Abstract
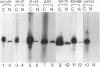
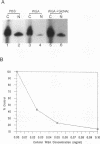
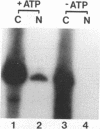
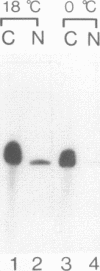
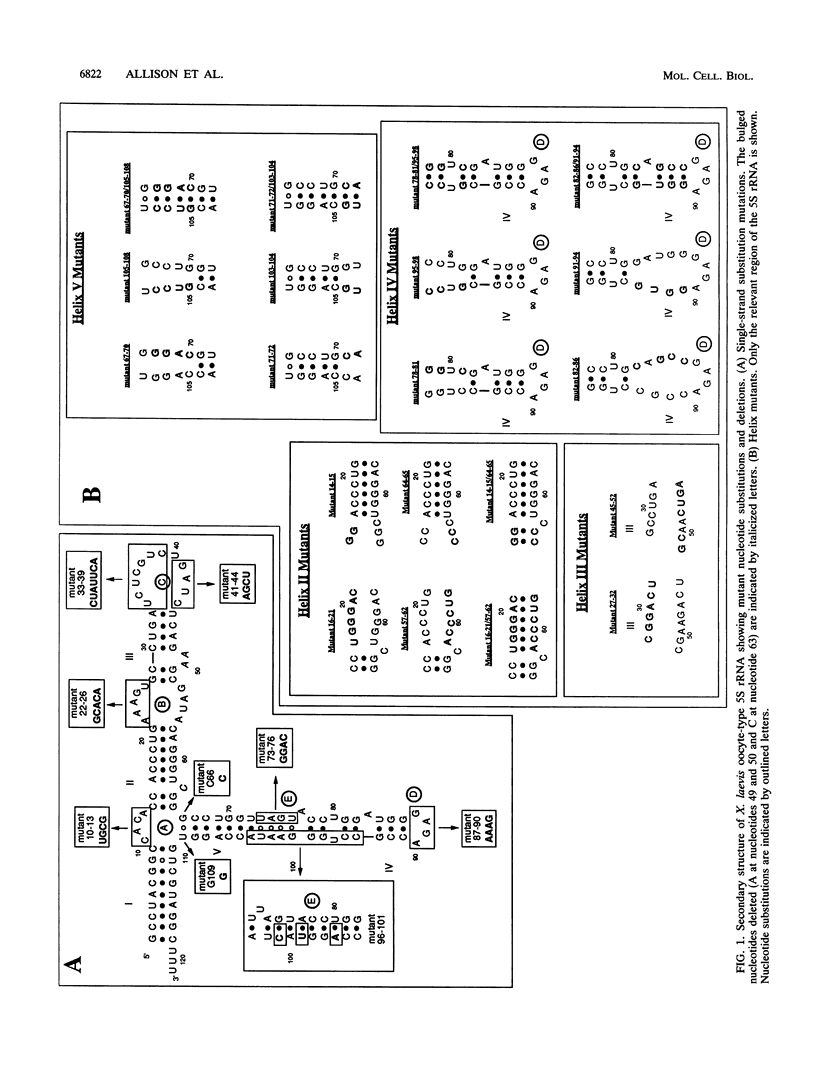
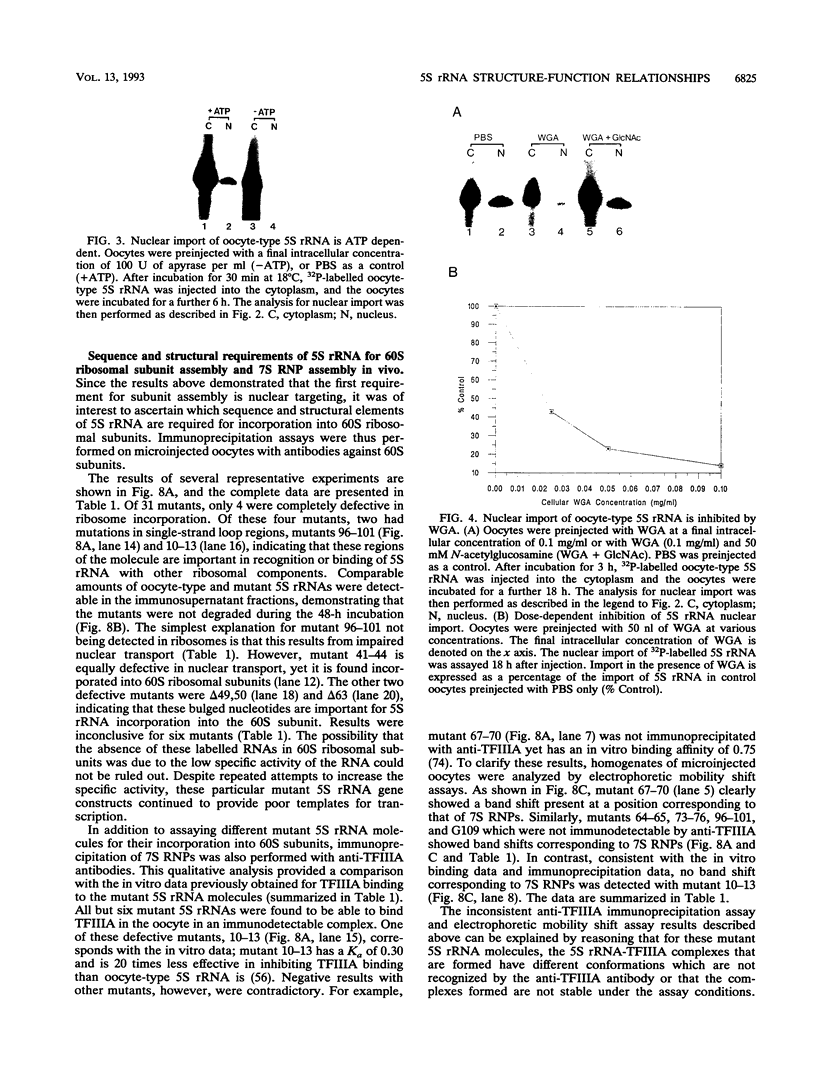
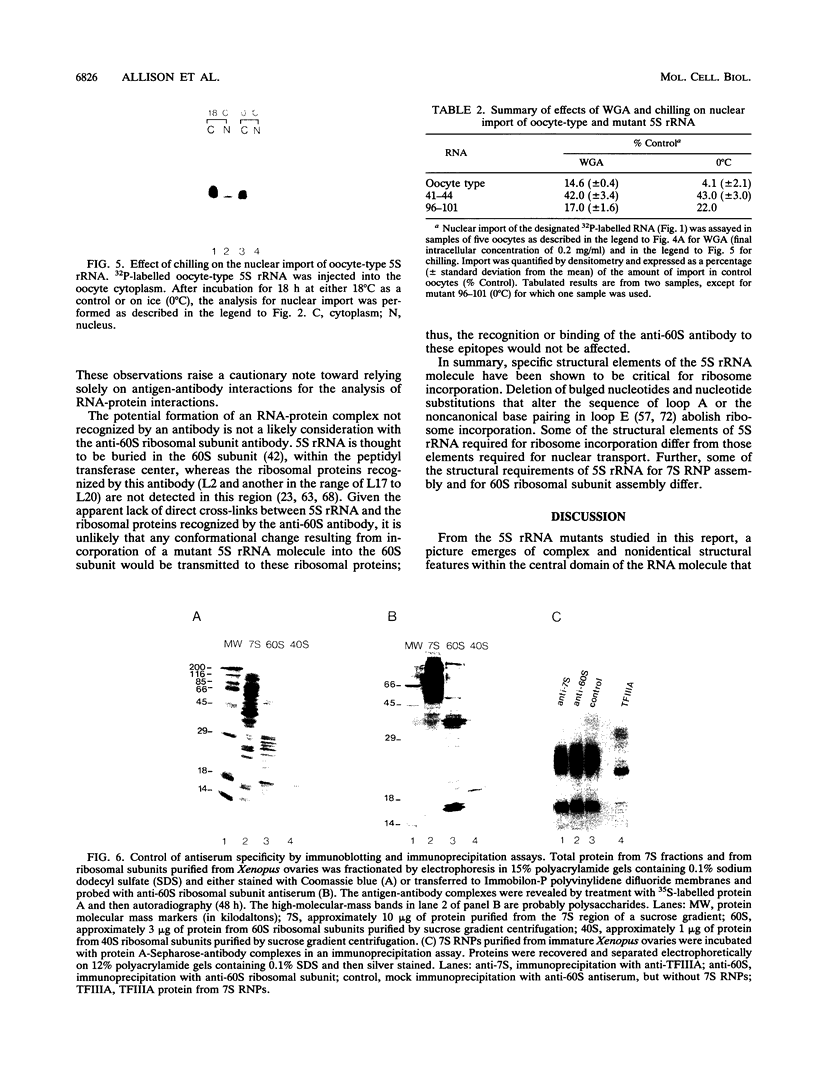
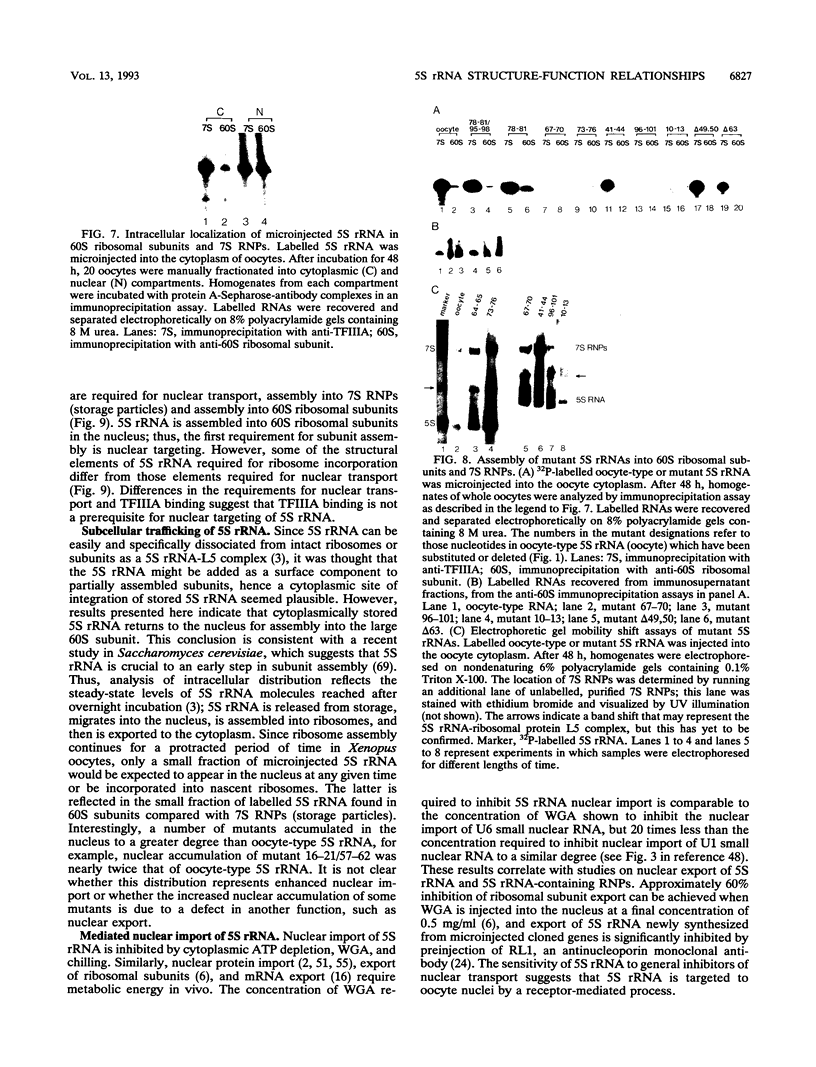
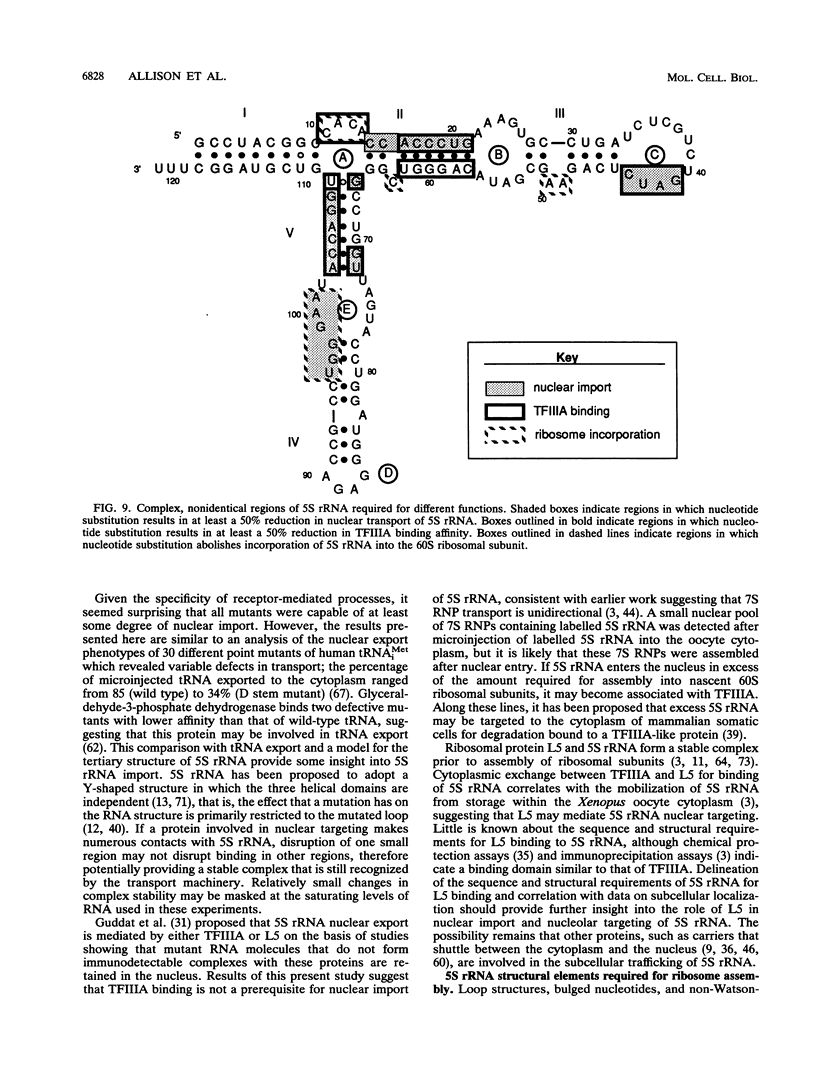
Structural requirements of 5S rRNA for nuclear transport and RNA-protein interactions have been studied by analyzing the behavior of oocyte-type 5S rRNA and of 31 different in vitro-generated mutant transcripts after microinjection into the cytoplasm of Xenopus oocytes. Experiments reveal that the sequence and secondary and/or tertiary structure requirements of 5S rRNA for nuclear transport, storage in the cytoplasm as 7S ribonucleoprotein particles, and assembly into 60S ribosomal subunits are complex and nonidentical. Elements of loops A, C, and E, helices II and V, and bulged and hinge nucleotides in the central domain of 5S rRNA carry the essential information for these functional activities. Assembly of microinjected 5S rRNA into 60S ribosomal subunits was shown to occur in the nucleus; thus, the first requirement for subunit assembly is nuclear targeting. The inhibitory effects of ATP depletion, wheat germ agglutinin, and chilling on the nuclear import of 5S rRNA indicate that it crosses the nuclear envelope through the nuclear pore complex by a pathway similar to that used by karyophilic proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Akey C. W., Goldfarb D. S. Protein import through the nuclear pore complex is a multistep process. J Cell Biol. 1989 Sep;109(3):971–982. doi: 10.1083/jcb.109.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison L. A., Romaniuk P. J., Bakken A. H. RNA-protein interactions of stored 5S RNA with TFIIIA and ribosomal protein L5 during Xenopus oogenesis. Dev Biol. 1991 Mar;144(1):129–144. doi: 10.1016/0012-1606(91)90485-l. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Zapp M. L., Green M. R., Szostak J. W. HIV-1 Rev regulation involves recognition of non-Watson-Crick base pairs in viral RNA. Cell. 1991 Nov 1;67(3):529–536. doi: 10.1016/0092-8674(91)90527-6. [DOI] [PubMed] [Google Scholar]
