geramizadeh B. Angiotensinogen, angiotensin converting enzyme and plasminogen activator inhibitor-1 gene polymorphism in chronic allograft dysfunction. Mol Biol Rep. 2009;36:909–15. doi: 10.1007/s11033-008-9262-z. [DOI] [PubMed] [Google Scholar]
- 39.Satoh S, Saito M, Inoue K, et al. Association of cytokine polymorphisms with subclinical progressive chronic allograft nephropathy in Japanese renal transplant recipients: preliminary study. International Journal of Urology. 2007;14:990–4. doi: 10.1111/j.1442-2042.2007.01886.x. [DOI] [PubMed] [Google Scholar]
- 40.Viklický OHJ, Kvasnicka J, Matl I, Voska L, Skibová J, Teplan V, Vítko S. Association of methylenetetrahydrofolate reductase T677 allele with early development of chronic allograft nephropathy. Clin Biochem. 2004;37:919–24. doi: 10.1016/j.clinbiochem.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Nikolova PN, Ivanova MI, Mihailova SM, et al. Cytokine gene polymorphism in kidney transplantation--impact of TGF-beta 1, TNF-alpha and IL-6 on graft outcome. Transplant Immunology. 2008;18:344–8. doi: 10.1016/j.trim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. American Journal of Transplantation. 2011;10:2066–73. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table 1. Multivariate Analysis Multivariate model showing all SNPs (ranked by p-value) associated with time to CGD, stratified by transplant center and adjusted for recipient race. Model also adjusted for confounders such as donor age and recipient characteristics such as African-American race, smoking status, recipient-donor CMV status, and age. Analysis conducted using a Cox proportional hazards model.
Supplement Table 2. Association of all SNPs with severity of ct-scores (chronic tubular atrophy) using an adjusted multinomial logistic regression with 3 outcome groups, ct score ≥ 2 (n=52), ct-score ≤ 1 (n=195) and no biopsy group (n=687). SNPs are ranked by p-values for the ct ≥ 2 versus ct ≤ 1 groups. The model was adjusted for transplant center, SPK versus kidney alone, donor age, recipient age, recipient African American race, and baseline creatinine at 3 months post-transplant, smoking status and steroid withdrawal status at 14 days post-transplant.