Abstract
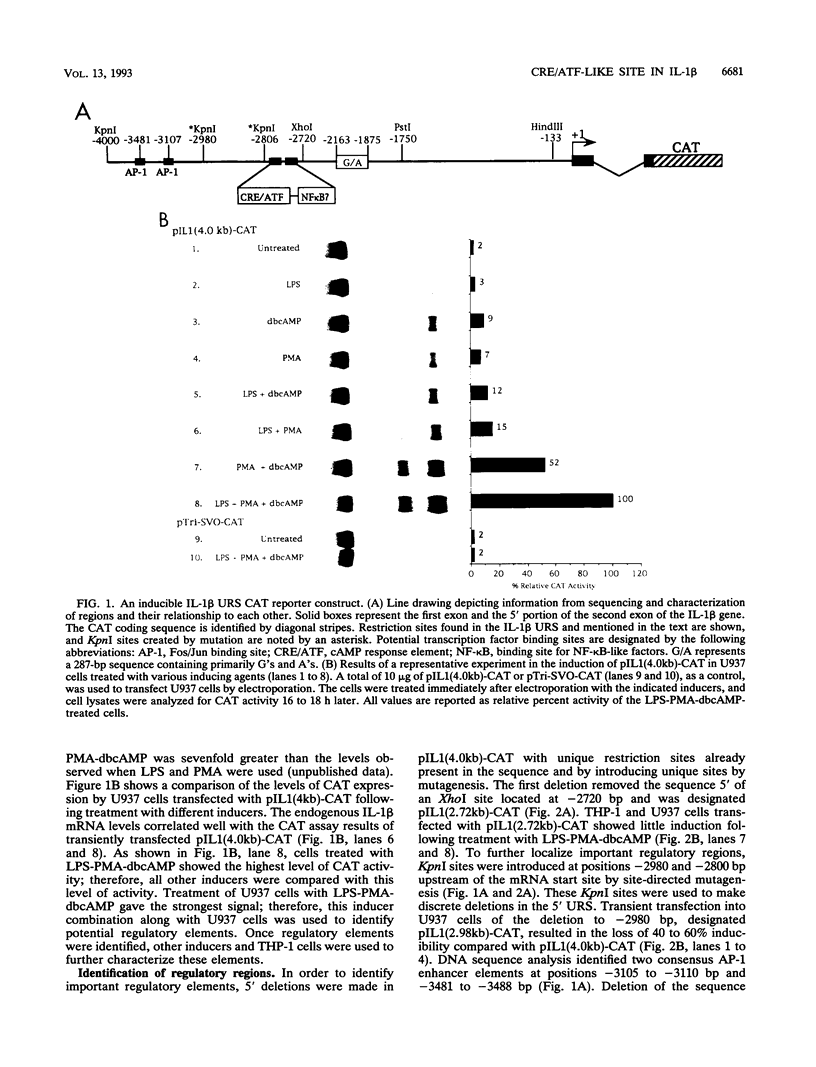
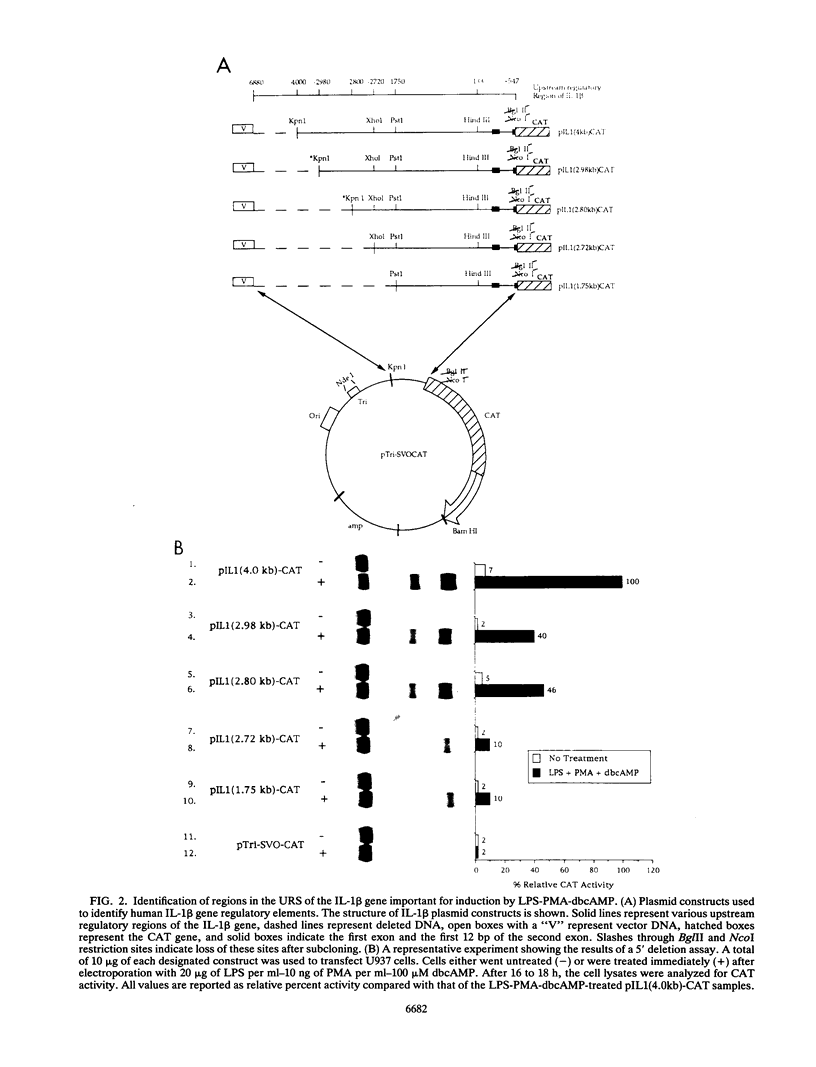
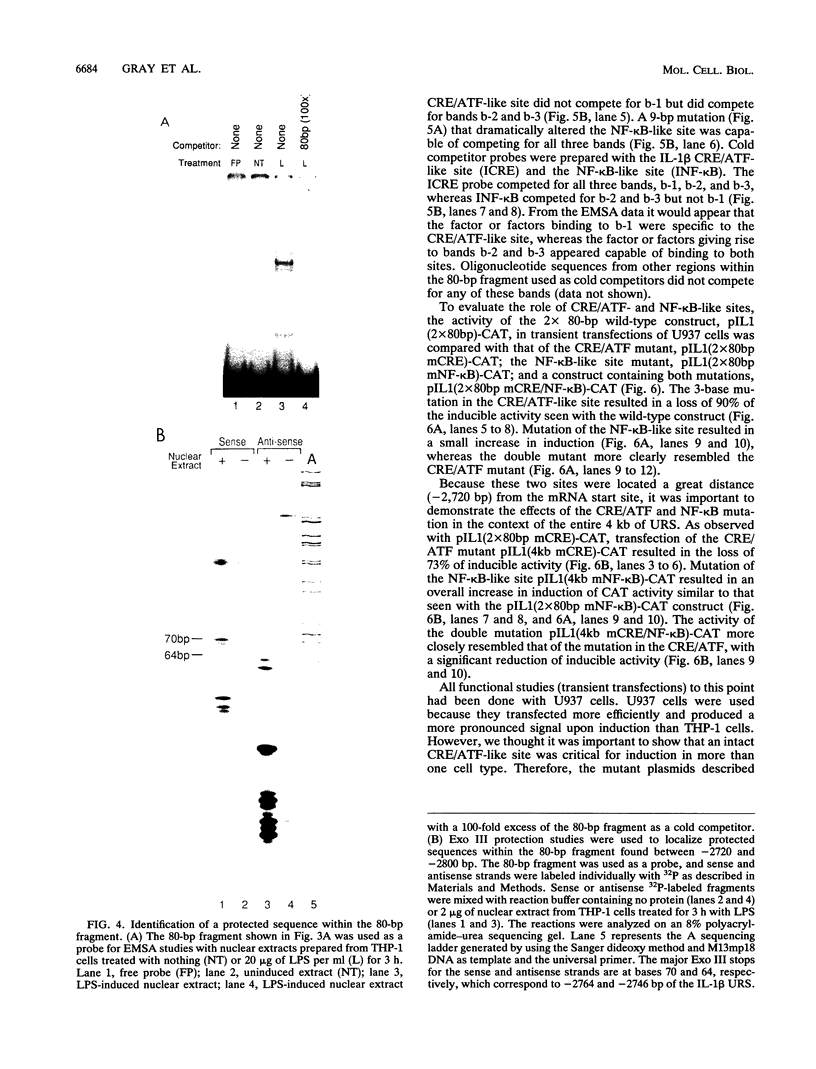
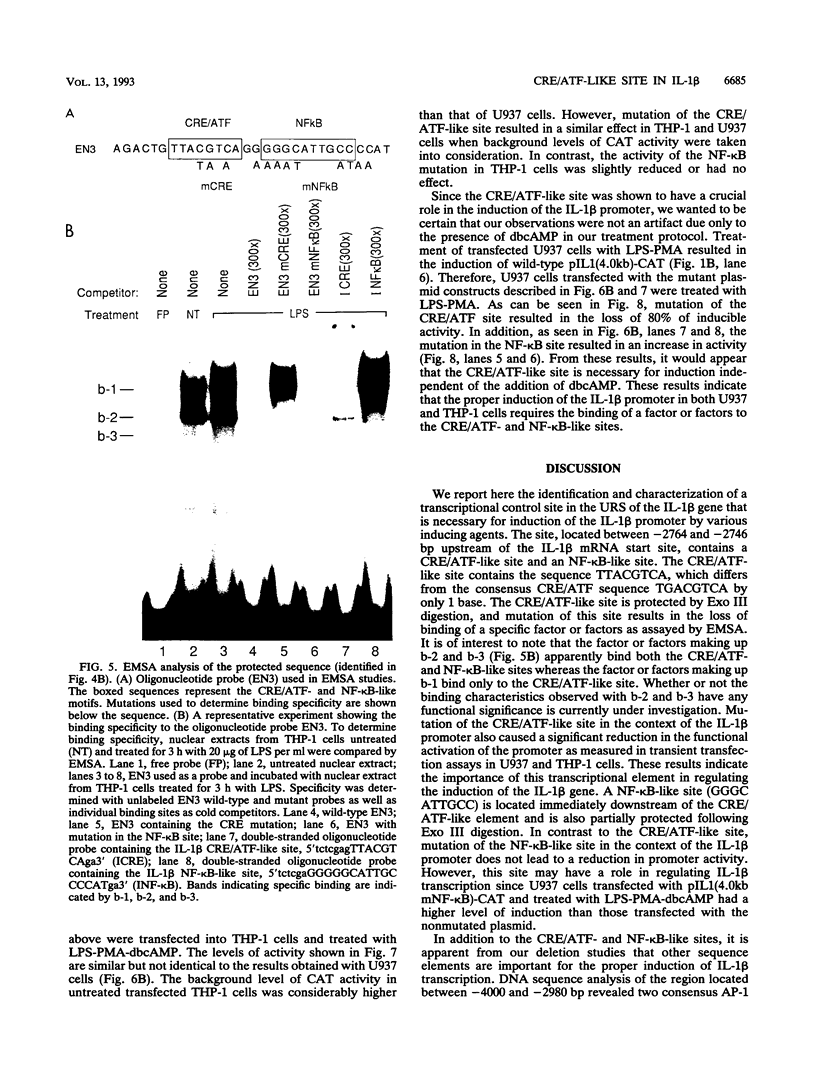
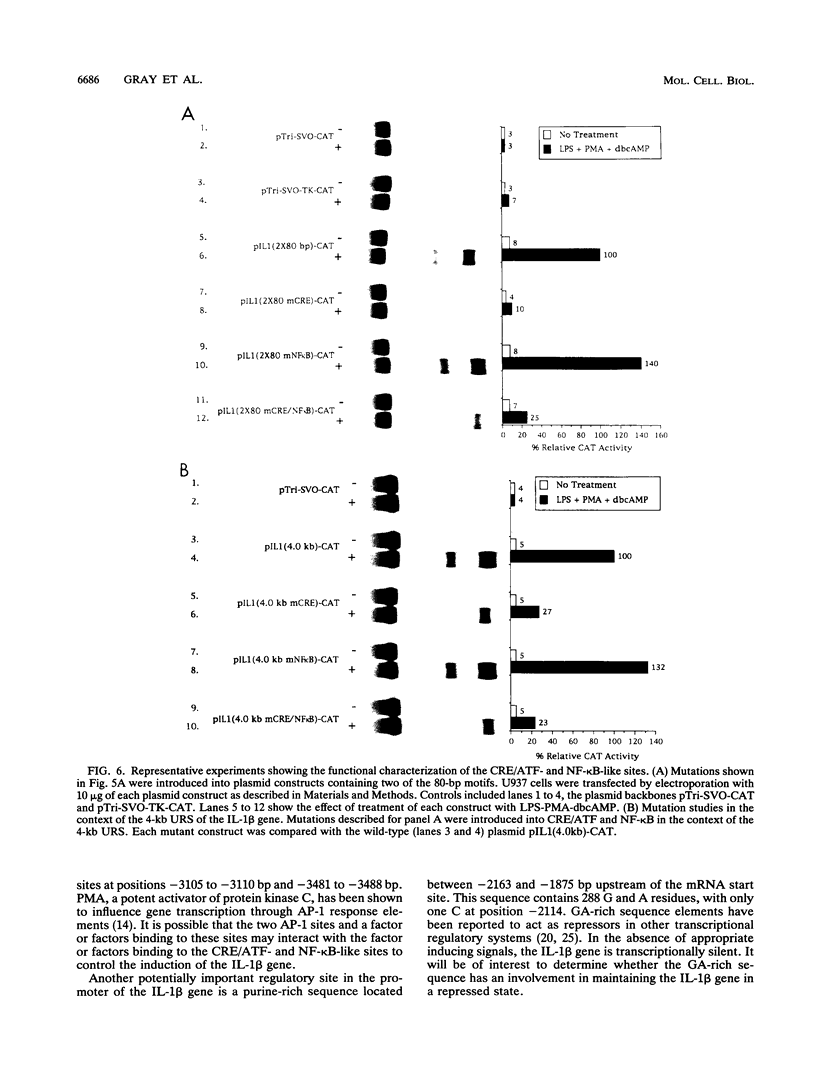
Transfection of U937 and THP-1 cells with a recombinant plasmid, pIL1(4.0kb)-CAT, containing 4 kb of the interleukin 1 beta (IL-1 beta) gene upstream regulatory sequence resulted in inducer-dependent expression of chloramphenicol acetyltransferase activity. Treatment of the transfected cells with various combinations of the inducers lipopolysaccharide, phorbol myristate acetate, and dibutyryl cyclic AMP upregulated the IL-1 beta promoter. In U937 and THP-1 cells, maximum stimulation of both the endogenous IL-1 beta gene and pIL1(4.0kb)-CAT transfectants was observed following treatment with the combination of inducing agents lipopolysaccharide-phorbol myristate acetate-dibutyryl cyclic AMP. This combination of inducing agents was used to identify and study, at the molecular level, some of the regulatory elements necessary for induction of the IL-1 beta gene. A series of 5' deletion derivatives of the upstream regulatory sequence were used in transient transfection assays to identify an 80-bp fragment located between -2720 and -2800 bp upstream of the mRNA start site that was required for induction. Exonuclease III mapping, electrophoretic mobility shift assays (EMSA), and DNA sequence analysis of this region were used to identify a transcription factor binding sequence which contained a potential cyclic AMP response element (CRE/ATF)- and NF-kappa B-like binding site. Site-directed mutagenesis