- Baserga S. J., Gilmore-Hebert M., Yang X. W. Distinct molecular signals for nuclear import of the nucleolar snRNA, U3. Genes Dev. 1992 Jun;6(6):1120–1130. doi: 10.1101/gad.6.6.1120. [DOI] [PubMed] [Google Scholar]
- Bataillé N., Helser T., Fried H. M. Cytoplasmic transport of ribosomal subunits microinjected into the Xenopus laevis oocyte nucleus: a generalized, facilitated process. J Cell Biol. 1990 Oct;111(4):1571–1582. doi: 10.1083/jcb.111.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin F., Romaniuk P. J. A difference in the importance of bulged nucleotides and their parent base pairs in the binding of transcription factor IIIA to Xenopus 5S RNA and 5S RNA genes. Nucleic Acids Res. 1989 Mar 11;17(5):2043–2056. doi: 10.1093/nar/17.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin F., Romaniuk P. J., Romby P., Brunel C., Westhof E., Ehresmann B., Ehresmann C. Involvement of "hinge" nucleotides of Xenopus laevis 5 S rRNA in the RNA structural organization and in the binding of transcription factor TFIIIA. J Mol Biol. 1991 Mar 5;218(1):69–81. doi: 10.1016/0022-2836(91)90874-6. [DOI] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Brow D. A., Geiduschek E. P. Modulation of yeast 5 S rRNA synthesis in vitro by ribosomal protein YL3. A possible regulatory loop. J Biol Chem. 1987 Oct 15;262(29):13953–13958. [PubMed] [Google Scholar]
- Brunel C., Romby P., Westhof E., Romaniuk P. J., Ehresmann B., Ehresmann C. Effect of mutations in domain 2 on the structural organization of oocyte 5 S rRNA from Xenopus laevis. J Mol Biol. 1990 Sep 5;215(1):103–111. doi: 10.1016/S0022-2836(05)80099-3. [DOI] [PubMed] [Google Scholar]
- Chow C. S., Hartmann K. M., Rawlings S. L., Huber P. W., Barton J. K. Delineation of structural domains in eukaryotic 5S rRNA with a rhodium probe. Biochemistry. 1992 Apr 7;31(13):3534–3542. doi: 10.1021/bi00128a030. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell. 1990 Nov 16;63(4):655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- Dabauvalle M. C., Schulz B., Scheer U., Peters R. Inhibition of nuclear accumulation of karyophilic proteins in living cells by microinjection of the lectin wheat germ agglutinin. Exp Cell Res. 1988 Jan;174(1):291–296. doi: 10.1016/0014-4827(88)90163-2. [DOI] [PubMed] [Google Scholar]
- Dargemont C., Kühn L. C. Export of mRNA from microinjected nuclei of Xenopus laevis oocytes. J Cell Biol. 1992 Jul;118(1):1–9. doi: 10.1083/jcb.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Denis H., le Maire M. Thesaurisomes, a novel kind of nucleoprotein particle. Subcell Biochem. 1983;9:263–297. doi: 10.1007/978-1-4613-3533-7_3. [DOI] [PubMed] [Google Scholar]
- Draper D. E. How do proteins recognize specific RNA sites? New clues from autogenously regulated ribosomal proteins. Trends Biochem Sci. 1989 Aug;14(8):335–338. doi: 10.1016/0968-0004(89)90167-9. [DOI] [PubMed] [Google Scholar]
- Dworetzky S. I., Feldherr C. M. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol. 1988 Mar;106(3):575–584. doi: 10.1083/jcb.106.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworetzky S. I., Lanford R. E., Feldherr C. M. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1988 Oct;107(4):1279–1287. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Ellmeier W., Birnstiel M. L. Mature mRNA 3' end formation stimulates RNA export from the nucleus. EMBO J. 1991 Nov;10(11):3513–3522. doi: 10.1002/j.1460-2075.1991.tb04915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabijanski S., Pellegrini M. Identification of proteins at the peptidyl-tRNA binding site of rat liver ribosomes. Mol Gen Genet. 1981;184(3):551–556. doi: 10.1007/BF00352539. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Darby M. K., Gerace L. A monoclonal antibody against the nuclear pore complex inhibits nucleocytoplasmic transport of protein and RNA in vivo. J Cell Biol. 1988 Oct;107(4):1289–1297. doi: 10.1083/jcb.107.