of the CRE/ATF-like site resulted in the loss of binding of a specific factor or factors as determined by EMSA. The loss of binding activity directly correlated with a loss of approximately 75% of promoter activity as determined in transient transfection assays. As determined by EMSA, the factor binding to the CRE/ATF-like site was present in nuclear extracts prepared from both uninduced and induced THP-1 and U937 cells. However, the intensity of the band appeared to be increased when nuclear extracts from induced cells were used. In contrast to the CRE/ATF mutation, which resulted in the loss of promoter activity, mutation of the NF-kappa B-like site resulted in a moderate increase in activity in U937 cells. A similar increase in promoter activity was not observed in THP-1 cells. From these studies, we conclude that a CRE/ATF-like site and a factor or factors interacting with this site are essential for the maximum induction of the IL-1 beta gene in stimulated U937 and THP-1 cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abmayr S. M., Workman J. L., Roeder R. G. The pseudorabies immediate early protein stimulates in vitro transcription by facilitating TFIID: promoter interactions. Genes Dev. 1988 May;2(5):542–553. doi: 10.1101/gad.2.5.542. [DOI] [PubMed] [Google Scholar]
- Auron P. E., Warner S. J., Webb A. C., Cannon J. G., Bernheim H. A., McAdam K. J., Rosenwasser L. J., LoPreste G., Mucci S. F., Dinarello C. A. Studies on the molecular nature of human interleukin 1. J Immunol. 1987 Mar 1;138(5):1447–1456. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensi G., Mora M., Raugei G., Buonamassa D. T., Rossini M., Melli M. An inducible enhancer controls the expression of the human interleukin 1 beta gene. Cell Growth Differ. 1990 Oct;1(10):491–497. [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G., Patel P., Kost T. A., Gray J. G. Large-scale purification of plasmid DNA by fast protein liquid chromatography using a Hi-Load Q Sepharose column. Anal Biochem. 1992 May 15;203(1):169–172. doi: 10.1016/0003-2697(92)90060-k. [DOI] [PubMed] [Google Scholar]
- Clark B. D., Collins K. L., Gandy M. S., Webb A. C., Auron P. E. Genomic sequence for human prointerleukin 1 beta: possible evolution from a reverse transcribed prointerleukin 1 alpha gene. Nucleic Acids Res. 1986 Oct 24;14(20):7897–7914. doi: 10.1093/nar/14.20.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump J. W., Geist L. J., Auron P. E., Webb A. C., Stinski M. F., Hunninghake G. W. The immediate early genes of human cytomegalovirus require only proximal promoter elements to upregulate expression of interleukin-1 beta. Am J Respir Cell Mol Biol. 1992 Jun;6(6):674–677. doi: 10.1165/ajrcmb/6.6.674. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton M. J., Clark B. D., Collins K. L., Webb A. C., Rich A., Auron P. E. Transcriptional regulation of the human prointerleukin 1 beta gene. J Immunol. 1987 Jun 1;138(11):3972–3979. [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Giri J. G., Lomedico P. T., Mizel S. B. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985 Jan;134(1):343–349. [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. R., Gaugler L., Deagostini-Bazin H., Bally-Cuif L., Goridis C. Identification of positive and negative regulatory elements governing cell-type-specific expression of the neural cell adhesion molecule gene. Mol Cell Biol. 1990 May;10(5):1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Monks B. G., Geist L. J., Monick M. M., Monroy M. A., Stinski M. F., Webb A. C., Dayer J. M., Auron P. E., Fenton M. J. The functional importance of a cap site-proximal region of the human prointerleukin 1 beta gene is defined by viral protein trans-activation. Mol Cell Biol. 1992 Aug;12(8):3439–3448. doi: 10.1128/mcb.12.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurme M. Modulation of interleukin-1 beta production by cyclic AMP in human monocytes. FEBS Lett. 1990 Apr 9;263(1):35–37. doi: 10.1016/0014-5793(90)80699-j. [DOI] [PubMed] [Google Scholar]
- Hurme M., Serkkola E., Ronni T., Silvennoinen O. Control of interleukin-1 beta expression by protein kinase C and cyclic adenosine monophosphate in myeloid leukemia cells. Blood. 1990 Dec 1;76(11):2198–2203. [PubMed] [Google Scholar]
- Iwamoto G. K., Monick M. M., Clark B. D., Auron P. E., Stinski M. F., Hunninghake G. W. Modulation of interleukin 1 beta gene expression by the immediate early genes of human cytomegalovirus. J Clin Invest. 1990 Jun;85(6):1853–1857. doi: 10.1172/JCI114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkis E., Riggs K. J., Gillespie W., Calame K. A transcriptional repressor of c-myc. Nature. 1989 Jun 29;339(6227):718–721. doi: 10.1038/339718a0. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Krane S. M., Conca W., Stephenson M. L., Amento E. P., Goldring M. B. Mechanisms of matrix degradation in rheumatoid arthritis. Ann N Y Acad Sci. 1990;580:340–354. doi: 10.1111/j.1749-6632.1990.tb17943.x. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt N., Briggs D., Gil A., Proudfoot N. J. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989 Jul;3(7):1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Saito K., Bonagura C. A., Galson D. L., Fenton M. J., Webb A. C., Auron P. E. The human prointerleukin 1 beta gene requires DNA sequences both proximal and distal to the transcription start site for tissue-specific induction. Mol Cell Biol. 1993 Mar;13(3):1332–1344. doi: 10.1128/mcb.13.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Fink J. S., Mandel G., Goodman R. H. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987 Jun 25;262(18):8743–8747. [PubMed] [Google Scholar]
- Turner M., Chantry D., Feldmann M. Post-transcriptional control of IL-1 gene expression in the acute monocytic leukemia line THP-1. Biochem Biophys Res Commun. 1988 Oct 31;156(2):830–839. doi: 10.1016/s0006-291x(88)80919-7. [DOI] [PubMed] [Google Scholar]
- Viherluoto J., Palkama T., Silvennoinen O., Hurme M. Cyclic adenosine monophosphate decreases the secretion, but not the cell-associated levels, of interleukin-1 beta in lipopolysaccharide-activated human monocytes. Scand J Immunol. 1991 Jul;34(1):121–125. doi: 10.1111/j.1365-3083.1991.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Yamato K., el-Hajjaoui Z., Koeffler H. P. Regulation of levels of IL-1 mRNA in human fibroblasts. J Cell Physiol. 1989 Jun;139(3):610–616. doi: 10.1002/jcp.1041390322. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]