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Forbes D. J. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990 Jan 12;60(1):17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Meier E., Bradley P., Horecka J., Forbes D. J. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991 Jul;114(1):169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Darzynkiewicz E., Tahara S. M., Dathan N. A., Lührmann R., Mattaj I. W. Diversity in the signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991 May;113(4):705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Sumpter V., Sekine M., Satoh T., Lührmann R. Nucleo-cytoplasmic transport of U snRNPs: definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G cap. EMBO J. 1993 Feb;12(2):573–583. doi: 10.1002/j.1460-2075.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D., Michaud N. Pathways for the nuclear transport of proteins and RNAs. Trends Cell Biol. 1991 Jul;1(1):20–24. doi: 10.1016/0962-8924(91)90065-h. [DOI] [PubMed] [Google Scholar]
- Guddat U., Bakken A. H., Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in xenopus oocytes. Cell. 1990 Feb 23;60(4):619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- Hamm J., Darzynkiewicz E., Tahara S. M., Mattaj I. W. The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell. 1990 Aug 10;62(3):569–577. doi: 10.1016/0092-8674(90)90021-6. [DOI] [PubMed] [Google Scholar]
- Hamm J., Mattaj I. W. Monomethylated cap structures facilitate RNA export from the nucleus. Cell. 1990 Oct 5;63(1):109–118. doi: 10.1016/0092-8674(90)90292-m. [DOI] [PubMed] [Google Scholar]
- Holmberg L., Melander Y., Nygård O. Ribosome-bound eukaryotic elongation factor 2 protects 5 S rRNA from modification. J Biol Chem. 1992 Oct 25;267(30):21906–21910. [PubMed] [Google Scholar]
- Huber P. W., Wool I. G. Use of the cytotoxic nuclease alpha-sarcin to identify the binding site on eukaryotic 5 S ribosomal ribonucleic acid for the ribosomal protein L5. J Biol Chem. 1986 Mar 5;261(7):3002–3005. [PubMed] [Google Scholar]
- Imamoto N., Matsuoka Y., Kurihara T., Kohno K., Miyagi M., Sakiyama F., Okada Y., Tsunasawa S., Yoneda Y. Antibodies against 70-kD heat shock cognate protein inhibit mediated nuclear import of karyophilic proteins. J Cell Biol. 1992 Dec;119(5):1047–1061. doi: 10.1083/jcb.119.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Cox R. A. The nucleotide sequence at the 3'-end of Neurospora crassa 18S-rRNA and studies on the interaction with 5S-rRNA. Nucleic Acids Res. 1982 Nov 11;10(21):6733–6745. doi: 10.1093/nar/10.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Gupta A., Ware V. C. Nucleocytoplasmic transport of ribosomes in a eukaryotic system: is there a facilitated transport process? Proc Natl Acad Sci U S A. 1989 Mar;86(6):1791–1795. doi: 10.1073/pnas.86.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagaye S., Barque J. P., le Maire M., Denis H., Larsen C. J. Characterization by human antibodies of two HeLa cell proteins which are related to Xenopus laevis transcription factor TFIIIA. Nucleic Acids Res. 1988 Mar 25;16(6):2473–2487. doi: 10.1093/nar/16.6.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A. C., Nazar R. N. Topography of 5.8 S rRNA in rat liver ribosomes. Identification of diethyl pyrocarbonate-reactive sites. J Biol Chem. 1982 Apr 10;257(7):3516–3524. [PubMed] [Google Scholar]
- Martin K., Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991 Oct 4;67(1):117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Zeller R., DeRobertis E. M. Nuclear exclusion of transcription factor IIIA and the 42s particle transfer RNA-binding protein in Xenopus oocytes: a possible mechanism for gene control? J Cell Biol. 1983 Oct;97(4):1261–1265. doi: 10.1083/jcb.97.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlin H., Daneholt B., Skoglund U. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell. 1992 May 15;69(4):605–613. doi: 10.1016/0092-8674(92)90224-z. [DOI] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992 Jul 10;70(1):127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- Michaud N., Goldfarb D. S. Multiple pathways in nuclear transport: the import of U2 snRNP occurs by a novel kinetic pathway. J Cell Biol. 1991 Jan;112(2):215–223. doi: 10.1083/jcb.112.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N., Goldfarb D. Microinjected U snRNAs are imported to oocyte nuclei via the nuclear pore complex by three distinguishable targeting pathways. J Cell Biol. 1992 Feb;116(4):851–861. doi: 10.1083/jcb.116.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Neuman de Vegvar H. E., Dahlberg J. E. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol Cell Biol. 1990 Jul;10(7):3365–3375. doi: 10.1128/mcb.10.7.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Peters R. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 1984 Aug;3(8):1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E. Messenger RNA for ribosomal proteins in Xenopus laevis oocytes. Eur J Biochem. 1980 May;106(2):603–611. doi: 10.1111/j.1432-1033.1980.tb04608.x. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., van Venrooij W. J. Analysis of protein--RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991 Oct 11;19(19):5173–5180. doi: 10.1093/nar/19.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J. The role of highly conserved single-stranded nucleotides of Xenopus 5S RNA in the binding of transcription factor IIIA. Biochemistry. 1989 Feb 7;28(3):1388–1395. doi: 10.1021/bi00429a067. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., de Stevenson I. L., Ehresmann C., Romby P., Ehresmann B. A comparison of the solution structures and conformational properties of the somatic and oocyte 5S rRNAs of Xenopus laevis. Nucleic Acids Res. 1988 Mar 25;16(5):2295–2312. doi: 10.1093/nar/16.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., de Stevenson I. L., Wong H. H. Defining the binding site of Xenopus transcription factor IIIA on 5S RNA using truncated and chimeric 5S RNA molecules. Nucleic Acids Res. 1987 Mar 25;15(6):2737–2755. doi: 10.1093/nar/15.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge K. D., Maxwell E. S. Intermolecular hybridization of 5S rRNA with 18S rRNA: identification of a 5'-terminally-located nucleotide sequence in mouse 5S rRNA which base-pairs with two specific complementary sequences in 18S rRNA. Biochim Biophys Acta. 1991 Jan 17;1088(1):57–70. doi: 10.1016/0167-4781(91)90153-d. [DOI] [PubMed] [Google Scholar]
- Shi Y., Thomas J. O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992 May;12(5):2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Gerbi S. A. Structural analysis of the peptidyl transferase region in ribosomal RNA of the eukaryote Xenopus laevis. J Mol Biol. 1991 Jan 5;217(1):93–112. doi: 10.1016/0022-2836(91)90614-c. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Berg C., Hendrick J. P., La Branche-Chabot H., Metspalu A., Rinke J., Yario T. A 5S rRNA/L5 complex is a precursor to ribosome assembly in mammalian cells. J Cell Biol. 1988 Mar;106(3):545–556. doi: 10.1083/jcb.106.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R., Blevitt J. M., Gerace L. O-linked glycoproteins of the nuclear pore complex interact with a cytosolic factor required for nuclear protein import. J Cell Biol. 1992 Jan;116(2):271–280. doi: 10.1083/jcb.116.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen O., Rudt F., Guddat U., Mentzel H., Pieler T. RNA and DNA binding zinc fingers in Xenopus TFIIIA. Cell. 1992 Nov 13;71(4):679–690. doi: 10.1016/0092-8674(92)90601-8. [DOI] [PubMed] [Google Scholar]
- Tobian J. A., Drinkard L., Zasloff M. tRNA nuclear transport: defining the critical regions of human tRNAimet by point mutagenesis. Cell. 1985 Dec;43(2 Pt 1):415–422. doi: 10.1016/0092-8674(85)90171-0. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Kikuchi M., Ogata K. Cross-linking study on protein neighborhoods at the subunit interface of rat liver ribosomes with 2-iminothiolane. J Biol Chem. 1986 Jul 25;261(21):9663–9667. [PubMed] [Google Scholar]
- Van Ryk D. I., Lee Y., Nazar R. N. Unbalanced ribosome assembly in Saccharomyces cerevisiae expressing mutant 5 S rRNAs. J Biol Chem. 1992 Aug 15;267(23):16177–16181. [PubMed] [Google Scholar]
- Viel A., Armand M. J., Callen J. C., Gomez De Gracia A., Denis H., le Maire M. Elongation factor 1 alpha (EF-1 alpha) is concentrated in the Balbiani body and accumulates coordinately with the ribosomes during oogenesis of Xenopus laevis. Dev Biol. 1990 Oct;141(2):270–278. doi: 10.1016/0012-1606(90)90383-t. [DOI] [PubMed] [Google Scholar]
- Westhof E., Romby P., Romaniuk P. J., Ebel J. P., Ehresmann C., Ehresmann B. Computer modeling from solution data of spinach chloroplast and of Xenopus laevis somatic and oocyte 5 S rRNAs. J Mol Biol. 1989 May 20;207(2):417–431. doi: 10.1016/0022-2836(89)90264-7. [DOI] [PubMed] [Google Scholar]
- Wimberly B., Varani G., Tinoco I., Jr The conformation of loop E of eukaryotic 5S ribosomal RNA. Biochemistry. 1993 Feb 2;32(4):1078–1087. doi: 10.1021/bi00055a013. [DOI] [PubMed] [Google Scholar]
- Wormington W. M. Developmental expression and 5S rRNA-binding activity of Xenopus laevis ribosomal protein L5. Mol Cell Biol. 1989 Dec;9(12):5281–5288. doi: 10.1128/mcb.9.12.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Q. M., Romaniuk P. J. The effects of disrupting 5S RNA helical structures on the binding of Xenopus transcription factor IIIA. Nucleic Acids Res. 1990 Sep 11;18(17):5055–5062. doi: 10.1093/nar/18.17.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carrier-mediated translocation process. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stevenson I. L., Romby P., Baudin F., Brunel C., Westhof E., Ehresmann C., Ehresmann B., Romaniuk P. J. Structural studies on site-directed mutants of domain 3 of Xenopus laevis oocyte 5 S ribosomal RNA. J Mol Biol. 1991 May 20;219(2):243–255. doi: 10.1016/0022-2836(91)90565-n. [DOI] [PubMed] [Google Scholar]
- le Maire M., Denis H. Biochemical research on oogenesis. Binding of tRNA to the nucleoprotein particles of Xenopus laevis previtellogenic oocytes. J Biol Chem. 1987 Jan 15;262(2):654–659. [PubMed] [Google Scholar]